Abstract
Progressive pulmonary inflammation and emphysema have been implicated in the progression of chronic obstructive pulmonary disease (COPD), while current pharmacological treatments are not effective. Transplantation of bone marrow mesenchymal stem cells (MSCs) has been identified as one such possible strategy for treatment of lung diseases including acute lung injury (ALI) and pulmonary fibrosis. However, their role in COPD still requires further investigation. The aim of this study is to test the effect of administration of rat MSCs (rMSCs) on emphysema and pulmonary function. To accomplish this study, the rats were exposed to cigarette smoke (CS) for 11 weeks, followed by administration of rMSCs into the lungs. Here we show that rMSCs infusion mediates a down-regulation of pro-inflammatory mediators (TNF-α, IL-1β, MCP-1, and IL-6) and proteases (MMP9 and MMP12) in lung, an up-regulation of vascular endothelial growth factor (VEGF), VEGF receptor 2, and transforming growth factor (TGFβ-1), while reducing pulmonary cell apoptosis. More importantly, rMSCs administration improves emphysema and destructive pulmonary function induced by CS exposure. In vitro co-culture system study of human umbilical endothelial vein cells (EA.hy926) and human MSCs (hMSCs) provides the evidence that hMSCs mediates an anti-apoptosis effect, which partly depends on an up-regulation of VEGF. These findings suggest that MSCs have a therapeutic potential in emphysematous rats by suppressing the inflammatory response, excessive protease expression, and cell apoptosis, as well as up-regulating VEGF, VEGF receptor 2, and TGFβ-1. J. Cell. Biochem. 114: 323–335, 2013. © 2012 Wiley Periodicals, Inc.
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide. It is characterized by a progressive, poorly reversible loss of lung function, and abnormal inflammatory response of the lung to noxious gases and particles [Buist et al., 2007; Yao et al., 2008; Stockley et al., 2009]. Tobacco smoke has been considered as a major etiologic factor of COPD. Various mechanisms including chronic inflammation; protease/antiprotease imbalance, involving destruction of the extracellular matrix; and cell apoptosis, affecting epithelial and/or endothelial cells, contribute to alveolar destruction, and destructive pulmonary function in COPD [Sethi and Rochester, 2000; Barnes et al., 2003; Tuder et al., 2003; Tuder et al., 2006]. Current therapies for COPD, primarily based on anti-inflammatory drugs, including corticosteroids, or theophylline with or without bronchodilators, have improved disease management, but there are no therapies that can prevent disease progression or reduce mortality. Therefore, there is a pressing need for the development of new therapies [de Boer et al., 2007; Barnes, 2008; Liu et al., 2011].
Mesenchymal stem cells (MSCs) have an immune modulatory function. They can modulate the activity of both innate and adaptive immune cells such as dendritic cells, T cells, and B cells [Aggarwal and Pittenger, 2005; Corcione et al., 2006; Krampera et al., 2006; Uccelli et al., 2006]. MSCs have also demonstrated suppression of inflammation in murine models of acute lung injury (ALI) [Gupta et al., 2007; Xu et al., 2007; Song et al., 2012]. Furthermore, a modulation of inflammatory response has been the basis for clinical trials of allogeneic human MSCs (hMSCs) administration to patients with several inflammatory and immune-mediated diseases including Crohn's and graft versus host disease [Kermani et al., 2008; Le Blanc et al., 2008]. These findings present an exciting therapeutic potential for MSCs treatment in COPD. “A phase II, multicenter, randomized, double-blind, placebo controlled study using allogeneic hMSCs in patients with moderate-severe COPD” was recently initiated [Iyer et al., 2009]. The medium-term evaluation demonstrated safety of hMSCs and that hMSCs infusion significantly decreased C reactive protein in COPD patients. However, the potential for MSCs improving pathological changes in COPD and the underlying mechanisms are not included in this study.
Here we evaluated whether the administration of rat MSCs (rMSCs) ameliorated emphysema and destructive pulmonary function induced by chronic cigarette smoke (CS) exposure, further studied the effects of rMSCs on chronic inflammation, protease expression, and lung cell apoptosis. We also explored whether the effects are mediated through paracrine mechanisms by up-regulating protective growth factors such as vascular endothelial growth factor (VEGF) and transforming growth factor-β1 (TGFβ-1).
MATERIALS AND METHODS
MSCs Culture and Characterization
Rat MSCs were isolated from tibias and femurs of 6 weeks old male Sprague–Dawlay (SD) rats weighing 150–180 g (n = 4; Chinese Academy of Science, Shanghai, China), and incubated in DMEM/F12 containing 10% FBS as previously reported [Gupta et al., 2007; Xu et al., 2007; Song et al., 2012]. The passage 2 rMSCs were stained with antibodies against CD11b/c (Invitrogen, Carlsbad, CA), CD45 (Invitrogen), CD29 (eBioscience, San Diego, CA), and CD90 (Biolegend, San Diego, CA) for flow cytometry, and also subject to osteogenic, adipogenic, and chondrogenic differentiation assays. hMSCs were isolated from bone marrow aspirates taken from the iliac crest of a 40 years old male healthy volunteer with informed consent, and cultured in DMEM/F12 containing 20% FBS as previously described [Le Blanc et al., 2008]. The passage 2 hMSCs were stained with antibodies (Becton, Dickinson, Franklin, NJ) CD11b/c, CD19, CD29, CD34, CD44, CD45, CD90, and CD105 for flow cytometry. The used MSCs in vivo and in vitro experiments were the cultured passage 2 rMSCs and hMSCs, respectively. All studies were subject to the Institutional Animal Care and Use Committee Review and Research Ethics Committee at the Shanghai Jiaotong University.
