BAI, A novel cyclin-dependent kinase inhibitor induces apoptosis in A549 cells through activation of caspases and inactivation of Akt
Abstract
Previously, we have synthesized a novel cyclin-dependent kinase (CDK) inhibitor, 2-[1,1′biphenyl]-4-yl-N-[5-(1,1-dioxo-1λ6-isothiazolidin-2-yl)-1H-indazol-3-yl]acetamide (BAI) and reported its anti-cancer activity in head and neck cancer cells. In this study, we further evaluated the effect of BAI on growth of various human cancer cell lines, including A549 (nonsmall cell lung cancer), HCT116 (colon), and Caki (kidney). Profoundly, results of XTT and clonogenic assays demonstrated that BAI at nanomolar concentrations (20–60 nM) inhibited growth of A549, HCT116, and Caki cells, suggesting the anti-cancer potency. We show that BAI induced a dose-dependent apoptotic cell death in these human cancer cells, as measured by fluorescence-activated cell sorting (FACS). Interestingly, further biochemical analysis showed that treatment with BAI at 20 nM induced apoptosis in A549 cells in association with activation of caspases, cleavage of phospholipase C-γ1 (PLC-γ1), and inhibition of Akt in A549 cells. Importantly, pharmacological inhibition study revealed that pretreatment with z-VAD-fmk, a pan caspase inhibitor strongly blocked the BAI-induced apoptosis in A549 cells. Transfection analysis with Akt cDNA encoding constitutively active Akt further addressed the significance of Akt inhibition in the BAI-induced apoptosis in A549 cells. Notably, disruption of the PI3K/Akt pathway by LY294002, a PI3K/Akt inhibitor potentiated apoptosis in A549 cells by BAI at a subcytotoxic concentration. These findings collectively suggest that BAI potently inhibits growth of A549, HCT116, and Caki cells, and that the BAI-induced apoptosis in A549 cells is associated with activation of caspases, and inhibition of Akt. J. Cell. Biochem. 114: 282–293, 2013. © 2012 Wiley Periodicals, Inc.
There are more than 500 protein kinases encoded by the human genome and this class of enzymes has been an area of intensive research for the development of novel anti-cancer agents [Dancey and Sausville, 2003]. One family of kinases that has attracted particular attention is the cyclin-dependent kinases (CDKs), which forms heterodimeric complexes composed of a catalytic kinase subunit and a regulatory cyclin subunit, and comprise a family divided into two groups based on their roles in cell cycle progression and transcriptional regulation [Meyerson et al., 1992; Sausville, 2002]. The inhibition of CDK, which plays an important role in cell cycle progression and gene transcription, has prompted intensive efforts to develop potent inducer of apoptosis in malignant cancer cells. For examples, flavopiridol, a CDK inhibitor is shown to induce apoptosis in lung cancer cells [Bible and Kaufmann, 1996], and the induction of apoptosis by other CDK inhibitors has also been widely reported [Mgbonyebi et al., 1999; Dai et al., 2002]. It has been reported that some of the CDK inhibitors, including flavopiridol [Morris et al., 2006; Lin et al., 2009], recently entering clinical trials have potency in clinical applicability [DePinto et al., 2006; Heath et al., 2008; Schwartz et al., 2011].
Cyclin E/CDK2 complexes play an important role in carcinogenesis. Cyclin E amplification has been identified in colon cancer [Leach et al., 1993] and CDK2 amplification is associated with concurrent cyclin E gene amplification in several tumors [Kitahara et al., 1995; Marone et al., 1998]. Furthermore, cyclin A/CDK2 complexes play a key role in human lung carcinoma cell proliferation [Dobashi, 2005] and tumor tissues which exhibited higher cyclin A and CDK2 expression also had higher CDK2 kinase activity [Dobashi et al., 1998]. Such lines of reasoning have led to the investigation and production of novel chemotherapeutic agents that target cyclin/CDK2 activity in an attempt to regulate aberrant cell cycle pathways. In order to achieve the aims, several researches have been mainly focused on the searching for ATP competitive inhibitors, which could inhibit CDK2 kinase activity [Clare et al., 2001; DePinto et al., 2006].
