Abstract
We used non-insulin producing pancreatic carcinoma cell line, MIA PaCa-2 and have modulated its culture conditions by using 1% matrigel as extracellular matrix, N2, B27 growth supplements and serum free conditions. Expression of markers was analyzed using qRT-PCR, immunofluorescence and in vitro functional assay for insulin and C-peptide release was assessed using insulin and C-peptide ELISA, respectively. The cells grown under this altered culture conditions have exhibited a transition in the morphology from mesenchymal to epithelial with extensive piling up of cells. A reduction in doubling time from 40 to 18 h, upregulation of beta islet specific markers like pancreatic duodenal homeobox-1 (Pdx-1), C-peptide, insulin, and disappearance of markers like vimentin were observed. On the functional level, the altered morphology bearing cells released high levels of insulin in response to 10 µM tolbutamide (an activator of insulin pathway) and reduced insulin secretion in response to 50 µM nifedipine (inhibitor of the pathway). On the contrary, the original cells (mesenchymal morphology) had failed to release any insulin in response to varying concentrations of glucose and also the activators and inhibitors of the insulin pathway. This investigation thus provides a basis for using this basic developmental biology phenomenon mesenchymal to epithelial transition as a strategy to generate a large number of functional islets from stem cells of mesenchymal origin. J. Cell. Biochem. 9999: XX–XX, 2013. © 2013 Wiley Periodicals, Inc. J. Cell. Biochem. 114: 1642–1652, 2013. © 2013 Wiley Periodicals, Inc.
Abbreviations used:
ES cells, embryonic stem cells; Pdx-1, pancreatic duodenal homeobox 1 transcription factor; MET, mesenchymal epithelial transition; EMT, epithelial mesenchymal transition; SCID, severe combined immunodeficiency syndrome; SFM, serum free media; SCM, serum containing media; DMEM, Dulbecco's modified eagle's medium; PBS, phosphate buffered saline; EDTA, ethylenediaminetetraacetic acid; qRT-PCR, quantitative reverse transcriptase polymerase chain reaction; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; CK-18, cytokeratin 18; CEA, carcinoembryonic antigen.
Beta islet cells are the main functional cells in the endocrine pancreas regulating glucose metabolism. However, these cells are destroyed by the autoimmune system in type-1 diabetes and become less efficient in type-2 diabetes. Many of the pancreatic cancers are known to be associated with impaired glucose metabolism [Schwartz et al., 1978; Permert et al., 1993]. MIA PaCa-2 cell line is one such pancreatic carcinoma cell line isolated by [Yunis et al., 1977]. It has a doubling time of 40 h and grows in complete medium containing DMEM, Fetal bovine serum and Horse serum. This cell line has been reported to secrete certain diabetogeneic factors [Basso et al., 1997] which were shown to induce diabetes in SCID mice [Basso et al., 1995]. However, the complete characterization of the cell line with respect to the presence of pancreatic markers as well as beta islet cell markers and operation of insulin pathway is not elucidated.
Out of various therapies for diabetes, islet transplantation is one. One of the main obstacles to successful islet transplantation both for type 1 and type 2 diabetes is the limitation of availability of insulin producing tissue [Weir and Bonner-Weir, 1997]. However, this limitation of availability of insulin producing tissue has given high priority efforts to stimulate the growth of new pancreatic islet tissue or other insulin producing tissues which will serve the purpose. Various attempts include differentiation of stem cells of embryonic, induced pluripotent, and mesenchymal origin into insulin producing clusters [Lumelsky et al., 2001; Chen et al., 2004; Bose et al., 2012; Jeon et al., 2012]. Cells producing insulin have also been obtained by transdifferentiation of ductal cells [Bonner-Weir et al., 2000] and freshly isolated acinar cells. Also, cells from rat acinar cell carcinoma cell line (ARJ) have been induced with growth factors like activin A and betacellulin to produce insulin [Mashima et al., 1996].