Rat Emphysema Induction and Treatment Protocols
Emphysema in rats was induced by chronic CS exposure. The 8 weeks old male SD rats weighing 224.2 ± 10.8 g were divided into three groups (n = 12 per group): sham exposed, smoke exposed with PBS treatment, and smoke exposed with rMSCs treatment. Six rats in a 6-L smoking chamber were exposed to CS generated by burning three commercial cigarettes (“Da Qianmen,” 1.25 mg nicotine, 12 mg tar oil, and 14 mg carbon monoxide per cigarette, Shanghai, China) at one time with fresh air being pumped, 6 times per day (2 h smoke exposure) divided into two 1 h round with a 5 h smoke-free interval without evidence of toxicity (blood carboxyhemoglobin levels <8%), 5 days a week for 11 weeks. Sham exposed rats were exposed to air. On the first day of smoke week 7, CS-exposed rats were anesthetized with pentobarbital (50 mg/kg) and infused with 6 × 106 rMSCs suspended in 0.15 ml PBS via the trachea in rMSCs treatment group, while the rats in other two groups were treated with 0.15 ml PBS. On the first day of week 12, the CS exposure for 11 weeks was ceased. On the first day of week 16, 4 weeks after the last CS exposure, the rats were anesthetized for the evaluation of pulmonary function (n = 5 per group) or sacrificed for sample collection (n = 7 per group). The inferior lobe of the right lung was prepared for histological and morphological analysis. After their areas of bronchial airways were removed, the left lungs were stored at −80°C for future molecular biological research.
Morphological Assessment
The paraffin sections were stained with hematoxylin and eosin (HE) for pulmonary inflammation and emphysema assessment. Emphysematous change was quantified by measurement of the mean linear intercept (MLI) previously described [Robbesom et al., 2003; Chen et al., 2009]. The MLI represents the average size of alveoli and was measured in Adobe Photoshop software by dividing the total length of a line drawn across the lung section by a total number of intercepts encountered in 24 lines per microscopic field at original magnification 100×. Images with large bronchi or showing compression of alveolar space were excluded from the measurement. A minimum of three microscope fields per specimen was analyzed.
MEASUREMENT OF RAT PULMONARY FUNCTION
The pulmonary function was evaluated using an invasive pulmonary function devices that is based on similar lung functional variables routinely used in humans (Buxco Research Systems, Wilmington, NC) as described previously with slight modification [Vanoirbeek et al., 2009]. Briefly, the rats were anesthetized, trachea-intubated, and placed in whole body in the system. An average breathing frequency of 50 breaths/min was imposed on the anesthetized rats. The following parameters were recorded by the software: functional residual capacity (FRC), total lung capacity (TLC), vital capacity (VC), inspiratory capacity (IC), forced VC (FVC), forced expiratory flows (FEVs), such as FEV in 100 ms (FEV100) and 200 ms (FEV200), maximal expiratory flow at 25% of vital capacity (FEF25), FEF50, and FEF75.
Measurement of mRNA Expression by Real-Time Quantitative PCR
Total RNA was extracted from lung tissues using trizol–chloroform–isopropanol extraction method. mRNA levels of TNF-α, IL-1β, MCP-1, IL-6, VEGF164,TGFβ-1, matrix metalloproteinase-9 (MMP9), and MMP12 in lung of experiment rats were measured by Real-time Quantitative PCR using SYBR Green PCR Mix (Takara, Dalian, China). The primers were as follows: TNF-α, 5′-ATACACTGGCCCGAGGCAAC-3′ (Forward), 5′-CCACATCTCGGATCATGCTTTC-3′ (Reverse); IL-1β, 5′-GCTGTGGCAGCTACCTATGTCTTG-3′ (Forward), 5′-AGGTCGTCATCATCCCACGAG-3′ (Reverse); MCP-1, 5′-CTATGCAGGTCTCTGTCACGCTTC-3′ (Forward), 5′-CAGCCGACTCATTGGGATCA-3′ (Reverse); IL-6, 5′-CCACTTCACAAGTCGGAGGCTTA-3′ (Forward), 5′-GTGCATCATCGCTGTTCATACAATC-3′ (Reverse); VEGF164, 5′-GTCCTCACTTGGATCCCGACA-3′ (Forward), 5′-CCTGGCAGGCAAACAGACTTC-3′ (Reverse); TGFβ-1, 5′-TGCGCCTGCAGAGATTCAAG-3′ (Forward), 5′-AGGTAACGCCAGGAATTGTTGCTA-3′ (Reverse); MMP9, 5′-ATGCGCTGGGCTTAGATCATTC-3′ (Forward), 5′-GAGCTGTCGGCTGTGGTTCA-3′ (Reverse); MMP12, 5′-CTCGATGTGGAGTGCCTGATGTA-3′ (Forward), 5′-ATCCGCACGCTTCATGTCTG-3′ (Reverse); GAPDH, 5′-GGCACAGTCAAGGCTGAGAATG-3′ (Forward), 5′-ATGGTGGTGAAGACGCCAGTA-3′ (Reverse). PCR was performed in the following conditions: 30 s incubation at 95°C, followed by 40 cycles of 95°C for 5 s and 64°C for 34 s. Gene expression was normalized to GAPDH and measured by 2−ΔΔCt.
Evaluation of Pro-Inflammatory Cytokines and Growth Factors in Lung Homogenate
The left lung tissues were homogenized in RIPA (Beyotime, Shanghai, China). The protein levels (pg/mg protein) of TNF-α, IL-1β, MCP-1, IL-6, VEGF164, and TGFβ-1 in lung homogenates were analyzed with ELISA kits (eBioscience or Genetimes Inc. Shanghai, China) according to the manufacturer's protocols, subsequently normalized to total protein content of lung homogenate measured by bicinchoninic acid protein assay kit.
Western Blot Analysis
Protein in lung homogenate was separated using SDS–PAGE and transferred to polyvinylidene difluoride membranes. The membranes were exposed to antibodies for MMP9, cleaved-caspase3, poly ADP-ribose polymerase (PARP), β-actin (Cell Signaling Technology, Boston, MA), MMP12, and VEGF receptors 2 (Abcom, London, UK) at 4°C overnight, then incubated with HRP-labeled antibodies. The protein bands were subsequently visualized with enhanced chemiluminescence reagents (Pierce Distribution Services Company, Rockford, IL).