Previously, we have synthesized novel CDK inhibitors 3,5-diaminoindazoles [Lee et al., 2008], and showed 2-[1,1′biphenyl]-4-yl-N-[5-(1,1-dioxo-1λ6-isothiazolidin-2-yl)-1H-indazol-3-yl]ac-etamide (BAI), a derivative of 3,5-diaminoindazoles, had potent anti-proliferative activity against head and neck cancer cells [Shin et al., 2009]. In this study, we further evaluated anti-proliferative and/or apoptosis inducing activities in various cancer cell lines, including lung (A549), colon (HCT116), and kidney (Caki).
MATERIALS AND METHODS
Cell Lines and Culture
A549 human nonsmall cell lung cancer cells and HCT116 human colorectal carcinoma cells were obtained from the American Type Culture Collection (ATCC; Rockville, MD) and grown in RPMI 1640 medium supplemented with 10% heated-inactivated FBS, 2 mM L-glutamine, 100 µg/ml streptomycin, and 100 µg/ml penicillin. Caki human renal clear cell carcinoma cells were obtained from ATCC and grown in Dulbecco's modified Eagle's medium, containing 10% heat-inactivated FBS, 20 mM HEPES buffer and 100 µg/ml streptomycin, and 100 µg/ml penicillin.
Drugs and Materials
2-[1,1′biphenyl]-4-yl-N-[5-(1,1-dioxo-1λ6-isothiazolidin-2-yl)-1H-indazol-3-yl] acetamide (BAI) was kindly supplied by Dr. Lee, JH (Keimyung University, Daegu, Korea; Fig. 1A). (R)-Roscovitine was purchased from Alexis (Farmingdale, NY). Mcl-1, heat shock conjugate protein (HSC) 70, phospholipase C-γ1 (PLC-γ1), pro-caspase-3 antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Phospho-Akt (p-Akt), Akt, cleaved caspase-3, and poly ADP-ribose polymerase (PARP) antibodies were purchased from Cell Signaling Technology (Beverly, MA). X-Linked Inhibitor of Apoptosis Protein (XIAP) was purchased from BD PharMingen (San Jose, CA). z-VAD-fmk and LY294002 (PI3K inhibitor) were purchased from Biomol (Plymouth Meeting, PA).
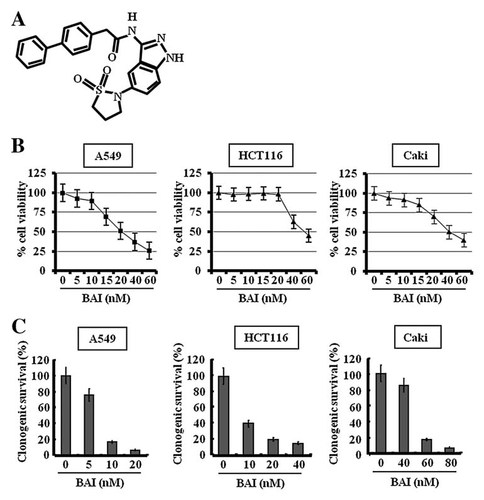
Effect of BAI on the cell viability in various human cancer cells. A: The structure of BAI. B: Exponentially growing cancer cells (A549, HCT116, and Caki) were treated with vehicle control (0.1% DMSO) or BAI at the indicated concentrations for 24 h. Cell viability was assessed by XTT assay. C: A549, HCT116, and Caki cells were incubated with vehicle control or BAI at the indicated concentrations for 24 h, after which the cells were trypsinized and plated in a clonogenic survival assay. The growth of cells without BAI was considered to be 100%. Data are mean values from three independent experiments and bars represent standard deviations.
