The role of hair follicle nestin-expressing stem cells during whisker sensory-nerve growth in long-term 3D culture†
Conflict of Interest: none.
Abstract
We have previously reported that nestin-expressing hair follicle stem cells can differentiate into neurons, Schwann cells, and other cell types. In the present study, vibrissa hair follicles, including their sensory nerve stump, were excised from transgenic mice in which the nestin promoter drives green fluorescent protein (ND-GFP mice), and were placed in 3D histoculture supported by Gelfoam®. β-III tubulin-positive fibers, consisting of ND-GFP-expressing cells, extended up to 500 µm from the whisker nerve stump in histoculture. The growing fibers had growth cones on their tips expressing F-actin. These findings indicate that β-III tubulin-positive fibers elongating from the whisker follicle sensory nerve stump were growing axons. The growing whisker sensory nerve was highly enriched in ND-GFP cells which appeared to play a major role in its elongation and interaction with other nerves in 3D culture, including the sciatic nerve, the trigeminal nerve, and the trigeminal nerve ganglion. The results of the present report suggest a major function of the nestin-expressing stem cells in the hair follicle is for growth of the follicle sensory nerve. J. Cell. Biochem. 114: 1674–1684, 2013. © 2013 Wiley Periodicals, Inc.
The intermediate filament protein, nestin, marks progenitor cells of the CNS. Such CNS stem cells can be selectively labeled by placing green fluorescent protein (GFP) under the control of the nestin promoter in transgenic mice, termed nestin-driven GFP (ND-GFP) mice. Our laboratory previously observed that in ND-GFP mice, small oval-shaped, nestin-expressing cells in the bulge area of the hair follicle surround the hair shaft and are interconnected by short dendrites. During telogen and in early anagen, the nestin cells are mainly located in the bulge area, but in mid- and late-anagen, the nestin-expressing cells are located in the outer-root sheath as well as in the bulge area, suggesting the nestin-expressing cells give rise to these structures [Li et al., 2003; Amoh et al., 2012].
In vitro, the hair follicle nestin-expressing cells differentiated into neurons, glia, keratinocytes, smooth muscle cells, and melanocytes [Amoh et al., 2005a]. When nestin-expressing cells from the mouse vibrissa bulge area were implanted into the gap region of the severed sciatic nerve, they effected functional nerve repair. The transplanted follicle bulge area cells transdifferentiated largely into Schwann cells, which are known to support neuron regrowth. The transplanted mice recovered the ability to walk normally [Amoh et al., 2005b]. Nestin-expressing mouse vibrissa cells from the bulge area were also transplanted to the injury site of mice in which the thoracic spinal cord was severed. Most of the transplanted cells also differentiated into Schwann cells that effected repair of the severed spinal cord. The rejoined spinal cord recovered and extensive hind-limb locomotor performance was re-established [Amoh et al., 2008].
Yu et al. [2010] cultured a population of human nestin-expressing hair follicle bulge area cells. The human bulge area cells proliferated as spheres, were capable of self-renewal, and differentiated into multiple lineages including myogenic, melanocytic, and neuronal cell lineages in vitro. Human hair follicle nestin-expressing cells were transplanted in the severed sciatic nerve of the mouse which differentiated into glial fibrillary-acidic-protein (GFAP)-positive Schwann cells and effected the functional recovery of the nerve [Amoh et al., 2009].
In the mouse whisker, nestin-expressing cells were found in the dermal papilla in early and middle anagen. In contrast to the dermal papilla, the bulge area had nestin expression throughout the hair cycle and to a greater extent than the dermal papilla. Nestin-expressing stem cells in the bulge area and dermal papilla have similar morphological features. The cells from both regions have a small body diameter of approximately 7 µm with long extrusions, as shown by confocal imaging. Nestin-expressing cells from both areas differentiated into neuronal cells at high frequency in vitro and differentiated into neuronal and glial cells after transplantation to the injured spinal cord and effected injury repair and locomotor recovery [Liu et al., 2011].
Using confocal imaging of whisker follicles from ND-GFP mice, it was found that the bulge area is the source of the nestin GFP-expressing cells of the hair follicle. The nestin GFP-expressing cells migrate from the bulge area to the dermal papilla as well as into the surrounding skin tissues including the epidermis, as well as during wound healing [Uchugonova et al., 2011].