Mesenchymal to epithelial and epithelial to mesenchymal transitions (MET/EMT) are phenomena well observed in vitro as well as in vivo during embryonic development [Hay, 1995] and carcinogenesis [Thiery, 2002, 2003]. EMT has been reported to generate proliferative human islet precursor cells [Gershengorn et al., 2004]. However, MET has been reported in mouse hepatic stem cells differentiation [Li et al., 2011]. Furthermore, there are no reports regarding MET to generate functional beta islet cells. In this present study, we used the MIA PaCa-2 pancreatic carcinoma cell line which when subjected to serum free conditions along with growth supplements underwent mesenchymal to epithelial transition and exhibited functional insulin signaling pathway. The epithelial transitioned cells responded well to activators and inhibitors of insulin pathway. Taken together, this strategy thus provides a simplistic method for generation of large quantities of functional insulin producing cells from mesenchymal like cells for cell-based therapies for diabetes. Moreover, this cell line can also be utilized for in vitro screening of different compounds, which may have a probable influence on insulin signaling pathway.
MATERIALS AND METHODS
Cell Culture Conditions
MIA PaCa-2 cells were procured from National Center for Cell Science (NCCS), Pune, India. The cells were cultured in T75 cm2 flask (Nunc, Denmark), in Dulbecco's modified Eagle's Medium (DMEM) High Glucose (Gibco BRL, USA), supplemented with 10% fetal bovine serum (Hyclone, Logan UT), 2.5% horse serum, 1 mM L-glutamine, 1% non-essential amino acids, 0.6% penicillin and streptomycin (all purchased from Gibco BRL) and 0.1 mM β-mercaptoethanol (Sigma, USA) under normal conditions. For serum free culture conditions, cells were cultured on 1% matrigel (BD Biosciences, USA) coated 60 mm dishes (Nunc, Denmark) in media containing DMEM, high glucose supplemented with N2 (1%), B27 (2%), and 1% penicillin and streptomycin (all purchased from Gibco BRL).
Dithizone Staining
The cells cultured in serum containing media (SCM) and those cultured in serum free media (SFM) were washed three times in PBS and then stained with Dithizone (Sigma) at a final concentration of 100 µg/ml of dithizone (DTZ) in DMEM without supplements for 15 min at 37°C. In order to rule out artifactual insulin staining in cells cultured in SFM with N2 and B27 supplements, SFM cells prior to DTZ staining were washed extensively with PBS and transferred to media containing DMEM with 1% penicillin–streptomycin 1 h. After DTZ staining, cells were washed twice with PBS and examined under normal inverted microscope. Only the insulin producing cells were stained bright red out of all the cells subjected to DTZ staining.
Growth Curve Analysis
MIA PaCa-2 cells were cultured in SFM and SCM, respectively in 60 mm tissue culture plates, at a seeding density of 2 × 103 cells/cm2 of surface area, in duplicates for every time point. Cells were trypsinized using 0.25% trypsin–EDTA (Gibco BRL) and counted at various times points 24, 48, 72, 96, and 120 h. Growth curve was plotted using the cell count in the Y-axis and time point in the X-axis, respectively.
Quantitative Real-Time-PCR
Total RNA extraction was done using RNeasy mini kit (Qiagen, Germany) as per manufacturer's instructions. One microgram of RNA was reverse transcribed into cDNA using random hexamers and superscript II (Invitrogen, USA). Real-time-PCR was performed with SYBR Green Platinum SYBR green qPCR Supermix-UDG (Invitrogen 11733–046) in an Eppendorf realplex/ABI 7000 (Eppendorf) equipment. Total RNA from human pancreas was purchased from (Ambion, USA) and served as control for qRT-PCR experiments. GAPDH was used as the housekeeping gene. ΔCt values >16 were considered not expressed (NE). The details of the primer pairs are summarized in Supplementary Table S1.
Immunofluorescence Microscopy
The cells grown in 2-welled chamber slides (NUNC, USA) were washed twice with PBS and fixed with 4% paraformaldehyde for 20 min. Cell permeabilization was done with 0.2% triton X-100 (Sigma) for 10 min. After one wash with PBS, the cells were blocked using 5% normal donkey serum for an hour at room temperature. Cells were then incubated with primary antibody (1:100 dilutions) at 4°C overnight. After three washes with PBS, appropriate secondary antibody incubation (1:500 dilutions) was done for 1 h. Cell nuclei was counterstained with DAPI and mounted with cover slips using immunofluor mounting media containing antifade agent. The cells were observed under Nikon fluorescence microscope (Nikon, Japan) and images were captured. Isotype controls were run in parallel. The details of the antibodies are summarized in Supplementary Table S2.