Cell Viability Assay
The cytotoxic effect of the BAI on various human cancer cells was investigated using a commercially available proliferation kit (WelCount Cell Viability Assay Kit, WELGENE, Daegu, Korea). Briefly, the cells were plated in 96-well culture plates at a density of 3,000 cells/well in phenol red free medium and allowed to attach for 10 h. After 24 h or more than 24 h treatment of BAI, 20 µl of XTT reaction solution (2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide inner salt and phenazine methosulfate; mixed in proportion 50:1) was added to the wells. The optical density was read at 450 nm wavelength in a spectrophotometer after 3 h incubation of the plates with XTT reaction solution in an incubator (37°C and 5% CO2 + 95% air).
Clonogenic Assay
A549, HCT116, and Caki cells were respectively seeded into six-well plates at 3,000, 3,000 and 1,500 per well in a final volume of 2 ml of medium containing appropriate drug concentrations. Triplicate cultures were used for each drug concentration and time tested. At the end of the drug exposures, the drug-containing medium was replaced with fresh media. All cultures were incubated for an additional 7 days. At this point, the medium was aspirated, and the dishes were washed once with PBS, fixed with 100% methanol for 30 min, and stained with a filtered solution of 0.5% (w/v) crystal violet (Sigma Chemical Co.) for 30 min. The wells were then washed with PBS and dried at room temperature. The colonies, defined as groups of ≥50 cells, were scored manually with the inverted microscope.
Annexin V and 7-Aminoactinomycin D Staining
Enumeration of apoptotic cells was done using FITC-conjugated Annexin V (BD PharMingen, CA) and 7-aminoactinomycin D (7-AAD). Cells were washed twice in cold PBS and resuspended in Annexin V-binding buffer (BD PharMingen, CA) at a concentration of 3 × 106 per ml. This suspension (100 µl) was stained with 5 µl of Annexin V-FITC and 5 µl 7-AAD. 7-AAD (BD PharMingen, CA) is a nucleic acid dye that was used for the exclusion of nonviable cells. The cells were gently vortexed and incubated for 15 min at room temperature in the dark. After addition of 400 µl of binding buffer to each tube, cells were analyzed by flow cytometry.
Western Blotting Analysis
Cellular lysates were prepared by suspending 0.8 × 106 cells in 80 µl of lysis buffer (137 mM NaCl, 15 mM EGTA, 0.1 mM sodium orthovanadate, 15 mM MgCl2, 0.1% Triton X-100, 25 mM MOPS, 100 µM phenylmethylsulfonyl fluoride, and 20 µM leupeptin, adjusted to pH 7.2). The cells were disrupted by sonication and extracted at 4°C for 30 min. The proteins were electrotransferred to Immobilon-P membranes (Millipore Corp., Bedford, MA). Detection of specific proteins was carried out with an ECL Western blotting kit according to the manufacturer's instructions.
Flow Cytometric Analysis
Approximately 0.8 × 106 A549 cells were suspended in 100 µl PBS, and 200 µl of 95% ethanol were added while vortexing. The cells were incubated at 4°C for 1 h, washed twice with PBS, and resuspended in 250 µl of 1.12% sodium citrate buffer (pH 8.4) together with 12.5 µg RNase. Incubation was continued at 37°C for 30 min. The cellular DNA was then stained by applying 250 µl propidium iodide (50 µg/ml) for 30 min at room temperature. The stained cells were analyzed by fluorescence-activated cell sorting (FACS) on a FACScan flow cytometer for relative DNA content based on red fluorescence.
RNA Isolation and Reverse Transcription-PCR
XIAP and Mcl-1(L) mRNA expressions were determined by reverse transcription-PCR. Total cellular RNA was extracted from cells using the TRIzol reagent (Molecular Research Center, Inc., Cincinnati, OH). Each cDNA was synthesized form 2 µg of total RNA using M-MLV reverse transcriptase (Promega, Madison, WI). The cDNAs for XIAP, and Mcl-1(L) were amplified by PCR with specific primers. The sequences of the sense and antisense primer for XIAP were 5′-CTTGAGGAGTGTCTGGTAA-3′ and 5′-GTGACTAGATGTCCACAAGGC-3′, respectively. The sequences of the sense and antisense primer for Mcl-1(L) were 5′-ATCTCTCGGTACCTTCGGGAG-3′ and 5′-ACCAGCTCCTACTCCAGCAAC-3′, respectively. PCR products were analyzed by agarose gel electrophoresis and visualized by ethidium bromide.