In 3D Gelfoam® culture, it was observed by confocal imaging over a 2-week period that the nestin-expressing cells trafficked from the bulge area toward the dermal papilla area [Duong et al., 2012].
In the present study, we demonstrate in 3D Gelfoam® histoculture that the nestin-expressing cells in the whisker follicle bulge traffic to the truncated whisker sensory nerve and effect nerve growth and interaction with other co-cultured nerves.
MATERIALS AND METHODS
Animals
Transgenic mice with nestin-driven GFP (ND-GFP), as well as red fluorescent protein (RFP) transgenic mice, at different ages (4 weeks up to 5 months), were used for this study. All animal studies were conducted in accordance with the principals and procedures outlined in the National Institute of Health Guide for the Care and Use of Animals under Assurance Number A3873-1.
Isolation of Vibrissa-Follicles (Whiskers), Trigeminal and Sciatic Nerves, and the Trigeminal Nerve Ganglion
The mice were anesthetized with a 30–50 µl ketamine solution (25 mg/ml) [Uchugonova et al., 2011; Duong et al., 2012]. Whisker pads from ND-GFP transgenic mice were sterilized with 70% isopropyl alcohol and washed in PBS three times. Using a binocular microscope (MZ6 Leica, Wetzlar, Germany), the whisker pad was dissected to obtain single vibrissae follicles with forceps and fine needles. The isolated vibrissae hair follicles with and without capsules were used for culture and imaging.
In order to excise the trigeminal nerves, whisker pads from RFP transgenic mice were sterilized with 70% isopropyl alcohol. The trigeminal nerve from the infraorbital foramen in each vibrissa, on the inside of the whisker pad, was exposed and removed.
In order to isolate the sciatic nerve, a skin incision was made in the medial side of the thigh of RFP transgenic mice. The nerve was exposed between the short adductor muscle and long adductor muscle.
Using the MZ6 binocular microscope (Leica), the trigeminal nerve and the sciatic nerve were both excised with fine forceps. The lengths of the excised trigeminal nerve and sciatic nerve were 1.5–2 and 3–4 mm, respectively.
In order to isolate the trigeminal nerve ganglion, a skin incision was made on top of the head and the skull was opened with a drill. The trigeminal nerve ganglion was exposed after the skull base was removed. Using the binocular microscope, the trigeminal nerve ganglion was excised with fine forceps.
The isolated nerves and ganglion were washed in PBS three times before being placed in Gelfoam® histoculture (please see below).
Gelfoam® Histoculture and Growth Medium
The vibrissae hair follicles, with or without capsules and containing their sensory nerve stumps, as well as trigeminal and sciatic nerves and the trigeminal nerve ganglion described above, were put on sterile Gelfoam® (Pharmacia and Upjohn Co., Kalamazoo, MI) hydrated in cell culture medium [Duong et al., 2012]. The whisker sensory nerve stump and the trigeminal nerve or sciatic nerve were arranged with stumps opposed to each other. The vibrissae hair follicle containing the sensory nerve stump and the trigeminal nerve ganglion were arranged with the nerve stump opposed to the ganglion. Culture medium used was DMEM-F12 medium (GIBCO/BRL) containing B-27 (2.5%) (GIBCO/BRL), N2 (1%) (GIBCO/BRL), and 1% penicillin and streptomycin (GIBCO/BRL). The culture was incubated at 37°C, 5% CO2 100% humidity. The medium was changed every other day [Hoffman, 2010; Duong et al., 2012].
Confocal Laser Scanning Microscopy
A confocal laser scanning microscope (Fluoview FV1000, Olympus Corp., Tokyo, Japan) was used for two- (X,Y) and three-dimensional (3D, X,Y,Z) high-resolution imaging of vibrissa follicles and nerves in histoculture. Fluorescence images were obtained using the 4×/0.10 Plan N, 10×/0.30 Plan-NEOFLUAR, 20×/0.50 UPlan FL N, and 20×/1.00w XLUMplan FL objectives.