Flow Cytometric Analysis
Cells fixed with 4% paraformaldehyde in PBS were blocked using 5% normal donkey serum and stained with respective primary antibodies Vimentin, Pdx1, Insulin, C-peptide, and MafA for 1 h. Following this, appropriate secondary antibody incubation was done for 20 min. Acquisition was done on Beckton Dickinson LSRII flow cytometer (BD USA) and the data was analyzed using the FACS DIVA software. The details of the antibodies are summarized in Supplementary Table II.
Insulin and C-Peptide Release Assay
MIA PaCa-2 cells were cultured in 60 mm dishes (Nunc, Denmark) at a cell density of 2 × 106. After 2 days, the culture medium was changed to no glucose media and incubated overnight. The cells were washed with Kreb's Ringer Bicarbonate buffer (130 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.5 mM CaCl2, 20 mM HEPES) and 0.1% BSA (w/v) for 2 h. The cells were treated with different concentrations of Glucose (3.3 and 27.7 mM) alone and also with a combination with Tolbutamide (10 µM) and Nifedipine (50 µM) and incubated for 1 h, respectively. Cell culture supernatants were collected from different treatments. Insulin and C-peptide levels were estimated separately using human Insulin ELISA kit and human C-peptide ELISA kit, respectively, from Mercodia (Sweden) as per manufacturer's instructions. Results were normalized to total protein concentration and expressed as milli units of insulin or pmol of C-peptide per milligram of protein.
Statistical Analysis
Data in this manuscript is reported as means ± SEM of three independent experiments. Statistical analysis includes Student's t-test. A value of *P < 0.05 was considered to be significant.
RESULTS
Altered Serum Conditions Have Resulted in Morphological and Physiological Transition of MIA PaCa-2 Cells
Morphological differences were profound when MIA PaCa-2 cells were cultured in serum containing media (SCM) and also in serum free media (SFM). MIA PaCa-2 when cultured in SCM media up to 72 h exhibited a combination of predominantly bipolar cells (an indicative of mesenchymal morphology), as well as some small round shiny cells (indicative of actively dividing cells; Fig. 1A). When the cell line was cultured in SFM for 72 h supplemented with N2 and B27 supplements the cell morphology shifted entirely towards the epithelial phenotype with extensive piling up of the cells (Fig. 1B). Furthermore, there was a total absence of cells with bipolar morphology (Fig. 1B). Cells with epithelial phenotype exhibited high nucleus to cytoplasmic ratio and stained red with dithizone, a zinc containing dye which stains insulin producing cells (Fig. 1C). Moreover, MIA PaCa-2 cells when cultured in SCM with mesenchymal morphology failed to exhibit any dithizone staining.
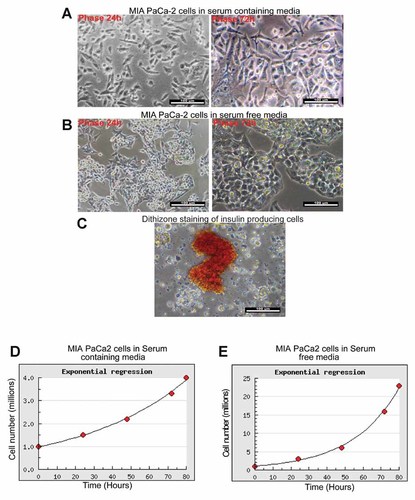
Morphology transition (MET) and subsequent insulin production of MIA PaCa-2 cells in SFM (A) MIA PaCa-2 cells cultured in SCM for 24 and 72 h with predominantly mesenchymal morphology. B: MIA PaCa-2 cells cultured in SFM for 24 and 72 h with predominantly epithelial morphology. C: MIA PaCa-2 cells cultured in SFM showing positive staining of insulin producing cells with Dithizone stain. D: Growth curve analysis of MIA PaCa-2 cells cultured in SCM. E: Growth curve analysis of MIA PaCa-2 cells grown in SFM.
