Cellular control of connective tissue matrix tension†
Conflict of interest: nothing to declare.
Abstract
The biomechanical behavior of connective tissue in response to stretching is generally attributed to the molecular composition and organization of its extracellular matrix. It also is becoming apparent that fibroblasts play an active role in regulating connective tissue tension. In response to static stretching of the tissue, fibroblasts expand within minutes by actively remodeling their cytoskeleton. This dynamic change in fibroblast shape contributes to the drop in tissue tension that occurs during viscoelastic relaxation. We propose that this response of fibroblasts plays a role in regulating extracellular fluid flow into the tissue, and protects against swelling when the matrix is stretched. This article reviews the evidence supporting possible mechanisms underlying this response including autocrine purinergic signaling. We also discuss fibroblast regulation of connective tissue tension with respect to lymphatic flow, immune function, and cancer. J. Cell. Biochem. 114: 1714–1719, 2013. © 2013 Wiley Periodicals, Inc.
The field of mechanobiology has provided a growing awareness that cells respond to mechanical forces, and that these forces influence a host of physiological and pathological processes including wound healing, inflammation, and cancer [Janmey et al., 2009]. In all organs, the connective tissue matrix or stroma is a key determinant of the transmission of these tissue forces. In other words, how much stress occurs in response to an externally applied deformation is determined by the material properties of the stroma (i.e., its intrinsic “hardness” or “softness”) which is largely determined by its matrix content and structure [Fung, 1981]. In addition, recent evidence is showing that tension within the stroma is under cellular control. This article will review the evidence suggesting that fibroblasts within areolar connective tissue play an active role in regulating tissue tension by adjusting to changes in tissue length. We speculate that this function of fibroblasts plays a protective role in minimizing osmotically driven swelling of tissue when the extracellular matrix is stretched. This role of fibroblasts in extracellular fluid dynamics could contribute to overall health and immune function as well as the body's defense against cancer.
MYOFIBROBLAST TRANSFORMATION AND CONNECTIVE TISSUE CONTRACTION
It has been known since the 1970s that, in response to injury, fibroblasts transform into contractile cells or myofibroblasts that can generate tissue tension during scar formation, as well as some pathological states including fibrosis and cancer [Gabbiani, 2003]. Importantly, tissue contraction produced by myofibroblasts is sustained, and is thought to continue unabated after the injury until the myofibroblast undergoes apoptosis [Tomasek et al., 2002]. Although the contractile function of myofibroblasts is vital to insure proper closure of wounds, it is also thought to have a “dark” side that can contribute to fibrotic and neoplastic disease [Hinz, 2007].
FIBROBLAST MODULATION OF CONNECTIVE TISSUE TENSION
Our recent work has shown that fibroblasts can dynamically influence the tension of connective tissue by rapidly remodeling their cytoskeleton, without transforming into myofibroblasts [Langevin et al., 2011]. Tension modulation by fibroblasts is rapid and occurs within minutes in response to sustained changes in tissue length within the physiological range. When whole areolar connective tissue is elongated, fibroblasts expand and flatten, such that their cross-sectional area in the plane of the tissue increases several fold [Langevin et al., 2005]. This expansion and flattening requires intact microtubules and actin microfilaments. Importantly, this change in fibroblast shape is accompanied by a lowering of connective tissue tension [Langevin et al., 2011]. Furthermore, these cytoskeletal responses appear to be specific to areolar or “loose” connective tissues, such as those forming interfaces between subcutaneous and perimuscular layers, and does not occur in more densely packed connective tissues such as dermis [Abbott et al., 2013].