Small Interfering RNA
The XIAP small-interfering RNA (siRNA) duplexes were obtained from Cell Signaling Technology. Control siRNA duplexes used in this study were purchased from Invitrogen (Calsbad, CA) and had the following sequences: green fluorescent protein (GFP), AAG ACC CGC GCC GAG GUG AAG. Cells were transfected with siRNA oligonucleotides using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's recommendations.
DNA Fragmentation Assay
After treatment with BAI, A549 cells were lysed in a buffer containing 10 mM Tris (pH 7.4), 150 mM NaCl, 5 mM EDTA, and 0.5% Triton X-100 for 30 min on ice. Lysates were vortexed and cleared by centrifugation at 10,000g for 20 min. Fragmented DNA in the supernatant was extracted with an equal volume of neutral phenol:chloroform:isoamyl alcohol mixture (25:24:1) and analyzed electrophoretically on 2% agarose gels containing 0.1 µg/ml of ethidium bromide.
DEVDase Activity Assay
To evaluate caspase-3 activity, cell lysates were prepared after their respective treatment with various drugs. Assays were performed in 96-well microtiter plates by incubating 20 µg cell lysates in 100 µl reaction buffer [1% NP-40, 20 mM Tris–HCl (pH 7.5), 137 mM NaCl, and 10% glycerol] containing the caspase-3 substrate (DEVD-pNA) at 5 µM. Lysates were incubated at 37°C for 2 h. Thereafter, the absorbance at 405 nm was measured with a spectrophotometer.
MCL-1(L) and Constitutively Active AKT Constructs and Stable Cell
The cDNA for Mcl-(L) was amplified by PCR with specific primers. The sequences of the sense and antisense primer for Mcl-1(L) were 5′-GCGACTGGCAAAGCTTGGCCTCAA-3′ and 5′-CAACTCTAGAAACTGGTTTTGGTG-3′, respectively. The PCR product was digested with Hind III and XbaI and cloned into the pFLAG-CMV-4™ expression vector (Sigma, USA), termed pFLAG-CMV-4/Mcl-1(L). The Caki cells were transfected in a stable manner with the pFLAG-CMV-4/Mcl-1(L) plasmid using LipofectAMINE as prescribed by the manufacturer (Invitrogen). After 48 h of incubation, transfected cells were selected in cell culture medium containing 700 µg/ml G418 (Invitrogen). After 2 or 3 weeks, single independent clones were randomly isolated, and each individual clone was plated separately. After clonal expansion, cells from each independent clone were tested for Mcl-1(L) expression by immunoblotting and were used in this study. Akt-overexpressing Caki cells were generated using a vector expressing Myc-His-tagged mouse Aktl (activated) under the control of the cytomegalovirus promoter (Upstate Biotechnology). The Caki cells were transfected in a stable manner with the pcDNA3.1-constitutive active Akt and control pcDNA3.1(+) vector using LipofectAMINE as prescribed by the manufacturer (Invitrogen). After 48 h of incubation, transfected cells were selected in cell culture medium containing 700 µg/m G418 (Invitrogen). After 2 or 3 weeks, single independent clones were randomly isolated, and each individual clone was plated separately. After clonal expansion, cells from each independent clone were tested for phospho-Akt expression by immunoblotting and were used in this study.
Transient Transfection
A549 cells were plated onto six-well plates at a density 7 × 105 cells/well and grown overnight. Constitutively active Myc-tagged form of Akt (myc-Akt) constructs (Upstate Biotechnology) were transiently transfected into the A549 cells using Lipofectamine reagent according to the manufacturer's instructions (Invitrogen).