Growth Pattern of the Cells
Growth curve analysis was done with the MIA PaCa-2 cells with mesenchymal morphology (SCM) as well as the transdifferentiated epithelial phenotype (SFM) cultured under altered serum conditions, respectively. The cells cultured in the normal SCM demonstrated a doubling time of 40 h (Fig. 1D). This was in accordance with the studies conducted by previous workers on MIA PaCa-2 cell line [Yunis et al., 1977]. In contrast, the cells cultured in SFM demonstrated a faster doubling time of just 18 h (Fig. 1E). To the author's best of knowledge, this is the first report in which MIA PaCa-2 cells were ever cultured in SFM supplemented with growth factors.
MIA PaCa-2 Cells Expressed Mesenchymal and Epithelial Genes Under Normal and Altered Serum Conditions, Respectively
Gene expression profiles conducted on MIA PaCa-2 cells when cultured in normal SCM exhibited a higher expression of mesenchymal markers like fibronectin, N-cadherin (Fig. 2A), vimentin, amylase, αSMA (Fig. 2C) and transcriptional factors including snail and slug (Fig. 2A). Concurrently, these MIA PaCa-2 cells did not express any epithelial related genes (Fig. 2B). Accordingly when the MIA PaCa-2 cells were cultured in SFM for 72 h showed a higher expression of epithelial markers like E-cadherin, Ep-CAM, and cytokeratins like CK8, CK19 (Fig. 2B), which were NE by cells cultured under normal SCM conditions. The markers Pdx1, insulin, Glut2, and Nkx 6.1 were expressed by the epithelial morphology only (Fig. 2C). Hnf3β was however expressed in both the phenotypes of the MIA PaCa-2 cells (Fig. 2C). Commercially available total RNA from human pancreas (Ambion, USA) was used as control.
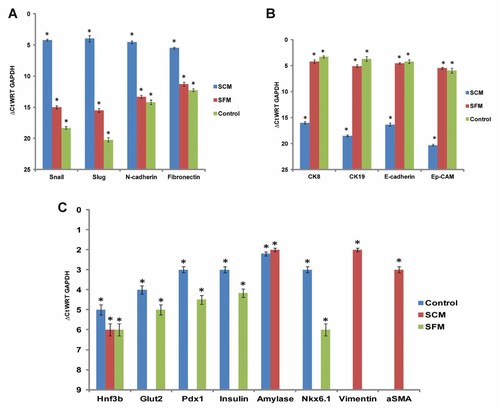
Gene expression analysis of MIA PaCa-2 cells cultured in SCM and SFM, respectively. A: MIA PaCa-2 cells cultured in SCM expressed mesenchymal markers snail, slug, N-cadherin, and fibronectin. B: MIA PaCa-2 cells cultured in SFM expressed epithelial markers CK-8, CK-19, E-cadherin, and Ep-CAM. C: MIA PaCa-2 cells cultured in SFM expressed early and mature pancreatic endocrine markers Hnf3β, Pdx1, Glut2, insulin concomitantly MIA PaCa-2 cells cultured in SCM expressed mesenchymal markers vimentin, amylase, αSMA. ΔCt were normalized with GAPDH and ΔCt values >16 were considered not expressed (NE).The values are mean ± standard error from three independent experiments. Total RNA from commercially available human pancreas was used as controls. A value of *P < 0.05 was considered to be significant.
Epithelial Phenotype of the MIA PaCa-2 Cells Under Serum Free Conditions Expressed Insulin and Pdx1
In order to confirm the cellular expression of the pancreatic markers, immunocytochemistry (ICC) was done with the MIA PaCa-2 cells with both types of morphology (mesenchymal and epithelial). The markers Pdx1 (Fig. 3C), insulin (Fig. 3D), E-cadherin (Fig. 3B), and CK-18, an endodermal epithelial marker (Fig. 3D) were expressed only by the epithelial phenotype of the cells growing in SFM. Moreover, no cells of mesenchymal phenotype expressed Pdx1, insulin, E-cadherin, and CK-18. However, vimentin and pancreatic amylase were predominantly expressed by the mesenchymal phenotype of MIA PaCa-2 cells (Fig. 3A) cultured in SCM. Pancreatic transcription factors MafA and Pdx1 (Fig. 4A) and mature pancreatic markers insulin, C-peptide (Fig. 4B) were expressed only by epithelial phenotypic cells cultured in SFM.