Histology and Immunofluorescence Staining
Tissues were fixed in pre-cooled 4% paraformaldehyde at room temperature (RT) for 2 h and embedded in tissue-freezing medium (Triangle Biomedical Science, Durham, NC) and frozen in nitrogen for 10 min at −80°C. Frozen sections of 7–10 µm thickness were prepared with a CM1850 cryostat (Leica). The frozen sections were washed with PBS three times. Some frozen sections were processed for hematoxilin and eosin staining. Immunofluoresence staining procedures were as follows: (1) 5% normal goat serum was applied at RT for 1 h. (2) Primary antibodies were applied at RT for 2 h. The primary antibodies used were anti-β III tubulin mAb (mouse, 1:100; Santa Cruz, Biotech, Dallas, TX); anti-glial fibrillary acidic protein (GFAP) mAb (mouse, 1:250; BD Pharmingen, San Jose, CA); anti-S100 mAb (mouse, 1:200; Millipore, Billerica, MA); anti-p75NTR mAb (rabbit, 1:3,200; Cell Signaling, Danvers, MA); anti-TrkA mAb (rabbit, 1:50; Santa Cruz Biotech) and anti-TrkB mAb (rabbit, 1:50; Santa Cruz Biotech). (3) Secondary antibodies used were: goat anti-mouse IgG (H + L) Alexa Fluor® 555 (1:1,000; Cell Signaling); goat anti-rabbit IgG (H + L) Alexa Fluor® 555 (1:1,000; Cell Signaling). Incubation conditions were: RT, dark, 1 h. (4) Alexa Fluor® 647 Phalloidin (1:40; Invitrogen, Grand Island, NY). For F-action detection, incubation conditions were: RT, dark, 1 h. (5) For DAPI (1:48,000; Invitrogen), incubation conditions were: RT, dark, 3 min. (6) Slides were mounted with Fluoromount (Sigma, St. Louis, MO) and observed under confocal laser scanning microscopy. Immunofluorescence staining was compared with positive and negative controls.
Imaging the Regenerating Trigeminal Nerve in Live ND-GFP Transgenic Mice
ND-GFP transgenic mice were anesthetized with 30–50 µl ketamine solution (25 mg/ml). A skin flap exposing the whisker pad and trigeminal nerve was made. The trigeminal nerve was then cut. The skin flap was subsequently closed with a 6-0 suture (Ethibond extra polyester suture; Ethicon, Somerville, NJ). All surgical procedures were performed under sterile conditions. The skin flap was opened one week later, and the whisker pad, including the regenerating trigeminal nerve, was removed and observed under fluorescence microscopy (MVX-10; Olympus Corp., Tokyo, Japan).
RESULTS
Imaging ND-GFP-Expressing Cells in the Vibrissa Hair Follicle at Initiation of 3D Histoculture
The nestin-expressing cells within the whiskers isolated from ND-GFP mice had round/oval-shaped bodies with a typical diameter of 7 µm and two-three long elongated processes containing club-like bodies (Fig. 1C). The ND-GFP-expressing hair follicle cells co-expressed S100, a Schwann cell marker, and p75NTR, a neural-crest and immature Schwann-cell marker, as well as nestin. The club bodies of the ND-GFP-expressing hair follicle cells co-expressed S100, p75NTR, and β-III tubulin, a neuron marker (Fig. 1D–F). TrkA and TrkB expression, receptors for neurotrophin, were negative in ND-GFP-expressing hair follicle cells (Supplementary Fig. 1).
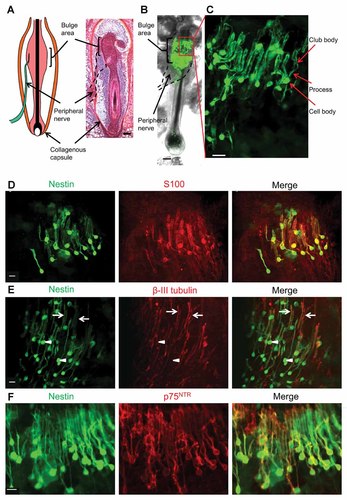
Characteristics of ND-GFP-expressing cells in the bulge area of the vibrissa hair follicle. A: The vibrissa (whisker) hair follicle is enclosed by a dense collagenous capsule. The vibrissa sensory nerve (black dash line) penetrates the capsule and is joined to the hair follicle bulge. H&E stain. Bar: 100 µm. B: A vibrissa hair follicle excised from the ND-GFP mouse and placed in 3D Gelfoam® histoculture. The hair follicle contained the bulge area and its sensory nerve stump. The bulge area contained variable numbers of ND-GFP-expressing cells. Bar: 100 µm. C: A magnified image of the area inside the box in (B) shows that ND-GFP-expressing cells have round/oval-shaped bodies with a typical diameter of 7 µm and with two-three elongated processes, which are club-like. White bar: 10 µm. D: The ND-GFP-expressing cells co-expressed S100. White bar: 10 µm. E: The club bodies (white arrows) of the ND-GFP-expressing cells co-expressed β-III tubulin. The cell bodies (arrowheads) of the ND-GFP-expressing cells did not express β-III tubulin. White bar: 10 µm. F: p75NTR staining was positive in the nestin GFP-expressing cells. White bar: 10 µm.