REGULATION OF INTERSTITIAL TISSUE FLUID PRESSURE
What role might this extensive cytoskeletal remodeling of fibroblast play in connective tissue physiology in vivo? An intriguing possibility is that this fibroblast response could actively regulate interstitial fluid pressure and flow. A defining characteristic of areolar connective tissue is its loose collagen mesh filled with polyanionic glycosaminoglycans that bind large quantities of water. It is a well-known phenomenon that an excised piece of connective tissue swells when placed in an isotonic bath, and extrudes water when compressed. The reasons for this swelling tendency are that (1) glycosaminoglycans have large numbers of negative charges that attract water and (2) the areolar connective tissue matrix is not maximally hydrated in vivo under normal physiological conditions. Although this has been known for quite some time, the mechanism responsible for this matrix “under-hydration” has only recently been elucidated. In a series of elegant experiments, Reed et al. showed that tension exerted by fibroblasts onto the collagen network actively restrains the loose matrix and prevents water from entering the tissue [Wiig et al., 2003; Reed et al., 2010]. Reed et al. also showed that a large initial drop in interstitial fluid pressure occurs at the onset of acute inflammation, and that this drop in fluid pressure is inhibited when cell–matrix contacts are disrupted using integrin blockers (Fig. 1) [Rodt and Reed, 1993; Reed and Rubin, 2010]. Thus, it appears that inflammatory edema is not simply due to increased vascular permeability, but is also caused by fluid being “sucked into” the tissue. This makes sense if one thinks of areolar connective tissue as a meshwork with pores partially occupied by cells (fibroblasts) that hold on to the sides of the pores. Keeping the mesh within a certain average pore size prevents swelling in the absence of inflammation. Inflammatory mediators unrestrain the matrix by disrupting integrin-mediated cell–matrix contacts, which causes a drop in matrix tension and allows the matrix to swell. The initial “loosening” of the matrix tension is then followed by an increase in matrix stiffness and turgidity, once water has entered into the tissue following the now accessible osmotic gradient created by the larger tissue pores.
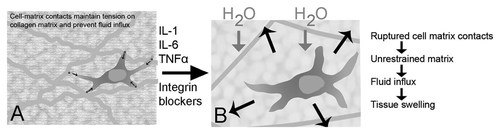
Role of fibroblasts in promoting fluid flux into the tissue during acute inflammation (adapted from Reed et al. [Wiig et al., 2003; Reed and Rubin, 2010]). A: In areolar connective tissue, the loose collagen mesh is filled with polyanionic glycosaminoglycans that attract large quantities of water. In the absence of inflammation, active tension exerted by fibroblasts onto the collagen network keeps the tissue under-hydrated by restraining the matrix, keeping pore size small and restricting water entry into the tissue. B: In the early stages of acute inflammation, inflammatory mediators cause the rupture of cell–matrix contacts. This unrestrains the matrix, allowing fluid to enter the tissue driven by the osmotic force created by the now accessible glycosaminoglycans. Integrin blockers have a similar effect by interfering with cell–matrix contacts.
The presence of such a mechanism, however, begs the following question: how does connective tissue maintain the critical pore size needed to avoid tissue swelling when the collagen matrix is mechanically stretched? As illustrated in Figure 2A,B, sustained stretching of the matrix for several minutes should cause the reverse of compression, that is, a decrease in matrix compaction and increase in pore size. If allowed to continue for several minutes, this decrease in matrix compaction would promote the flow of fluid from the capillary bed into the extracellular space. How would fibroblasts within the tissue respond to such a situation? One possibility is that the fibroblasts might “let go” of the cell–matrix contacts in response to matrix stretching. This would have the drawback of further unrestraining the matrix, causing an even greater transient drop in interstitial fluid pressure, an increase in transcapillary fluid flow and further swelling, similar to what happens during inflammation (Fig. 2C). Alternatively, fibroblasts could remodel and expand, maintaining and remodeling their cell–matrix contacts while continuing to exert counter-tension until a new tension equilibrium is reached (Fig. 2D). This would restrain the matrix and avoid tissue swelling. This mechanism, if true, would require fibroblasts to be able to (1) maintain cell–matrix contacts in the face of increased tissue strain (and, presumably, increased local stress) and (2) expand to accommodate the increased tissue pore size.
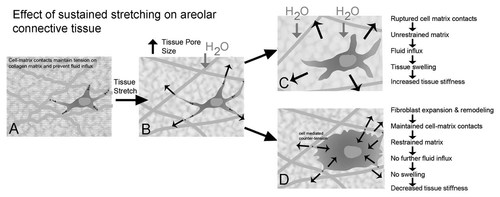
Proposed mechanism for fibroblast control of matrix tension and fluid flux in response to tissue stretch. A: Fibroblasts maintain tension on the extracellular matrix and prevent fluid influx into the tissue. B: Sustained stretching of the matrix for several minutes decreases matrix compaction and increase in pore size, allowing water to flow in. C: Fibroblasts “letting go” of the cell–matrix contacts would further unrestrain the matrix and cause further swelling. D: Fibroblast remodeling, expansion, and maintenance of cell–matrix contacts would keep the matrix restrained and reduces water influx into the tissue.