Zymography
MMP9 and MMP-12 enzymatic activity were determined by gelatin zymography according to the manufacturer's instruction (Invitrogen). Briefly, 40 µg total protein in lung homogenates was electrophoresed by SDS–PAGE gels containing 2 mg/ml and 0.5 mg/ml gelatin for MMP9 and MMP12, respectively. After electrophoresis, the separated proteins were renatured in Renaturing Buffer and then incubated in Developing Buffer at 37°C for 48 h. Subsequently, gels were stained in 0.1% Coomassie Brilliant Blue R-250. Gelatin-degrading enzymatic activity was indicated by the appearance of clear bands against a blue background and quantified by relevant intensity and the bands area.
Immumohistochemistry
To evaluate the apoptosis of endothelial cells in lungs, sections were processed for immunohistochemistry staining with the anti-cleaved-caspase3 monoclonal antibody (Cell Signaling Technology) according to standard protocol. Apoptotic endothelial cells were evaluated by the number of anti-cleaved-caspase3 positive cells per 500 counted endothelial cells.
Co-Culture of Cigarette Smoke Extract (CSE)-Stimulated EA.hy926 With hMSCs
In order to confirm whether MSCs can regulate apoptosis of vascular endothelial cells by paracrine mechanism, Transwell chambers (Costar, Corning, NY) were used where vascular endothelial cells and MSCs were co-cultured in the lower and upper chambers, respectively. Since rat pulmonary vascular endothelial cells are difficult to primary culture, in the present study, the human cell lines of human umbilical vein endothelial cells (called EA.hy926, provided by the Chinese Academy of Science, Shanghai, China) were used to co-culture with hMSCs. Firstly, CSE solution was prepared by drawing 200 ml CS into 20 ml serum-free DMEM/F12 with vigorous shaking to obtain 100% CSE. The cells were starved with DMEM/F12 containing 0.5% FBS for 36 h, then subject to the following conditions: cultured EA.hy926 alone without or with 10% CSE, EA.hy926 co-cultured with hMSCs exposed to 10% CSE, cultured hMSCs alone without or with 10% CSE. After 24 h incubation, VEGF165 mRNA levels in EA.hy926 and hMSCs were detected by Real-time PCR as previously described and the primers (Invitrogen) were as follows:VEGF165, 5′-GAGCCTTGCCTTGCTGCTCTAC-3′ (Forward), 5′-CACCAGGGTCTCGATTGGATG-3′ (Reverse); GAPDH, 5′-GCACCGTCAAGGCTGAGAAC-3′ (Forward), 5′-TGGTGAAGACGCCAGTGGA-3′ (Reverse). In addition, VEGF165 protein levels (pg/ml) in culture supernatant were assessed by ELISA as previously described without normalization.
In order to evaluate whether hMSCs could inhibit the apoptosis of EA.hy926 via VEGF165, another in vitro experiment was designed (similar to above, cells were starved for 36 h in advance): cultured EA.hy926 alone without or with 10% CSE, 5 or 25 ng/ml recombinant VEGF165 (Peprotech, Rocky Point, NJ) were added into cultured EA.hy926 alone with 10% CSE, EA.hy926 co-cultured with hMSCs exposed to 10% CSE, anti-VEGF165 neutralizing antibody (2 µg/ml) were added to co-cultured EA.hy926 with hMSCs exposed to 10% CSE. After the 24 h incubation, the cells were collected for apoptosis detection by FITC-annexinV-PI (BD) and cleaved-caspase3 and PRAP levels detection in EA.hy926 by Western Blot. The number of the used EA.hy926 and hMSCs in vitro study were 1 × 106/well and 2 × 105/well, respectively.
Statistical Analysis
The data was analyzed using SPSS11.5 software and shown as mean ± SD. The analysis between the different groups was performed using a one-way analysis of variance followed by LSD test with equal variances and Dunnet's T3 test with unequal variances. A value of P < 0.05 was considered as statistically significance.
RESULTS
rMSCs and hMSCs Characterization
Morphologically, the primary rMSCs (Fig. 1Aa) and passage 1 hMSCs (Fig. 1C left) had a spindled-fibroblast appearance in their culture. Differentiation assays demonstrated that rMSCs retained their ability to differentiate into osteoblasts, adipocytes, and chondrocytes (Fig. 1Ab–d). The flow cytometry analysis demonstrated that rMSCs did not express the cell surface markers CD11b/c and CD45, but expressed CD29 and CD90 (Fig. 1B). hMSCs did not express the cell surface markers CD11b/c, CD19, CD34, and CD45, but expressed CD29, CD44, CD90, and CD105 (Fig. 1D). All characterizations are consistent with international standards of MSCs [Horwitz et al., 2005].
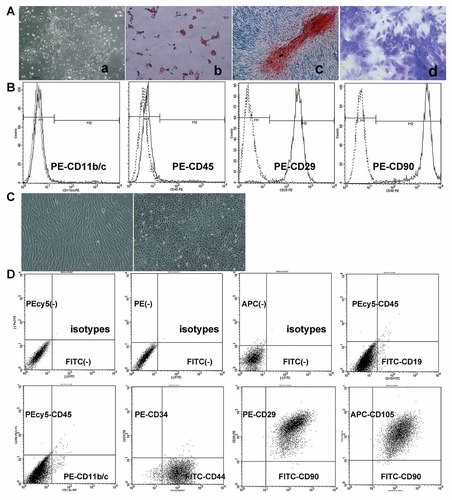
The characterization of MSCs. A: A colony-forming unit of cultured primary fibroblast-like rMSCs (40×) was observed (a). The multi-potential differentiation of rMSCs was verified by oil red stain (200×) for adipocytes (b), alizarin S stain (100×) for osteoblasts (c), and toluidine blue stain (100×) for chondrocytes (d). B: The rMSCs did not express CD11b/c and CD45, but expressed CD29 and CD90 by flow cytometry. C: The fibroblast-like hMSCs (left) and paving stone-like growth of EA.hy926 (right) were observed. D: The hMSCs did not express CD11b/c, CD19, CD34, and CD45, but expressed CD29, CD44, CD90, and CD105.