The ND-GFP-Expressing Hair Follicle Cells Extended Their Processes and Migrated Toward the Whisker Sensory Nerve Stump in 3D Histoculture
The vibrissa sensory nerve penetrates the capsule at the lower part of the vibrissa. It is joined to the hair follicle bulge area via a cavernous sinus (Fig. 1A,B). The processes from the ND-GFP-expressing cells in the vibrissa hair follicle bulge area began to extend toward the nerve stump by Day 4 in 3-D Gelfoam® histoculture (Fig. 2A). By Day 9, ND-GFP-expressing cells reached the whisker sensory nerve stump (Fig. 2B). The processes extending from the ND-GFP-expressing cells co-expressed β-III tubulin, p75NTR, and TrkB but no longer expressed S100 (Fig. 2C and Supplementary Fig. 2A,C).
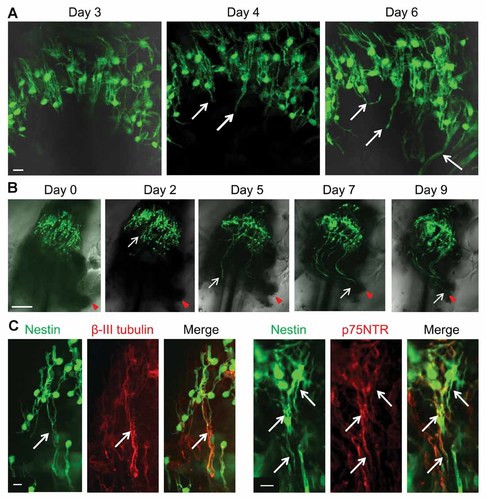
ND-GFP-expressing hair follicle cells extended their processes and migrated toward the whisker sensory nerve stump in histoculture. A: Vibrissa hair follicle without its capsule in 3D Gelfoam® histoculture. The processes (white arrows) from the ND-GFP-expressing cells appeared and began to extend by Day 4 of 3D Gelfoam® histoculture. White bar: 10 µm. B: The processes (white arrow) extended from the ND-GFP-expressing cells by Day 2 in 3D Gelfoam® histoculture. By Day 9, ND-GFP-expressing cells (white arrow) reached the whisker sensory nerve stump (red arrowhead). White bar: 100 µm. C: Vibrissa hair follicles cultured in 3D Gelfoam® histoculture for one week. The processes (white arrows) extending from the ND-GFP-expressing cells co-expressed β-III tubulin and p75NTR. White bar: 10 µm.
ND-GFP-Expressing Cells Proliferated at the Whisker Sensory Nerve Stump and Formed Cord-Like Structures in 3D Histoculture
The whisker sensory nerve stump became enriched with ND-GFP-expressing cells in 3D Gelfoam® histoculture. Higher-magnification images of the whisker sensory nerve stump demonstrated the presence of both spindle and spherical shaped cells (Fig. 3C Day 8). The ND-GFP-expressing cells formed cord-like structures extending further into the Gelfoam® from the sensory nerve stump (Fig. 3B Day 16). The spindle-shaped cells highly expressed ND-GFP. However, only some of the round-shaped cells expressed ND-GEP, which eventually was extinguished (Fig. 3C). The cord-like structures continued to extend at Day 21 in Gelfoam® culture.