Statistical Analysis
All data are presented as mean ± SD. Significant differences between the groups were determined using the unpaired Student's t-test. A value of *P < 0.05 was accepted as indication of statistical significance. All the figures shown in this article were obtained from at least two independent experiments with a similar pattern.
RESULTS
BAI Potently Inhibits Growth of Various Human Cancer Cells
Previously, we have shown that BAI has a potent anti-proliferative activity in human head and neck cancer cells [Shin et al., 2009]. To further extend the anti-cancer property of BAI, we herein investigated the effect of BAI on proliferation in multiple human cancer cell lines, including lung (A549), colon (HCT116), and kidney (Caki), using XTT assay. As shown in Figure 1B, treatment with BAI markedly inhibited proliferation of A549, HCT116, and Caki cells in a concentration-dependent manner. Notably, the IC50 value of BAI was found to be 20, 40, and 50 nM in A549, Caki, and HCT 116 cells, respectively. Clonogenic assay was next utilized to assess the effectiveness of BAI on the reproductive potential (the survival and proliferation) of A549, HCT116, and Caki cells. To this end, A549 cells (3,000 cells/well), HCT116 cells (3,000 cells/well), and Caki (1,500 cells/well) were seeded, and then treated with the indicated concentrations of BAI. As shown in Figure 1C, clonogenic survival of respective cancer cell lines was also decreased by BAI at nanomolar concentrations. These results thus collectively demonstrate the ability of BAI to potently inhibit growth of those human cancer cells. Since BAI had the most potency to reduce viability and clonogenic survival of A549 cells, we further concentrated on the anti-cancer properties of BAI in A549 cells and possible molecular and cellular mechanisms associated.
BAI Induces Apoptosis in Various Human Cancer Cells
We next investigated whether BAI induces apoptosis in several types of human cancer cells. We first analyzed the occurrence of apoptosis in A549, HCT116, and Caki cells using flow cytometric analysis to detect hypodiploid cell populations. As shown in Figure 2A, treatment of A549, HCT116, and Caki cells with BAI resulted in a markedly increased accumulation of sub-G1 phase cells in a dose-dependent manner. Next, A549 cells were exposed to BAI at different times, and then the amount of sub-G1 phase in the conditioned cells was analyzed by flow cytometry. As shown in Figure 2B, the exposure of BAI to A549 cells resulted in markedly increased accumulation of the sub-G1 phase in a time-dependent manner. The maximal apoptotic effects on A549 cells were shown by treatment with BAI at 20 nM for 24 h. Results of DNA fragmentation assay further demonstrated a dose-dependent genomic DNA fragmentation in A549 cells treated with BAI for 24 h (Fig. 2C). In addition, treatment of A549 cells with 10 and 20 nM BAI resulted in progressive morphological changes typical of apoptosis, including cell shrinkage, rounding, and detachment of the cells from the plate, as observed with light microscopy (Fig. 2C). To next clearly distinguish whether BAI-induced A549 cell death is due to apoptosis or necrosis, we examined the translocation of membrane phosphatidylserine (PS) from the inner to outer leaflet of the plasma membrane. Given that translocation of PS to external cell surface occurs during early apoptosis and necrosis, Annexin V-FITC and vital dye, such as 7-AAD, were used to distinguish apoptotic cells from necrotic cells [Darzynkiewicz et al., 1997]. As shown in Figure 2D, the incidence of apoptotic 7-AAD−/Annexin V+ cells was increased in BAI-treated cells but the incidence of necrotic 7-AAD+/Annexin V− or 7-AAD+/Annexin V+ cells was not, which indicates that BAI induces cell death in A549 cells through induction of apoptosis but not via necrosis.