MECHANISMS ALLOWING MAINTENANCE OF CELL–MATRIX CONTACTS IN STRETCHED TISSUE
Intuitively, it is clear that there is a level of stress at which any cell–matrix contact will fail, either through pure mechanical failure, regulated disassembly/release, or a combination of both. While the numerical value of such a “failure point” has not been experimentally determined for fibroblasts in a 3D matrix, it would undoubtedly vary as a function of the qualitative and quantitative molecular composition of both sides of the cell–matrix contacts [Pedersen and Swartz, 2005; Harunaga and Yamada, 2011] as well as the milieu of soluble factors surrounding the cell during the applied stress. Furthermore, while the mechanism for such release also has not been directly investigated, it would likely invoke some or all of the pathways described for focal adhesion disassembly/turnover (e.g., Rho- and myosin IIB-dependent contractility, calpain-mediated proteolysis of focal adhesion proteins such as talin and FAK [Wehrle-Haller, 2012]). It has long been known that the abrupt release of a highly strained cellular contact can allow and even promote membrane and cytoskeletal remodeling in the rest of the cell [Chen, 1981a, 1981b] and that regulated dissolution of adhesive contacts is a bona fide protective mechanism in response to excessive matrix stretching and tension [Liu et al., 2011]. On the other hand, the ability of cells to respond to applied mechanical stress by strengthening the strained adhesive contacts also is well documented [Bershadsky et al., 2006; Geiger et al., 2009; Parsons et al., 2010] and provides a clear pathway for active tensional regulation. While this response is becoming increasingly well understood at discrete cellular, molecular, and biophysical mechanistic levels [Schober et al., 2007; Solon et al., 2007; Aratyn-Schaus and Gardel, 2010; Dumbauld et al., 2010; Grashoff et al., 2010; Maruthamuthu et al., 2010; Stricker et al., 2011; Watanabe-Nakayama et al., 2011; Wolfenson et al., 2011], considerable additional effort will be required to form a unifying theoretical mechanism and even more effort to apply its tenets to mechanoresponses in 3D.
MECHANISMS LEADING TO FIBROBLAST EXPANSION
If one assumes that fibroblasts are able to maintain cell–matrix contacts in the stretched tissue, what mechanism would trigger the increase in cell size? An interesting possibility is that the initial fluid influx (and drop in tissue osmotic pressure) at the onset of stretching could itself constitute the mechanism triggering fibroblast remodeling and expansion. This hypothesis is supported by the observation that increasing the osmolarity of the tissue bath inhibits fibroblast expansion in response to tissue stretch (Fig. 3). Koyama et al. showed that, in cultured fibroblasts, hypotonic stress causes a change in cell shape from stellate to expanded and flattened that is similar to the remodeling of fibroblasts that occurs in whole connective tissue in response to static stretch. Furthermore, this osmotically induced cytoskeletal remodeling was accompanied by a Rho-dependent sustained extracellular release of ATP. Several lines of evidence suggest that changes in cell volume, triggered by either changes in osmolarity or mechanical stress are linked to a sustained extracellular release of ATP [Dezaki et al., 2000; Koyama et al., 2001; Luckprom et al., 2011; Lu et al., 2012]. ATP can then activate purine receptors on the cell surface followed by an increase in cytosolic Ca2+ concentration [Pedersen et al., 1999]. This autocrine signaling pathway appears to be adaptive, as the elevation in Ca2+ activate membrane channels and co-transporters necessary for restoring or altering cell volume [Feranchak et al., 1998; Dezaki et al., 2000], and at the same time may facilitate reorganization of the actin cytoskeleton. In support of this mechanism, we have recently shown that exposure of cultured fibroblast to purine receptor agonists induced both Ca2+ increases and a transient disassembly of the actin cytoskeleton [Goldman et al., 2013]. The Ca2+ pump inhibitor, cyclopiazonic acid (CPA) eliminated both the purine induced Ca2+ increases and the actin cytoskeleton disassembly. Interestingly, however, elevations of cytosolic Ca2+ were in themselves not sufficient for this transient cytoskeletal remodeling, since the Ca2+ ionophore A-23187 did not induce significant alterations in the fibroblast actin organization, despite triggering robust increases in cytosolic Ca2+. Possible mechanisms by which Ca2+ increases may trigger a transient remodeling/severing of actin filaments include activation of gelsolin [Kinosian et al., 1998] and/or ADF/cofilin [Wang et al., 2005]. Ca2+ influx also can promote adhesion disassembly through multiple mechanisms [Bhatt et al., 2002; Easley et al., 2008], which may be necessary for remodeling of the cell structure to maintaining tissue tension. In further support of the possible role of ATP signaling in stretch-induced fibroblast expansion, we have recently found that stretching of whole connective tissue ex vivo is accompanied by a sustained Rho-dependent release of extracellular ATP [Langevin et al., in press]. We also showed that both suramin (inhibitor of ATP binding to cell surface receptors) and apyrase (enzyme degrading extracellular ATP) inhibit fibroblast expansion in response to tissue stretch ex vivo. Taken together, these findings suggest that the remodeling of fibroblasts induced by mechanical stretching of connective tissue may be the consequence ATP autocrine signaling triggered by the initial drop in tissue osmotic pressure that occurs as water enters into the stretched tissue.