Administration of rMSCs Ameliorated Smoke-Induced Emphysema and Pulmonary Inflammation
Emphysema is a structural disorder characterized by destruction of the alveolar walls and enlargement of the alveolar spaces. For histological evaluation, rats in different groups were weighed and sacrificed at the designated endpoint. We firstly found that CS-exposed rats significantly lost their body weights compared with sham exposed rats (respectively 306.9 ± 22.1 and 415.8 ± 22.4 g, P < 0.0001), while rMSCs infusion significantly rescued the body weight loss (353.3 ± 16.4 g, P < 0.0001). A microscopic analysis of lung tissue sections revealed significant emphysematous change and pulmonary inflammation with inflammatory cells of peribronchial and alveolar structures after 11 weeks of CS exposure, while rMSCs administration improved the alveolar structure destruction and pulmonary inflammation (Fig. 2A). Furthermore, MLI which is a measurement of inter-alveolar wall distance widely used to examine air space enlargement was measured to evaluate emphysema severity (n = 7). The MLI were increased in the CS exposed group (51.7 ± 4.1 vs. 90.7 ± 8.2 µm, P < 0.05), while rMSCs transplantation attenuated the increase (71.8 ± 5.1 µm, P < 0.05). These results suggest that rMSCs are able to ameliorate CS-induced emphysema and pulmonary inflammation.
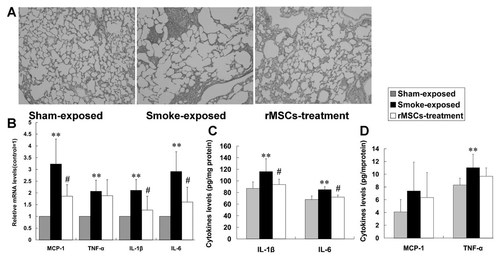
rMSCs improved the emphysema and lung inflammation. A: Lung histopathology changes were observed by hematoxylin–eosin stain (100×). The representative images showed the significant emphysema and inflammatory cells of peribronchial and alveolar structures, while rMSCs administration improved emphysema and lung inflammation. B: The mRNA (by Real-time PCR) and protein (by ELISA) levels of proinflammatory factors in lung tissue. Data are expressed as mean ± SD. **P < 0.01 versus sham exposed rats, #P < 0.05 versus CS-exposed rats.
Infused rMSCs Improved Smoke-Induced Destructive Pulmonary Function
Table I showed the pulmonary function results (n = 5). A statistically significant decline in IC, VC, FVC, and TLC was found in CS-exposed rats when compared with sham-exposed rats, while a statistically significant increase in FRC was observed. In addition, the markers for airflow obstruction during expiration (FEV100, FEV200, FEV100/FVC, FEV200/FVC, FEF25, FEF50, and FEF75) were statistically significant reduction in CS-exposed rats. Interestingly, rMSCs administration significantly improved the airflow obstruction supported by statistically higher FEV100, FEV200, FEV100/FVC, FEV200/FVC, FEF25, FEF50, and FEF75 with a non-statistically significant reduction in FRC compared with CS-exposed rats.
| Parameters | Sham-exposed | Smoke-exposed | Smoke exposed+rMSCs |
|---|---|---|---|
| IC (ml) | 18.8 ± 0.6 | 14.3 ± 0.3* | 15.6 ± 0.2*** |
| VC (ml) | 20.7 ± 1.3 | 15.7 ± 0.9* | 18.6 ± 0.1** |
| FVC (ml) | 18.4 ± 1.5 | 14.3 ± 0.9* | 16.6 ± 0.2** |
| FRC (ml) | 4.3 ± 0.9 | 6.9 ± 1.5* | 5.6 ± 0.5 |
| TLC (ml) | 22.6 ± 0.9 | 19.4 ± 1.3* | 22.2 ± 1.7** |
| FEV100 (ml) | 8.4 ± 3.3 | 4.0 ± 0.4* | 6.2 ± 0.1*** |
| FEV200 (ml) | 15.3 ± 1.1 | 7.9 ± 0.5* | 12.7 ± 0.1*** |
| FEV100/FVC (%) | 44.6 ± 1.3 | 28.3 ± 3.8* | 37.6 ± 1.0** |
| FEV200/FVC (%) | 83.7 ± 1.2 | 55.8 ± 7.3* | 76.3 ± 1.7** |
| FEF25 (ml/sec) | 62.8 ± 13.7 | 36.9 ± 1.1* | 62.2 ± 1.9*** |
| FEF50 (ml/sec) | 86.9 ± 4.4 | 40.4 ± 2.8* | 65.7 ± 1.7*** |
| FEF75 (ml/sec) | 91.5 ± 4.1 | 42.0 ± 3.6* | 67.9 ± 1.8*** |
- The pulmonary function of rats was evaluated by Buxco system, the following parameters were reccorded: inspiratory capacity (IC), vital capacity (VC), forced VC (FVC), functional residual capacity (FRC), total lung capacity (TLC), forced expiratory flow (FEV) at 100 ms (FEV100) and 200 ms (FEV200), maximal expiratory flow at 25% of vital capacity (FEF25), FEF50, and FEF75.
- Data are expressed as mean ± SD (n = 5).
- * P < 0.01 versus sham exposed rats.
- ** P < 0.05,
- *** P < 0.01 versus smoke exposed rats treated by PBS.
rMSCs Administration Decreased the Levels of Pro-Inflammatory Mediators in Lung
In order to evaluate the anti-inflammatory action of rMSCs, pro-inflammatory mediators were measured in lung homogenates (n = 7). The chronic CS exposure mediated a significant mRNA up-regulation of IL-1β, MCP-1, IL-6, and TNF-α in the lungs. Meanwhile CS-exposed rats treated with rMSCs had significantly lower mRNA levels of IL-1β, MCP-1, and IL-6, except that the TNF-α mRNA showed a trend toward reduction without statistically significance (Fig. 2B). Similarly, protein levels of IL-1β and IL-6 in lung homogenate were consistent with their mRNA level changes (Fig. 2C). MCP-1 and TNF-α levels were lower in the rMSCs-treated rats, but the differences were not statistically significant (Fig. 2D). The results showed that rMSCs transplantation reduced the pro-inflammatory cytokines in lungs of CS-exposed rats.
rMSCs Administration Decreased Levels of Proteases in Lung
Matrix metalloproteinases (MMPs) which regulates extra-cellular matrix homeostasis have been implicated in CS-induced emphysema. MMP9 and MMP12 levels in the lungs were tested in current study. Real-time PCR (n = 7) and Western Blot analysis (n = 5) demonstrated that the mRNA (Fig. 3A) and protein (Fig. 3B) levels of MMP9 and MMP12 were significantly higher in CS-exposed rats compared with sham exposed rats, while they were significantly lower after rMSCs infusion. Furthermore, we also found that the results of MMP9 and MMP12 enzymatic activity determined by gelatin zymography (n = 5) were consistent with that of protein levels (Fig. 3C).