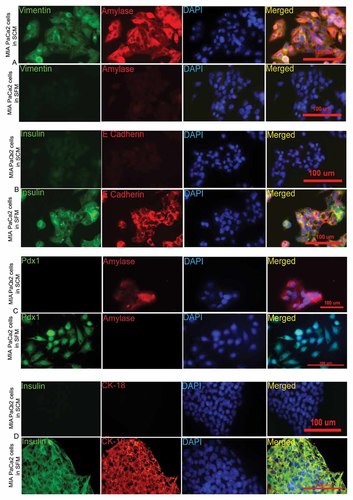
MIA PaCa-2 cells expressed mesenchymal markers and amylase under SCM, while these cells expressed epithelial markers and insulin under SFM. Immunofluorescence pictures of MIA PaCa-2 cells cultured in SCM and SFM stained simultaneously for (A) vimentin (green), amylase (red), DAPI (blue) and merged (B) insulin (green), E-cadherin (red), DAPI (blue) and merged (C) Pdx1 (green), amylase (red), DAPI (blue) and merged (D) insulin (green), CK18 (red), DAPI (blue) and merged.
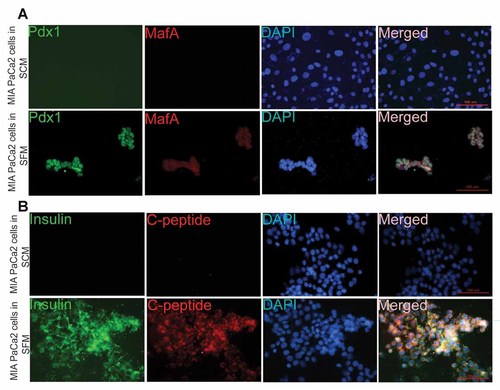
Immunofluorescence images of MIA PaCa-2 cells cultured in SCM and SFM stained simultaneously for (A) Pdx1 (green), MafA (red), DAPI (blue) and merged (B) insulin (green), C-peptide (red), DAPI (blue), and merged.
Furthermore, FACS data corroborated the data obtained by qRT-PCR and ICC. MIA PaCa-2 cells cultured in SCM media did not express any of the pancreatic marker proteins but expressed only vimentin (92%; Fig. 5A) suggesting that the cells were of mesenchymal phenotype. Simultaneously, when the MIA PaCa-2 cells were cultured in SFM, they lost vimentin expression and started expressing early pancreatic marker, Pdx1 (84.9%; Fig. 5B), and mature pancreatic markers like Insulin (45.2%), C-peptide (48.6%), and MafA (60.3%; Fig. 5B). Therefore, the expression of the aforesaid marker proteins in cells cultured in SFM, indicated an attainment of epithelial beta cell phenotype.
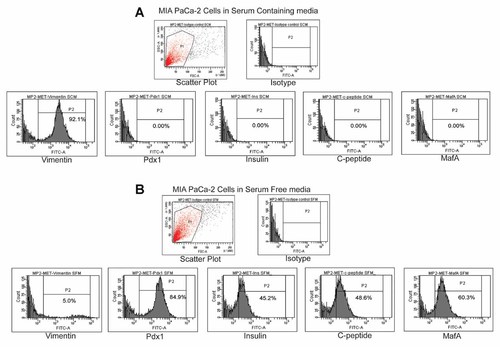
Flow cytometric analysis of MIA PaCa-2 cells cultured in SCM and SFM (A) MIA PaCa-2 cells cultured in SCM expressed vimentin and did not express Pdx1, insulin, C-peptide, MafA. B: MIA PaCa-2 cells cultured in SFM expressed Pdx1, insulin, C-peptide, MafA and did not express vimentin. Percentage of positive cells is indicated for each antigen studies.