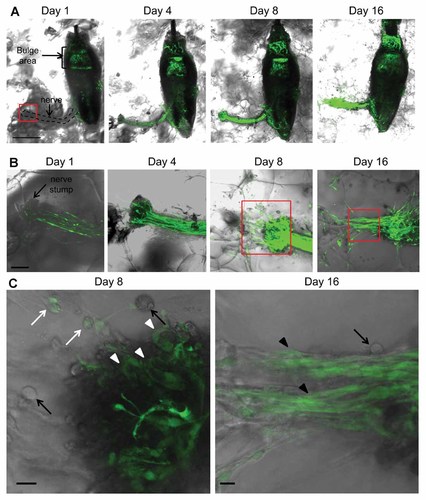
ND-GFP-expressing cells proliferated at the whisker sensory nerve stump and formed cord-like structures in histoculture. A: Time course imaging of ND-GFP-expressing cell trafficking in a histocultured vibrissa hair follicle from an ND-GFP mouse. The nerve stump became enriched with ND-GFP-expressing cells by Day 8 in 3D Gelfoam® histoculture. Bar: 500 µm. B: Magnified images of the nerve stump. By Day 8, the ND-GFP-expressing cells proliferated at the nerve stump and migrated into the Gelfoam®. The ND-GFP-expressing cells formed cord-like structures extending 500 µm from the whisker sensory nerve stump by Day 16. Bar: 100 µm. C: Higher magnification images of the area inside the boxes in (B). By Day 8, the ND-GFP-expressing cells grew out from the nerve stump (white arrow heads). Some round-shaped cells expressed nestin (white arrows) and some did not (black arrows). By Day 16, the cord-like structure contained spindle-shaped cells expressing ND-GFP (black arrow heads). In addition, there were round-shaped cells without nestin expression (black arrow). Bar: 10 µm.
The Whisker Sensory Nerve Interacted With the Trigeminal Nerve, Mediated by ND-GFP-Expressing Cells, in 3D Histoculture
The vibrissa follicle obtained from the ND-GFP transgenic mice, containing its sensory nerve stump, was co-cultured on Gelfoam® along with the trigeminal nerve from RFP transgenic mice (Fig. 4A). The GFP-expressing cord-like structures of the whisker sensory nerve intermingled with the ends of the co-cultured RFP-expressing trigeminal nerve by Day 12 of 3D-Gelfoam® histoculture (Fig. 4A,B). The ND-GFP-expressing cells proliferated at the whisker sensory nerve stump, forming radial cords which extended the nerve. By Day 10, the thickest cord extended and intermingled with the RFP-expressing trigeminal nerve. Two types of cells were observed in the histocultured whisker nerve intermingling with the trigeminal nerve; one was spindle-shaped highly expressing ND-GFP throughout the nerve, and the other was round-shaped with less ND-GFP expression (Fig. 4B).
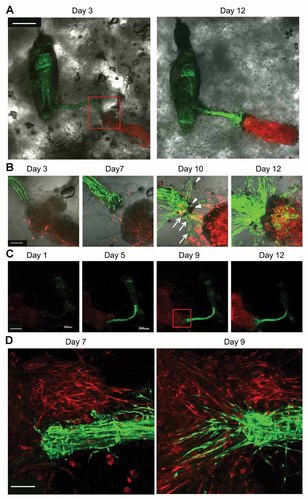
The extending whisker sensory nerve joined with other nerves in 3D Gelfoam® histoculture. A: A whisker sensory nerve of a vibrissa hair follicle from an ND-GFP transgenic mouse was placed on Gelfoam® next to the trigeminal nerve from an RFP transgenic mouse. By Day 12, in 3D Gelfoam® histoculture the whisker sensory nerve was enriched with ND-GFP-expressing cells and intermingled with the RFP-expressing trigeminal nerve. White bar: 500 µm. B: Magnified images of the area inside the box in (A) show that the ND-GFP-expressing cells proliferated at the whisker sensory nerve stump, forming radial cords which extended the nerve. By Day 10, the thickest cord extended and intermingled with the RFP-expressing trigeminal nerve. By Day 12, the two nerves intermingled. Fibers containing ND-GFP-expressing cells migrated into the RFP-expressing trigeminal nerve. There were two types of cells in the growing whisker sensory nerve; one was spindle-shaped, expressing ND-GFP throughout (white arrows), and the other was round-shaped with less nestin expression (white arrow heads). Both cell types were present at the junction of the two nerves. White bar: 100 µm. C: Time course imaging of the intermingling of the whisker sensory nerve containing ND-GFP cells and the sciatic nerve from an RFP mouse in Gelfoam® histoculture. White bar: 500 µm. D: High-magnification images show that ND-GFP-expressing cells migrated from the nerve stump of the vibrissa and invaded deeply into the RFP-expressing sciatic nerve shown at Days 7 and 9 of 3D Gelfoam® histoculture. White bar: 100 µm.