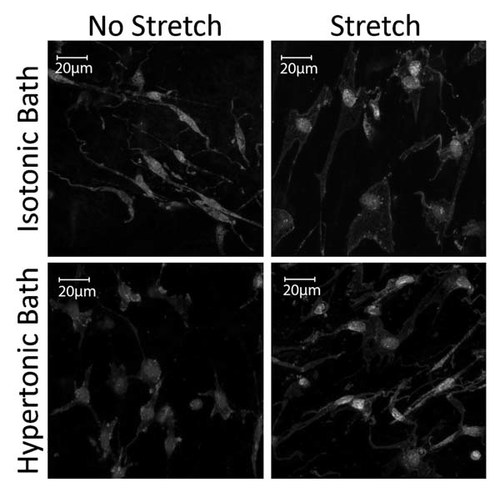
Response to fibroblasts to tissue stretch ex vivo in isotonic and hypertonic tissue bath. Raising the osmolarity of the bath by 20–40% (by adding bovine serum albumin) inhibited the expansion of fibroblasts in response to tissue stretch.
POTENTIAL CONSEQUENCES FOR INTERSTITIAL FLUID FLOW, LYMPHATIC FLOW AND IMMUNE FUNCTION
A change in fibroblast shape occurring in response to sustained stretching of areolar connective tissue would serve two related purposes: (1) preserve cell–matrix contacts and (2) prevent tissue swelling. Interstitial fluid slowly and constantly moves through the interstitial space, returning to the vascular compartment via the lymphatics at a speed of ∼0.1–2 µm/s [Swartz and Fleury, 2007]. Flow into lymphatics is determined by pressure gradients between the interstitium and lymphatics, with an increase in interstitial pressure driving fluid flow [Swartz and Fleury, 2007]. Compression of tissues during body movements (e.g., walking) plays a well-established role in promoting interstitial fluid flow. However, tissue stretching during these same movements might have effects opposite of those of compression (i.e., transiently decreasing interstitial fluid pressure and “pooling” fluid into the tissues). If connective tissue is in fact like a sponge, taking up water every time tissues are stretched for more than a few minutes might not be beneficial (e.g., during changes in posture or sleep position). Although increased tissue tension due to tissue swelling would ultimately increase interstitial fluid pressure and restore lymphatic drainage, this would come at the cost of an expanded, swollen matrix. Therefore, connective tissue needs a mechanism that would allow it to be stretched without becoming “water-logged.”
If the fibroblast cytoskeletal mechanisms outlined above are correct, fibroblast remodeling might serve as a protective mechanism against stretch-induced fluid stasis by keeping the matrix under tension equilibrium. Importantly, inflammatory mediators such as PGE1, IL-1, IL-6, and TNF-α have been shown to have the same effect as integrin blockers in lowering interstitial fluid pressure [Reed et al., 2010]. It, therefore, is plausible that even low levels of inflammation in the tissue could predispose to swelling and fluid stasis when connective tissue is being stretched. Although the consequences of fluid stasis are not well understood, we know that lymph stasis due to lymphatic duct obstruction interferes with immune surveillance and predisposes to fibrosis and fat accumulation into the tissues [Alitalo, 2011]. Fibroblast dysfunction, therefore, could contribute to tissue inflammation, fatty infiltration, and fibrosis, which, importantly, all are thought to promote cancer formation.