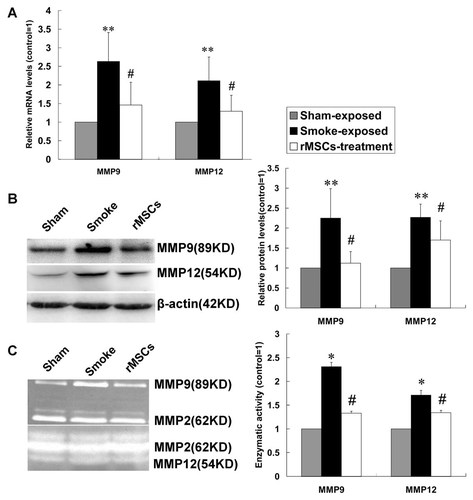
rMSCs down-regulated the levels of MMP9 and MMP12 in lung tissue. The mRNA (A) and protein (B) levels of MMP9 and MMP12 were analyzed by Real-time PCR and Western Blot, respectively. C: Enzymatic activity of MMP9 and MMP12 was measured by gelatin zymograph. Data are expressed as mean ± SD. *P < 0.05, **P < 0.01 versus sham exposed rats, #P < 0.05 versus CS-exposed rats.
rMSCs Infusion Up-Regulated VEGF164 and TGFβ-1 Levels
To investigate VEGF164 and TGFβ-1 levels in lungs, Real-time PCR (n = 7) for VEGF164 and TGFβ-1 mRNA levels and ELISA for protein levels (n = 7) were performed. The mRNA and protein levels of VEGF164 in lungs were significantly lower in CS-exposed rats compared with sham exposed rats, while they were significantly higher in rMSCs-treated rats (Fig. 4A–B). TGFβ-1 mRNA level was higher in CS-exposed rats and further higher in rMSCs-treated rats, however, the difference was not statistically significant (Fig. 4A). Of note, TGFβ-1 protein level was significantly lower after CS exposure, while it was significantly higher after rMSCs administration (Fig. 4C).
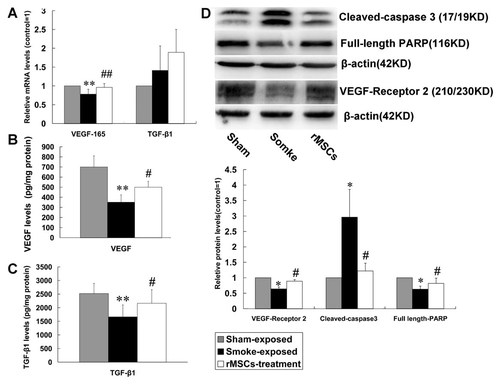
rMSCs up-regulated the levels of VEGF164, VEGF receptor 2, and TGFβ-1 in lung tissue, while reduced lung cells apoptosis. A: The mRNA levels of VEGF164 and TGFβ-1 were evaluated by Real-time PCR. B and C: The protein levels of VEGF164 and TGFβ-1 were analyzed by ELISA. D: The levels of apoptosis-related proteins and VEGF receptor 2 were evaluated by Western Blot. Data are expressed as mean ± SD. *P < 0.05, **P < 0.01 versus sham exposed rats, #P < 0.05 versus CS-exposed rats.
Effect of rMSCs Infusion on Apoptosis of Lung Cells
Apoptosis-related proteins (cleaved-caspase3 and PARP) in lungs were assessed by Western Blot (n = 5). The results showed that cleaved-caspase3 were significantly higher in CS-exposed rats. The CS-induced effect was significantly inhibited by rMSCs. Subsequently, full-length PARP in the downstream substrate of cleaved-caspase3 in CS-exposed rats was significantly reduced, while it was higher in rMSCs-treated rats (Fig. 4D). In addition, CS exposure induced the reduction in VEGF receptor 2 level, and rMSCs significantly up-regulated VEGF-receptor 2 level (Fig. 4D). Furthermore, a significant increase in the number of cleaved-caspase3 positive pulmonary vascular endothelial cells was detected in CS exposed rats with respect to control rats (11.3 ± 3.0 vs. 82.3 ± 13.8, n = 5, P < 0.0001), while rMSCs infusion significantly reduced the number of apoptotic cells (27.7 ± 5.0, P < 0.0001; Fig. 5).
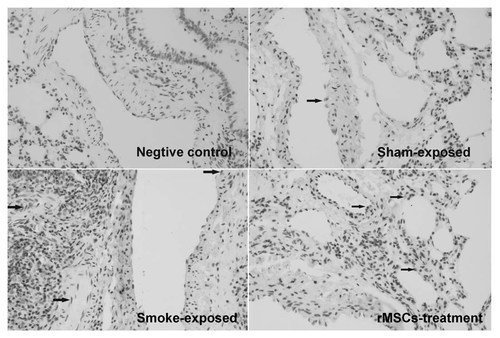
rMSCs reduced the apoptosis of pulmonary vascular endothelial cells. The apoptotic endothelial cells were identified by cleaved-caspase3 immunohistochemistry assay. CS exposure induced the endothelial cells apoptosis (arrow), which were reduced after rMSCs infusion.