Insulin and C-Peptide Release in Response of MIA PaCa-2 Cells to Activators and Inhibitors of Insulin Pathway
To examine whether this transdifferentiated epithelial phenotype induced by the altered serum conditions has resulted in functional hormone producing cells, insulin release assay was performed using ELISA. Cells with both types of morphology (mesenchymal and epithelial) were induced with two different concentrations of glucose (3.3 and 27.7 mM) alone and also in combination of the insulin pathway activator, tolbutamide (10 µM) and insulin pathway inhibitor, nifedipine (50 µM), respectively. Results indicated that the cells cultured in SCM have expressed no insulin under all conditions, that is, in untreated controls as well as in all the treatments (Fig. 6A) confirming their non beta cell nature. While in case of the epithelial transdifferentiated cells, cultured in SFM, a very high basal insulin levels were observed in untreated cells (180 mU/mg of total protein). These epithelial transdifferentiated cells released insulin in a dose dependent manner when stimulated with low, 3.3 mM glucose (insulin release-150 mU/mg of total protein) and high, 27.7 mM glucose (insulin release-730 mU/mg of total protein), respectively. Moreover, a proportionate increase and decrease in insulin release was observed when these cells (epithelial morphology in SFM) were treated with tolbutamide (activator) and nifedipine (inhibitor), respectively, along with 3.3 and 27.7 mM glucose (Fig. 6), respectively. Furthermore, in order to rule out the chances of false positivity regarding insulin expression attributed to the culture media containing N2 and B27 supplements, we also checked for the levels of C-peptide as a marker for de novo insulin production by the cells. Results indicated that the pattern of C-peptide release was similar to that of insulin release in response to different concentrations of glucose and treatment with activators and inhibitors of insulin pathway (Fig. 6B). This indeed confirms the transdifferentiated morphology (epithelial) of MIA PaCa-2 cells were functionally active insulin producing cells.
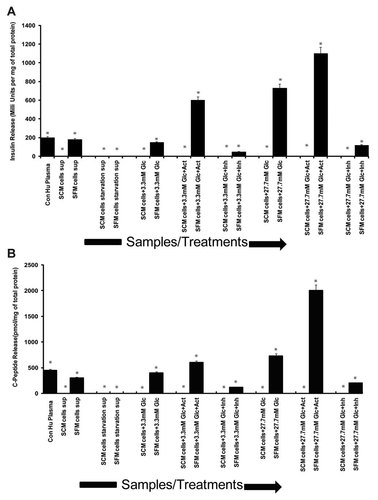
In vitro functional assay of MIA PaCa-2 SCM/SFM cells in response to different concentrations of glucose alone or in combination with activator and inhibitor of insulin pathway (A) insulin ELISA showing insulin release expressed in terms of milli units per mg of total protein and (B) C-peptide ELISA showing C-peptide release expressed in terms of pmol per mg of total protein respectively in the Y-axis and various samples/treatments respectively in the X-axis. A value of *P < 0.05 was considered to be significant. Con Hu plasma, control human plasma; Sup, supernatant; Glc, glucose; Act, activator (10 µM tolbutamide); Inh, inhibitor (50 µM nifedipine).
DISCUSSION
EMT is an important change in the cell phenotype which allows the escape of epithelial cells from the structural constraints imposed by tissue architecture. Reversals of these changes termed MET also occur and are important in tissue construction in normal development [Hugo et al., 2007]. Several oncogenic pathways may induce EMT which leads to increase in transcription factors like snail and slug. These transcription factors activate desmosomal disruption, cell separation, inhibition of cytokeratin expression, and activation of vimentin expression [Boyer et al., 1989; Savagner et al., 1997]. The concept of epithelial–mesenchymal transition (EMT) was also demonstrated to be useful in generation of endocrine progenitor cells from human pancreatic islets [Gershengorn et al., 2004, 2005]. However, there has been significant debate in understanding the proliferative potential of “terminally” differentiated cells, such as the insulin-producing β-cells. Russ et al. [2009] and Gershengorn et al. [2004] now confirm that human pancreatic insulin-producing cells proliferate and undergo EMT in vitro. These groups have also indicated that mesenchymal cells derived from pancreatic islets can undergo reverse EMT or MET to generate islet-like cell aggregates. Although such islet-like aggregates showed very low levels of insulin, the concept of generating progenitors from insulin-producing cells by EMT may help in generation of lineage-committed islet progenitor cells. However, further optimization of differentiation protocols to obtain large quantities of functional beta islets using EMT phenomenon is required. Such cells may have potential for replacement therapy in diabetes. Accordingly, our results indicate a successful mesenchymal epithelial transition, MET to produce large numbers of functional insulin producing cells from non-insulin producing cells.