The Whisker Sensory Nerve Intermingled With the Sciatic Nerve, Mediated by ND-GFP-Expressing Cells, in 3D Histoculture
Figure 4C shows a time course of the interaction of the GFP-expressing whisker sensory nerve and a sciatic nerve from an RFP transgenic mouse in 3D Gelfoam® histoculture. By Day 9 of 3D Gelfoam® histoculture, the ND-GFP-expressing cells migrated from the whisker sensory nerve stump of the vibrissa hair follicle and invaded deeply into the RFP-expressing sciatic nerve (Fig. 4D).
ND-GFP-Expressing Spindle-Shaped Cells Expressed Neuronal Markers and Nestin-Negative Round-Shaped Cells Expressed Glial Markers in the Extending Whisker Sensory Nerve Interacting With the Trigeminal Nerve in 3D Histoculture
Two whisker sensory nerves were arranged on either side of a trigeminal nerve on Gelfoam® and cultured. The nerves intermingled within one month of histoculture (Fig. 5A,B). The ND-GFP-expressing cells in the whisker sensory nerve, intermingling with the trigeminal nerve, co-expressed p75NTR, TrkB, and β-III tubulin (Fig. 5D–F). High-magnification images show that spindle-shaped cells expressing ND-GFP in the whisker nerve, co-expressed p75NTR, TrkB, and β-III tubulin (Fig. 5H–J). Most of the many round-shaped cells expressed GFAP, but not nestin (Fig. 5G,K).
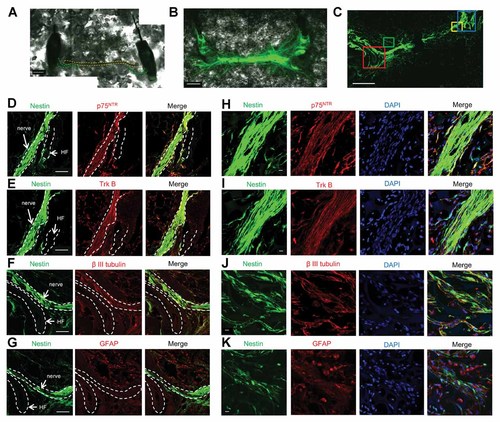
Nestin-positive spindle-shaped cells expressed neuronal markers and nestin-negative round-shaped cells expressed a glial marker in the growing whisker sensory nerve intermingling with the trigeminal nerve in 3D histoculture. A: Two whisker sensory nerves (enclosed by green dashed lines) were arranged on either side of a trigeminal nerve (enclosed by yellow dashed lines) on Gelfoam®. White bar: 500 µm. B: The nerves interminled by one month of Gelfoam® histoculture. White bar: 500 µm. C: One section from (B). White bar: 500 µm. D–F: Magnified images from the blue box in (C). F: contains magnified images from the red box in (C). The ND-GFP expressing cells in the nerve co-expressed p75NTR, TrkB, and β-III tubulin. White bar: 100 µm. G: A magnified image from the red box in (C). There were many round-shaped cells proliferating around the hair follicle and the nerve. Most of these cells expressed GFAP but not ND-GFP. White bar: 100 µm. H,I: High-magnification images of the area inside the yellow box in (C) show that the spindle-shaped cells, expressing ND-GFP in the nerve, co-expressed p75NTR and TrkB. White bar: 10 µm. J: Magnified image from the area inside the green box in (C). The spindle-shaped cells expressing nestin-GFP co-expressed β-III tubulin. White bar: 10 µm. K: Magnified image of the area inside the green box in (C). The nestin-negative round-shaped cells expressed GFAP. White bar: 10 µm. HF: hair follicle.
The Growing Whisker Sensory Nerve Expressed β-III Tubulin and Phalloidin-Positive F-Actin
By Day 15 in 3D Gelfoam® histoculture (Fig. 6A), live imaging and immunofluorescence staining of β-III tubulin demonstrated that many β-III tubulin-positive fibers extended from the nerve stump. The fibers consisted of ND-GFP expressing cells (Fig. 6B,C). By 28 days of 3D Gelfoam® histoculture, the β-III tubulin-positive fibers extended widely and radially around the hair follicle sensory nerve (Fig. 6D). The tips of the β-III tubulin-positive fibers expressed phalloidin-positive F-actin suggesting that the β-III tubulin-positive fibers were axons growing from the whisker sensory nerve stump (Fig. 6E).