VEGF165 Produced by hMSCs
All data of in vitro study were from four independent experiments (n = 4). Firstly, we confirmed that 10% CSE did not affect the viability of hMSCs and EA.hy926. Then, a significantly increased VEGF165 mRNA level in EA.hy926 exposed to 10% CSE was observed compared with EA.hy926 without CSE and a further increased expression in EA.hy926 co-cultured with hMSCs (Fig. 6A). Although VEGF165 protein levels in culture supernatant were consistent with mRNA results, however, we observed that cultured EA.hy926 alone with or without CSE produced a very small amount of VEGF165 (5.5 ± 0.6 and 10.3 ± 0.9 pg/ml, respectively). Interestingly, VEGF165 in cell suspension of CSE-stimulated EA.hy926 co-cultured with hMSCs significantly increased to 288.7 ± 25.7 pg/ml, which suggested VEGF165 in co-culture medium was mainly secreted by hMSCs (Fig. 6B).
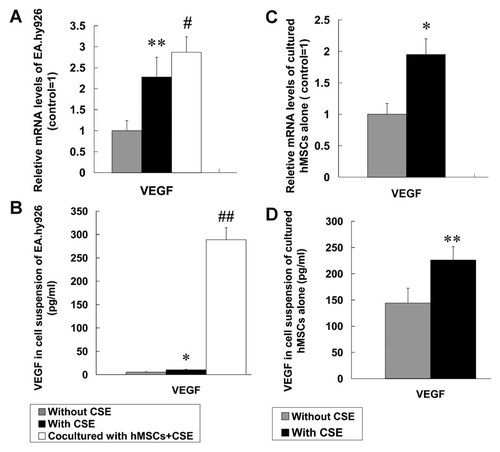
VEGF165 levels in cell culture medium. The EA.hy926 and rMSCs were respectively cultured alone (with or without CSE) and co-cultured with CSE. After 24 h culture with cell starvation for 36 h in advance, the cells and cell supernatant were collected. The mRNA expression of VEGF165 in EA.hy926 (A) and hMSCs (C) was evaluated by Real-time PCR. The concentrations of VEGF165 in cell supernatant were analyzed by ELISA (B, D). Data are expressed as mean ± SD. *P < 0.05, **P < 0.01 versus without CSE, #P < 0.05, ##P < 0.01 versus with CSE.
To further identify the VEGF165 source, VEGF165 mRNA, and protein levels of the cultured hMSCs alone were also evaluated. The results demonstrated that VEGF165 mRNA in hMSCs exposed to 10% CSE were increased compared with hMSCs without CSE (Fig. 6C), and the level (ΔCt = 4.80 ± 0.36) was significantly higher in abundance compared with that in EA.hy926 exposed to 10% CSE (ΔCt = 9.61 ± 0.41; about 28-fold). In addition, compared with the negligible amount of VEGF165 released from EA.hy926, we also found hMSCs cultured alone without or with 10% CSE released large amounts of VEGF165 (226.2 ± 25.6 and 144.1 ± 28.3 pg/ml, respectively; Fig. 6D). These results further indicated that VEGF165 in the co-culture system was released mainly from hMSCs.
VEGF165 Secreted by hMSCs Partly Inhibited the Apoptosis of EA.hy926
In order to test whether hMSCs can inhibit EA.hy926 apoptosis by secreting VEGF165, we also used recombinant human VEGF165 and anti-VEGF165 antibody. EA.hy926 apoptosis was examined by flow cytometry and Western Blot for cleaved-caspase3 and PARP. We found that the number of apoptotic cells in cultured EA.hy926 with 10% CSE (16.9 ± 0.6%) was significantly greater than that of apoptotic cells in EA.hy926 without CSE (13.0 ± 1.8%). When different concentrations of VEGF165 (5 and 25 ng/ml) were added to the medium of EA.hy926 with 10% CSE, the percentage of apoptotic cells significantly reduced in a concentration-dependent manner (9.9 ± 0.4% and 4.3 ± 0.6%, respectively). The number of apoptotic cells in EA.hy926 exposed to 10% CSE co-cultured with hMSCs was significantly lower (1.7 ± 0.4%). In addition, cell starvation and CSE stimulation did not induce co-cultured hMSCs apoptosis, which was supported by low apoptosis index (3.46 ± 0.58%). Importantly, the inhibition of apoptosis in co-cultured EA.hy926 was partly blocked by added anti-VEGF165 neutralizing antibody (4.0 ± 0.7%; Fig. 7).
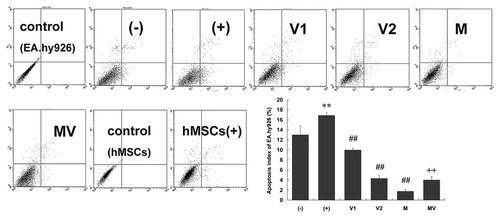
VEGF165 partly inhibited EA.hy926 apoptosis induced by cell starvation and CSE stimulation. The EA.hy926 were cultured alone without CSE (called group “−”) and exposed to CSE without (“+”) or with 5 ng/ml (“V1”) and 25 ng/ml (“V2”) recombinant VEGF group. The EA.hy926 were co-cultured with hMSCs without (“M”) or with 2 µg/ml anti-VEGF neutralizing antibody (“MV”) was added into the co-culture system. After the two kinds of cells were cultured for 24 h with cell starvation for 36 h in advance, the cells were harvested to evaluate cell apoptosis index using flow cytometry. Data are expressed as mean ± SD. **P < 0.01 versus without CSE, ##P < 0.01 versus with CSE, ++P < 0.01 versus co-cultured EA.hy926 with hMSCs.
Consequently, we measured cleaved-caspase3 and full-length PARP levels in EA.hy926. Higher cleaved-caspase3 and lower full-length PARP after CSE stimulation were found. Recombinant VEGF165 and co-cultured hMSCs significantly reduced cleaved-caspase3 level and increased full-length PARP level in EA.hy926. In particular, the cleaved-caspase3 in EA.hy926 co-cultured with hMSCs was too low to be detectable. The changes of cleaved-caspase3 and full-length PARP in EA.hy926 co-cultured with hMSCs were partly blocked by an addition of anti-VEGF neutralizing antibody (Fig. 8). Our findings suggested that VEGF165 secreted by hMSCs was crucial for the inhibition of EA.hy926 apoptosis induced by cell starvation and CSE stimulation.