There are different kinds of pancreatic cancer cell lines available including insulinoma cell lines which secrete insulin in response to various secretagogues, for example, RIN-m, CM cell lines, etc. [Sakurai et al., 2004]. These insulinoma cells have been either isolated from pancreatic cancers of human origin or have been induced by the retroviruses. Many of the pancreatic cancers are by and large associated with co-occurrence of diabetes in patients. Several studies have demonstrated that diabetes in pancreatic cancer patients is characterized by peripheral insulin resistance [Permert et al., 1993]. Accordingly, the pancreatic carcinoma cell lines isolated from such cancers also should show non-responsiveness to insulin secretion and thus behave diabetic. Likewise, the MIA PaCa-2 cell line used for our studies was also reported secreting certain diabetogeneic factors [Valerio et al., 1999]. The cell culture supernatants from these cells also induce diabetes in SCID mice [Basso et al., 1995]. The basal levels of insulin secreted by this cell line under normal culture conditions were negligible. In correlation to the previous literature on MIA PaCa-2 cells, our studies indicated that when this cell line was cultured under normal serum conditions, it did not respond to glucose at all and also the activators and the inhibitors of the insulin signaling pathway. In this study, we have focused on the probability of modulating this non-insulin producing cell line to produce insulin.
Transplantation of insulin producing cells is one of the major therapies for type1 and in some cases type 2 diabetes, as well. In 1966, the first pancreatic transplantation was performed, where the entire pancreas including the exocrine gland was transplanted [Kelly et al., 1967]. However, the availability of such cadaveric pancreas remains a serious problem. Various approaches, therefore, can be adopted to solve this problem. These are: (a) Obtaining insulin producing cell lines by differentiation of embryonic stem cells [Assady et al., 2001; Lumelsky et al., 2001; Bose et al., 2012] or mesenchymal stem cells [Chen et al., 2004]; pancreatic stem cells [Gershengorn et al., 2004] or other types of cells like ductal cells; hepatic cells etc; and (b) genetically engineering immortal beta cell lines into glucose responsive cell lines [McClenaghan and Flatt, 1999] by simultaneously addressing the c-myc associated glucose response and oncogenic issues [Jonas et al., 2001]. Although this study is not identical to the previous studies conducted by various groups, authors attempted to address the phenomenon of insulin production by non-insulin producing pancreatic carcinoma cell line MIA PaCa-2 when subjected to simple culture modifications. The phenomenon authors have proven here is mesenchymal to epithelial transition, which can be applicable to various differentiation protocols, to obtain functional beta islet cells. However, MIA PaCa-2 cells, which authors thus, modulated to secrete insulin in a dose dependent manner, in response to various secretagogues, may find its application for drug testing studies only. Such cells cannot be used for cell transplantation due to their cancerous nature.