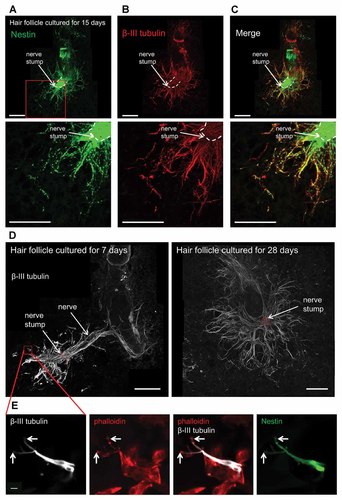
Fibers of cord-like structures extending from the nerve stump expressed β-III tubulin and contained tips expressing phalloidin-positive F-actin. A: A vibrissa hair follicle with its sensory nerve from an ND-GFP mouse was histocultured for 15 days on Gelfoam®. The nerve stump became enriched with ND-GFP-expressing cells and many cord like structures extended from the nerve stump. White bar: 500 µm. B: Immunofluorescence staining images of β-III tubulin (red) demonstrated that many β-III tubulin-positive fibers extended from the nerve stump. White bar: 500 µm. C: Merged images of (A,B) demonstrated that β-III tubulin-positive fibers consisted of ND-GFP-expressing cells. White bar: 500 µm. D: β-III tubulin (white) immunofluorescence staining of the hair follicle sensory nerve histocultured for 7 and 28 days. The β-III tubulin-positive fibers grew out from the whisker sensory nerve stump of the hair follicle histocultured for 7 days. By 28 days of culture, the β-III tubulin-positive fibers extended widely and radially around the hair follicle. White bar: 500 µm. E: Tips of the β-III tubulin (white)-positive fibers had phalloidin (red)-positive F-actin. The presence of F-actin indicates that the tips are axon growth cones. These images are high-magnification images of the area inside the red box in (D). White arrows: tips of the β-III tubulin-positive fibers. White bar: 10 µm.
The Extending Hair Follicle Sensory Nerve Interacted With the Trigeminal Nerve Ganglion in 3D Histoculture
A vibrissa hair follicle, containing its sensory nerve stump, was co-cultured on Gelfoam® with a trigeminal nerve ganglion, both isolated from an ND-GFP transgenic mouse (Fig. 7A and Supplementary Fig. 3). The extending GFP-expressing cord-like structures of the whisker sensory nerve intermingled extensively with the co-cultured trigeminal nerve ganglion by Day 41 of 3D Gelfoam® histoculture (Fig. 7A). Immunofluorescence staining demonstrated that many β-III tubulin-positive fibers extended from both the trigeminal nerve ganglion and the hair follicle sensory nerve. The fibers consisted of ND-GFP-expressing cells (Fig. 7B). The β-III tubulin-positive fibers extending from the nerve stump of the whisker spread widely like a fan and extended toward the trigeminal nerve ganglion (Supplementary Fig. 3). In long-term 3D Gelfoam® histoculture, there was a thick bundle of fibers linking the trigeminal nerve ganglion and the whisker sensory nerve stump (Fig. 7E).
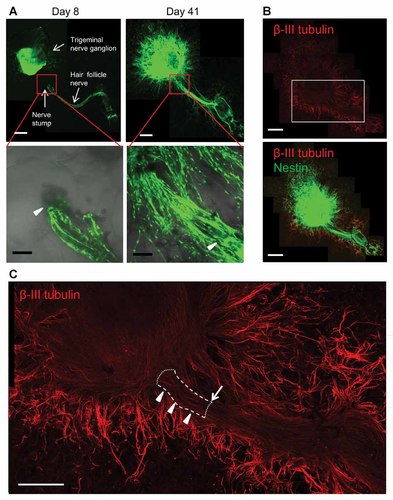
The extending hair follicle sensory nerve intermingled with the trigeminal nerve ganglion in 3D Gelfoam® histoculture. A: A whisker sensory nerve of a vibrissa hair follicle was placed on Gelfoam® next to a trigeminal nerve ganglion, both from an ND-GFP transgenic mouse. By Day 41, the whisker sensory nerve and the trigeminal nerve ganglion intermingled. White arrow heads: whisker sensory nerve stump. White bar: 500 µm. Black bar: 100 µm. B: Immunofluorescence staining of β-III tubulin (red) demonstrated that many β-III tubulin-positive fibers extended from both sides of the hair follicle and the trigeminal nerve ganglion. The fibers consisted of ND-GFP expressing cells. White bar: 1 mm. C: Magnified image of the area inside the box in (B) shows that many β-III tubulin-positive fibers extended widely and radially from the trigeminal nerve ganglion and the hair follicle. There was a thick bundle of fibers linking the ganglion and the nerve (white arrow heads). White arrow: whisker sensory nerve stump. White bar: 500 µm.