The levels of apoptosis-related proteins in EA.hy926. The experiment protocol was in agreement with that of Figure 7. The cultured EA.hy926 were harvested for Western Blot detection for apoptosis related proteins (cleaved-caspase3 and full-length PARP). A higher cleaved-caspase3 level and lower full-length PARP level suggested more apoptotic cells in EA.hy926. Data are expressed as mean ± SD. **P < 0.01 versus without CSE, ##P < 0.01 versus with CSE, ++P < 0.01 versus co-cultured EA.hy926 with hMSCs.
DISCUSSION
There are three major findings in this study: (1) the intrapulmonary administration of rMSCs ameliorated the severity of emphysema and destructive pulmonary function induced by CS; (2) the beneficial effects were primarily exerted by reducing the levels of pro-inflammatory mediators (TNF-α, IL-1β, MCP-1, and IL-6), MMP9 and MMP12, whereas up-regulating VEGF164, VEGF receptors, and TGFβ-1, subsequently reducing lung cells apoptosis; (3) the in vitro study demonstrated that anti-apoptosis effect of hMSCs on endothelial cells was mediated partly by paracrine VEGF165.
The current evidence supporting the application of MSCs as a potential biologic therapy for a variety of diseases includes: ease of accessibility for isolation, enormous expansion potential in culture, multi-differentiation capacity, immunosuppressive properties, paracrine-mediated effects, homing, and migratory behavior to sites of tissue injury as well as host compatibility [Salem and Thiemermann, 2003]. Recent studies have demonstrated that the use of MSCs has been attempted in the treatment of lung diseases including ALI, lung fibrosis, and COPD on the basis of their capacity to modulate local inflammation and immune responses [Sueblinvong and Weiss, 2009].
Alveolar destruction (emphysema) with no apparent fibrosis is one of major pathological presentations of COPD [Yao et al., 2008]. Therefore, our primary endpoint was the effect of rMSCs on emphysema. The results showed that rMSCs significantly protected against emphysema induced by chronic CS exposure, which is consistent with previous report that rMSCs attenuated lung emphysema caused by papain or elastase [D'Agostino et al., 2010; Zhen et al., 2010; Katsha et al., 2011].
On the other hand, COPD is characterized by slowly progressive and poorly reversible airflow limitation and pulmonary function measure is indispensable in COPD diagnosis. Therefore, we also evaluated the effect of rMSCs on the pulmonary function. The decline of FEV100, FEV200, FEV100/FVC, FEV200/FVC, FEF25, FEF50, and FEF75 and increase of FRC in CS-exposed rats are in agreement with diagnosis of human COPD. In addition, the lower IC, VC, FVC, and TLC, together with about 50% decline of FEV200/FVC were consistent with the changes in severe COPD patients. It is important to point out that the administration of rMSCs significantly improved the airflow obstruction induced by CS exposure and improvement in pulmonary function will significantly improve the lift quality of COPD patients.
We then explored the possible underlying mechanisms of rMSCs improving the destructive pulmonary function and emphysema. To date, it is considered that COPD is associated with chronic inflammation of airway and lung parenchyma, characterized by increased numbers of neutrophils, activated macrophages, and T-lymphocytes. Many pro-inflammatory mediators including TNF-α, IL-1β, MCP-1, IL-6, MIP-1α, and MIP-1β are involved in pathogenesis of COPD. Furthermore, the activated macrophages and neutrophils release elastolytic enzymes, particularly neutrophil elastase, MMP-9, and MMP12 that in turn result in enhanced inflammatory cell recruitment [Sethi and Rochester, 2000; Barnes et al., 2003; Yoshida and Tuder, 2007; Yao et al., 2008]. Therefore, these changes in the experimental rats were observed. We found that rMSCs have the capacity to suppress TNF-α, IL-1β, MCP-1, and IL-6 expression in the lungs of CS-exposed rats, which had a beneficial effect on chronic lung inflammation. Gupta et al. [2007] reported that intrapulmonary administration of MSCs reduced TNF-α and MIP-2 levels in the bronchoalveolar lavage fluid and the severity of endotoxin-induced ALI. A similar study showed that systemic administration of MSCs reduced the systemic inflammatory responses (lower IL-1β, IL-6, MIP-1α, and IL-8 levels in serum) of endotoxin-induced ALI [Xu et al., 2007].
It has been demonstrated that MSCs can release several types of growth factors to protect injured tissues [Chen et al., 2008; Crisostomo et al., 2008]. A recent study indicated that MSCs improved elastase-induced emphysema in mice by increasing hepatocyte growth factor and epidermal growth factor [Katsha et al., 2011]. On the other hand, Zandvoort et al. [2006] reported that TGFβ-1 and TGFβ-1 receptor type I in lung tissue were significantly lower in stage II COPD patients than controls. Here we observed that chronic CS reduced TGFβ-1 protein level and rMSCs significantly up-regulated the level. In addition, we also observed rMSCs infusion reduced MMP12 and MMP9 expression in lungs of CS-exposed rats. Notably, it is demonstrated that TGFβ-1 can inhibit MMP9 and MMP12 expression in alveolar macrophage and monocyte, meanwhile the inhibition of TGFβ-1 signal leads to increased MMP12 expression and subsequent extra-cellular matrix degradation contributing to the development of emphysema [Morris et al., 2003; Roberts, 2003; Morty et al., 2009]. The anti-inflammatory capacity of TGFβ-1 has been suggested by the report that TGFβ-1 null mice died of excessive inflammation within 1 month after birth [Shapiro, 2007]. Therefore, our results suggest that decreased MMP9, MMP12, and pro-inflammatory mediators by rMSCs transplantation are perhaps partly due to increased TGFβ-1 production.