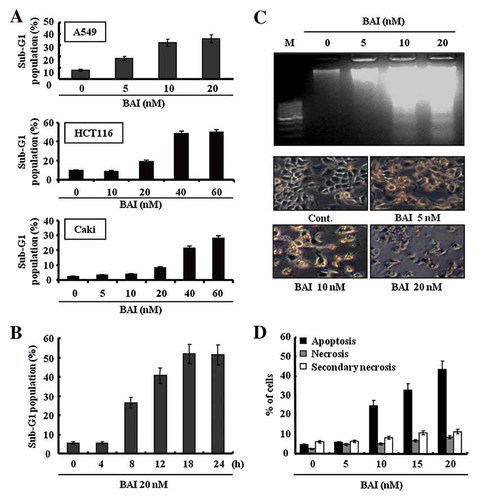
Induction of apoptosis in various human cancer cells by BAI. A: A549, HCT116, and Caki cells were treated with BAI at the indicated concentrations for 24 h. The conditioned cells were then harvested and analyzed apoptosis by fluorescence-activated cell sorting (FACS) analysis. B: A549 cells were for the indicated time points with 20 nM BAI. The fraction of sub-G1 population (apoptotic cells) in the conditioned cells was determined by FACS analysis. C: Fragmentations of genomic DNA in A549 cells treated for 24 h with BAI. Fragmented DNA was extracted and analyzed on 2% agarose gel. Morphological change was visualized using a light microscopy. D: A549 cells were treated with the indicated concentrations of BAI for 24 h. The conditioned cells were then harvested and stained with 7-AAD and Annexin-V. Apoptotic or necrotic cell death was determined by FACS. Primary apoptotic cells, Annexin-V+/7-AAD−; necrotic cells, Annexin-V−/7-AAD+; secondary necrotic cells, Annexin-V+/7-AAD+. Values correspond to the percentage of cells in those quadrants. Data are mean values from three independent experiments and bars represent standard deviations.
BAI Dose not Affect Growth of Untransformed Cells and has Greater Apoptotic Potency Than Roscovitine, A Well-Known CDK Inhibitor in A549 Cells
At present, only a few agents are known to possess the potential for selective/preferential elimination of cancer cells without affecting the normal cells [Donnerstag et al., 1996; Ahmad et al., 1997]. To thus evaluate whether BAI induces apoptosis in untransformed cells, two types of untransformed cells, human skin fibroblasts (HSF) and mesangial cells were exposed to BAI at 100–1,000 nM for 24 h. Clearly, as shown in Figure 3A, treatment with BAI at the higher doses (100–1,000 nM) induced apoptosis in HSF and mesangial cells by less than 15% and 30%, respectively. Considering BAI as the CDK inhibitor, we next compared the apoptotic potency between BAI and roscovitine, a well-known CDK inhibitor in A549 cells. For this, A549 cells were exposed to BAI at 20 nM or roscovitine at 5–50 µM, followed measurement of apoptosis in the conditioned cells by flow cytometry. As shown in Figure 3B, though BAI at 20 nM induced apoptosis in A549 cells by more than 40%, roscovitine at 5 µM had little effect on induction of apoptosis in A549 cells. However, treatment with roscovitine at higher doses (10–50 µM) was able to induce apoptosis in A549 cells.
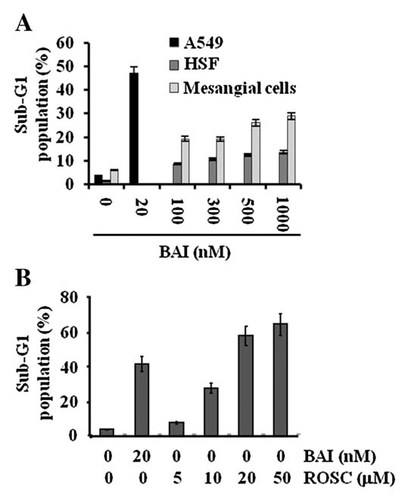
Effect of BAI on growth of untransformed cells and the difference between BAI and roscovitine on induction of apoptosis in A549 cells. A: Untransformed human skin fibroblast (HSF) and mesangial cells were treated for 24 h with the indicated concentrations of BAI for 24 h. The fraction of sub-G1 population in the conditioned cells was determined by FACS analysis. B: A549 cells were treated with the indicated concentrations of BAI or roscovitine for 24 h, and then apoptosis was analyzed by FACS. Data are mean values from three independent experiments and bars standard deviations.