Hormones producing beta islets or islet like clusters or pancreatic endocrine progenitors are mainly epithelial in morphology [Selander and Edlund, 2002]. At certain stages, mesenchymal signals such as follistatin induce exocrine differentiation and repress endocrine cells. Mesenchyme removal had resulted in the generation of four times more number of beta cells in vivo as compared to when mesenchyme was intact [Soria et al., 2001]. Epithelial to mesenchymal transitions and vice versa are reported during many events in early embryogenesis [Hay, 1995] and carcinogenesis [Kierner et al., 2001]. This transition is a complex process occurring as a result of the dynamic equilibrium state reached between cells transcription and transduction machinery and local environment [Hay, 1995; Newgreen and Minichiello, 1995]. After EMT induction and the other way round (mesenchymal to epithelial), the cells lose or gain their epithelial features or mesenchymal characteristics depending on the transition. Vimentin filaments are mainly associated with mesenchymal phenotype [Kalluri and Neilson, 2003]. During this investigation also, we had observed a complete loss of vimentin filaments when the cells were switched from SCM to SFM conditions where morphology of the cells got transitioned from mesenchymal to epithelial. MIA PaCa-2 cell line proliferates normally on plastic with a doubling time of 40 h under SCM. This cell line has been partially characterized as adenocarcinoma based on morphology [Yunis et al., 1977]. However, there are no reports regarding detailed characterization for epithelial markers being expressed by MIA PaCa-2. Lack of expression of carcinoembryonic antigen (CEA) in MIA PaCa-2 has been reported earlier [Yunis et al., 1977]. Expression of CEA has been predominantly associated with cancers of epithelial in origin [Chapin et al., 2012]. As per our studies, when this cell line was cultured in SCM, there was an abundance of all the mesenchymal markers like snail, slug, N-cadherin, and fibronectin. Subsequently, there was a transition of this cell line to epithelial phenotype when subjected to serum free media as evident from the marker analysis. Moreover, we also observed an expression of E-cadherin only when this MIA PaCa-2 cell line was cultured in SFM (epithelial) and not during culturing of the cell line in SCM. This corroborates the previous reports of Wells et al. [2008] that re-expression of E-cadherin is a hallmark of induction of mesenchymal to epithelial transition in cancer cells. The presences of the extracellular matrices have been known to promote the acquiring of epithelial phenotype [Vogel and Hedgecock, 2001]. In our case, it was 1% matrigel, in addition to, the serum free conditions. Taken together, we have proved at transcript as well as protein level for the appearance of various markers of insulin producing cells with concomitant disappearance of markers of non-insulin producing cells in the altered morpho/physiology of MIA PaCa-2 cell line.
The different secretagogues, which modulate insulin pathway, are potassium and calcium channel blockers, in addition to, various concentrations of glucose. Sulfonylurea group of drugs come under potassium channel blockers which stimulate insulin secretion by regulating K+ATP channel activity [Mortensen et al., 2006]. These drugs act much like the rise in extracellular glucose, inhibit K+ATP channels, cause membrane depolarization, activation of voltage sensitive Ca2+ influx and rise in Ca2+ that initiates insulin release [Mortensen et al., 2006]. Tolbutamide [Kalkhoff et al., 1983], a drug from sulfonylurea group at, 10 µM concentrations had shown insulin secretion in the epithelial phenotype of the cells growing under serum free conditions. The mesenchymal counterpart failed to show any such response. Calcium channel blockers are likewise inhibitors of insulin pathway [Minami et al., 2002]. Nifedipine, a drug from the “dihydropyridine calcium channel blocking agent” group was used in our studies at 50 µM final concentrations. It has shown a reduction in secretion of insulin in the MIA PaCa-2 cells grown in SFM and not those growing in SCM.
In conclusion, we describe culture induced MET (mesenchymal to epithelial transition) using SFM, extracellular matrix and growth supplements for a pancreatic carcinoma cell line, MIA PaCa-2. The newly transitioned cells were capable of secreting insulin in a dose dependent manner in response to various regulators of insulin pathway. This investigation thus provides a basis for protocol optimization for generating terminally differentiated insulin producing functional beta islet like cells from ES cells or adult mesenchymal like stem cells. Such transitioned cells can be used for cell based therapy in diabetes. Since the altered morphology of this cell line can be obtained in large quantities, these cells can be commercially exploited to screen new compounds having a probable influence on the insulin signaling pathway as well as for basic research.
Acknowledgements
The authors gratefully acknowledge the funding and support of Reliance Life Sciences Pvt. Ltd (RLS), in carrying out this research work. Authors had carried out this project as a part of basic research interest on diabetes with no applied aspect towards which company holds no direct interest or any patentability issues. Accordingly, RLS has no role in designing this work. The authors would also like to thank Ms Aishwarya Movva (summer trainee) for her excellent technical assistance provided during the study.