Apoptosis of epithelial and endothelial cells is increasingly recognized as an important contributor to alveolar destruction in COPD [Plataki et al., 2006; Park et al., 2007; Yoshida and Tuder, 2007]. Activation of caspase-3 requires proteolytic processing of its inactive zymogen into activated fragments (cleaved-caspase3). Cleaved-caspase3 is primarily responsible for the cleavage of full-length PARP, which plays a central role in the execution of the apoptotic program. Therefore, activation of caspase-3 suggests cells apoptosis. Our results showed that rMSCs administration inhibited lung cells apoptosis supported by reduced cleaved-caspase3 and increased full-length PARP levels. In addition, VEGF is a pluripotent growth factor that is critical for endothelial cell proliferation, lung development, and several lung disorders including COPD, lung cancer, and ALI [Voelkel et al., 2006], and the most studied molecule of the VEGF family is VEGF-A (VEGF165 in human and VEGF164 in rats). Moreover, a previous report showed that VEGF and VEGF receptor 2 were decreased in lungs of COPD patients, and inhibition of VEGF receptors induced alveolar endothelial cells apoptosis and subsequent emphysema [Kasahara et al., 2000, 2001]. In the current study, CS reduced the expression of VEGF and its receptor 2, while rMSCs significantly up-regulated their levels.
VEGF-dependent homeostasis of alveolar walls has been considered as a potential mechanism in the pathogenesis of COPD: reduced VEGF might result in pulmonary endothelial cell death and subsequently impair normal microcirculation and epithelial cell repair [Kanazawa, 2007]. In the present study, we also observed that CS induced the apoptosis of vascular endothelial cells, rMSCs inhibited their apoptosis. To identify whether MSCs have the capacity to inhibit endothelial cell apoptosis by paracrine VEGF, we used a Tran-swell system to physically separate EA.hy926 and hMSCs. Firstly, co-cultured hMSCs protected against cell apoptosis in EA.hy926 induced by cell starvation and CSE stimulation. Furthermore, to confirm the anti-apoptosis role of hMSCs was related to their ability to secrete VEGF165, the addition of recombinant VEGF165 to the medium of cultured EA.hy926 alone and anti-VEGF165 antibody to the co-culture system were performed. The recombinant VEGF165 exerted the anti-apoptosis effect in a concentration-dependent manner, and the reduced apoptosis index of EA.hy926 by co-cultured hMSCs increased again after adding the anti-VEGF165 neutralizing antibody. These findings indicated that paracrine VEGF165 by hMSCs was crucial for the inhibition of cell apoptosis in EA.hy926. It is necessary to point out that the concentration of VEGF165 (pg/ml) released by hMSCs was significantly lower than that of added recombinant VEGF165 (ng/ml), while co-cultured hMSCs more strongly inhibited cell apoptosis. These findings suggested that other protective cytokines were involved in MSCs-mediated anti-apoptosis effect.
Furthermore, it has been reported that lung presents the highest level of VEGF among normal tissues and alveolar epithelial cells are the main sites of VEGF production. The altered function of epithelial cells by chronic smoke exposure leads to the decline of VEGF production[Kanazawa, 2007], subsequently the down-regulated VEGF/VEGF receptors signaling may result in emphysema due to pulmonary endothelial apoptosis. Since co-cultured hMSCs can promote VEGF release from endothelial cells, we presume that rMSCs in vivo experiment can promote VEGF release from alveolar epithelial cells by regulating lung local microenvironment, together with VEGF release from rMSCs, which may account for the elevated VEGF in lungs. VEGF receptor 2 is almost exclusively expressed on endothelial cells, therefore, the enhanced VEGF and its receptor 2 improved excessive cell apoptosis of lung cells including vascular endothelial cells in CS-exposed rats, which contributed to improvement of emphysema. A similar study from Zhen et al. [2010] reported that the protective effect of rMSCs on papain-induced emphysema was partly mediated by up-regulating VEGF164 and inhibiting the apoptosis of lung cells. The difference from the current study was that they detected the apoptosis of the cultured lung cells isolated from papain-induced emphysematous lungs.
MSCs in the lung can engraft as type I and II epithelial cells and endothelial cells [Ortiz et al., 2003; Rojas et al., 2005], however, the engraftment is rare and of unclear physiologic and therapeutic significance, so structural repair or replacement of injured lung epithelial cells by exogenous stem cells is now felt to be less likely [Sueblinvong and Weiss, 2009]. The present authors investigated rMSCs infusion reduced the expression of pro-inflammatory mediators and pulmonary inflammation, increased protective growth factors (VEGF and TGFβ-1) and VEGF receptor 2 levels, subsequently reduced MMP9, MMP12 levels, and lung cells apoptosis. In vitro results showed that hMSCs can inhibit EA.hy926 apoptosis in a contact-independent manner partly by secreting VEGF. All these findings suggest that the administration of MSCs does attenuate lung injury and promote functional recovery on basis of their capacity to modulate local microenvironment [Uccelli et al., 2007; Sueblinvong and Weiss, 2009].
However, we also found that MSCs could not completely reverse smoke-induced emphysema and is not omnipotent in disease treatment. It is also important to recognize certain limitations of the present research. Firstly, the CS-induced rat model cannot fully reproduce the complexity of COPD patients and there remains a long way to go for a full understanding of the MSCs role in COPD before clinical use. Furthermore, rMSCs were infused only once during CS exposure, therefore, it will be crucial to define a therapeutic window of intervention and a long-term effect of repeatedly infused rMSCs at second or three time point. Moreover, alveolar epithelial cells and macrophages are the main cells involved in the pathogenesis of COPD and there is a need to further study the effects of co-cultured MSCs on the function of these cells in the future.
CONCLUSION
Intrapulmonary rMSCs can sense the local microenvironment and regulate the production of pro-inflammatory mediators and growth factors such as VEGF and TGFβ-1, then mediate the anti-inflammatory, anti-protease, and anti-apoptosis effects. They contribute to an attenuation of emphysema and destructive pulmonary function in COPD. Therefore, MSCs-based cell therapy will shed light on the prevention and treatment of COPD currently lacking efficient intervention.
Acknowledgements
All authors thank all members of their laboratory for helpful discussion.