It is noteworthy that in both the trigeminal nerve in 3D Gelfoam® histoculture (Fig. 7A) and in the regenerating trigeminal nerve in vivo (Supplementary Fig. 4), nestin-expressing cells were very prominent.
DISCUSSION
Li et al. [1992a, b] reported that mouse skin and human scalp histocultured on Gelfoam® showed continuous hair growth for up to 40 days in vitro, thereby demonstrating that Gelfoam®-supported histoculture can be used to evaluate the dynamic state of hair follicles. Duong et al. [2012] showed that isolated mouse whiskers could be histocultured for long periods on Gelfoam® and that trafficking of ND-GFP-expressing cells could be imaged longitudinally within the histocultured whisker by confocal microscopy. Gelfoam®-supported histoculture is therefore useful for long-term culture and imaging of cellular dynamics within the hair follicle.
The present study imaged the behavior of ND-GFP-expressing cells in the elongating whisker sensory nerve in 3D Gelfoam® histoculture using confocal microscopy. The ND-GFP-expressing cells extended their processes, which expressed the neuron marker β-III tubulin and migrated toward the nerve stump of the injured nerve in 3D Gelfoam® histoculture. The enrichment and proliferation of the ND-GFP-expressing cells in the whisker sensory nerve stump and their organization into cord-like structures enabled the nerve stump to elongate in 3D Gelfoam® histoculture. Fibers extending from the elongating whisker nerve contained spindle-shaped cells expressing ND-GFP and β-III tubulin, features of axons [Avwenagha et al., 2003]. The ND-GFP-expressing spindle-shaped cells also co-expressed p75NTR and TrkB, which are receptors for BDNF, and expressed mainly by neurons. p75NTR and TrkB expression are found in early stages of development of the trigeminal nerve [Kirstein and Fariñas, 2002]. Neural precursor cells express p75NTR and TrkB [Hosomi et al., 2003]. The elongating fibers also expressed F-actin on their tips detected by phalloidin staining, further suggesting the fibers were growing axons.
The cord-like structures could intermingle with the trigeminal nerve, the sciatic nerve and the trigeminal nerve ganglion. If the axis of the nerves were arranged at right angles, the cord-like structure of the whisker sensory nerve still grew toward and intermingled with the trigeminal nerve suggesting possible guidance factors (Supplementary Fig. 5).
In previous studies, injected ND-GFP expressing cells in the severed sciatic nerve/spinal cord differentiated to Schwann cells in vivo [Amoh et al., 2005b, 2008; Liu et al., 2011], whereas the present study observed differentiation to both neuronal cells and glial cells during nerve elongation in Gelfoam® histoculture. These different conditions may account for the different fate of ND-GFP expressing cells.
There have been numerous studies on explanted nerves and peripheral nerve recovery in vitro from the proximal side but only a few are from the distal side [Cajal, 1968; Bray et al., 1980; Nurcombe et al., 1984; Heaton and Wayne, 1986; Kapfhammer et al., 1986; Kapfhammer and Raper, 1987; Thomson et al., 2006; Wang and Marquardt, 2012]. In the present study, we observed what appear to be growing axons from the distal stump of an injured peripheral nerve, in this case the whisker sensory nerve in Gelfoam® histoculture. We also observed that the elongating whisker sensory nerve can interact with other nerves in Gelfoam® histoculture. The results of the present report suggest that Gelfoam® 3D histoculture is a physiologic system to support nerve growth and interaction and should have broad application.
The present study suggests a major role for the nestin-expressing cells in the hair follicle is the extension of the whisker sensory nerve. The observations in the present report should enable future in vitro studies of the elongation and interaction of other nerves as well.