CONTRASTING BEHAVIOR OF FIBROBLASTS AND MYOFIBROBLASTS
The presence of myofibroblasts in tissues increases matrix stiffness in both chronically inflamed and cancer-associated tissues [Hinz, 2007; Swartz and Lund, 2012]. There is recent evidence that this increase in stiffness is accompanied by increased interstitial pressure and lymphatic flow, and that these factors may predispose an individual to cancer invasion and metastasis [Swartz and Lund, 2012]. If both fibroblasts and myofibroblasts can exert tension on the matrix, how could this protect against cancer in one case and promote it in another? This apparent paradox can be resolved if one considers that the primary function of myofibroblasts is to close wounds. In order to perform this task, myofibroblasts need to pull continuously on the matrix until the job is done. Furthermore, myofibroblasts seem to operate via a positive feedback mechanism, that is, the more tension there is in the matrix, the harder they pull [Tomasek et al., 2002]. This contrasts with the dynamic regulation of tissue tension that results from cytoskeletal remodeling in normal fibroblasts. In the model proposed here, areolar connective tissue fibroblasts use their cytoskeletal machinery to adjust the tension of the matrix, not simply pull on it. The feedback loop allowing this tension regulation appears to involve osmosensation and ATP signaling, reminiscent of mechanisms used by astrocytes to protect the brain from swelling in the presence of hyponatremia [Risher et al., 2009; Thrane et al., 2011]. In the case of myofibroblasts, continuous stretching over several days may give rise to sustained release of ATP and prolonged and activation of P2Y2 receptors causing an increase alpha-smooth muscle actin (alpha-SMA) production and collagen accumulation [Lu et al., 2012]. One can also speculate that alteration in purinergic signaling of myofibroblast could contribute to the increased interstitial pressure and matrix stiffness characteristic of inflamed and cancer-associated tissues.
It is important to note, however, that a great deal of our current knowledge of “fibroblasts” from cell culture models applies more to myofibroblasts than to fibroblasts. There is a tendency in the literature to attribute myofibroblast behaviors (e.g., stress fiber formation, sustained matrix traction) to normal fibroblasts. However, stress fibers are not present in fibroblasts within loose connective tissues, or fibroblasts grown in loose compliant 3D gels, in the absence of a stimulus (e.g., TGFβ) leading to myofibroblast transformation [Grinnell, 2003]. It is, therefore, important that our conclusions regarding the function of fibroblasts need to take these considerations into account, especially with respect to mechanosensing and mechanotransduction.
- (1)
The sponge's pore size is regulated to admit an optimal amount of water molecules according to whether the sponge is in a stretched or compressed state.
- (2)
Opposing forces that determine water flux into the sponge are the osmotic pull of under-hydrated glycosaminoglycans and active restraint of the collagen mesh by fibroblasts.
- (3)
Water flux in and out of the tissue also serves as the mechanism by which fibroblasts sense a change in osmotic pressure and adjust their size, driven by ATP autocrine signaling.
- (4)
Expansion of fibroblasts in response to a lower tissue osmotic pressure allows maintenance of cell–matrix contacts and prevents further water influx and swelling.
Areolar connective tissue wears many hats: as part of the musculoskeletal system, it forms compliant layers that can stretch linearly up to 60% of their length without tearing [Iatridis et al., 2003], yet can bear significant loads when subjected to shear stress. Areolar connective tissue also is the “container” through which metabolic, endocrine, and immune exchanges take place in all tissues, as well as the conduit through which water, proteins, and immune cells return to the blood via lymphatics. Reconciling connective tissue's biomechanical, metabolic, and immune functions demands mechanisms ensuring that these roles do not interfere with one another. The mechanisms proposed here could play an important role in maintaining a healthy interstitial fluid environment, metabolic homeostasis, and immune surveillance, all the while allowing the body to move and stretch.
Acknowledgements
The authors thank Cathryn Koptiuch for technical assistance and manuscript preparation, Dr. Rosalyn Abbott for helpful discussions, Nicole Bouffard for creating the illustrations, and Ben Kress for editing the manuscript. This work was funded by the National Institutes of Health Center for Complementary and Alternative Medicine research Grant RO1-AT01121. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.