BAI Induces Caspase-Dependent Apoptosis in A549 Cells
We next analyzed whether BAI induces activation of caspases, a key executioner of apoptosis in A549 cells. In this study, activation of caspase-3 was assessed by not only an in vitro caspase-3 (DEVDase) activity measurement using DEVD as a caspase-3 substrate but also the altered expression levels of pro-caspase-3 (inactive) and cleaved caspases-3 (active) in the BAI-treated A549 cells. As shown in Figure 4A, the exposure of BAI to A549 cells led to a dose-dependent activation of caspase-3, as evidenced by the reduced amounts of pro-caspase-3 in parallel with the increased levels of cleaved caspase-3. The dose-dependent increase in the cleavage of PLC-γ1, a substrate protein of caspases further supported activation of caspase-3 by BAI. Moreover, results of an in vitro caspase-3 activity measurement clearly demonstrated that BAI stimulated DEVDase activity in A549 cells in a dose-dependent manner (Fig. 4B). These results suggest that BAI treatment leads to activation of caspase-3 in A549 cells. We next investigated the significance of caspases activation in BAI-induced apoptosis in A549 cells using z-VAD-fmk, a general and potent inhibitor of caspases. Profoundly, as shown in Figure 4C–E, pretreatment with z-VAD-fmk strongly abrogated the ability of BAI to reduce the expression levels of pro-caspase-3 in parallel with the increased levels of cleaved caspase-3, to cleave PLC-γ1, to stimulate the DEVDase activity, and to induce apoptosis in A549 cells. These data thus suggest that activation of caspases is critical for the BAI-induced apoptosis in A549 cells.
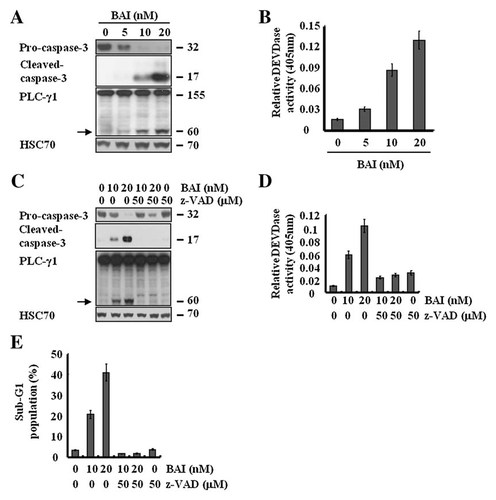
Caspase-dependent apoptosis in the BAI-treated A549 cells. A: A549 cells were treated with the indicated concentrations of BAI for 24 h. Whole cell lysates were isolated and analyzed by Western blot with respective antibody. Cleaved form of PLC-γ1 is indicated by arrow. HSC70 was used as a protein loading control. B: An aliquot of proteins from A was analyzed by an in vitro DEVDase activity using 200 µM chromogenic substrate (DEVD-pNA). The release of chromophore pNA was monitored spectrophotometrically at 405 nm. C: A549 cells were pretreated with z-VAD-fmk or vehicle for 1 h and then treated with BAI (10, 20 nM) for 24 h. Whole cell lysates were then isolated and analyzed by Western blot with respective antibody. Cleaved form of PLC-γ1 is indicated by arrow. HSC70 was used as a protein loading control. D: An aliquot of proteins from C was analyzed by an in vitro DEVDase activity using 200 µM DEVD-pNA. The release of chromophore pNA was monitored spectrophotometrically at 405 nm. E: A549 cells were pretreated with z-VAD-fmk or vehicle for 1 h and then treated with BAI (10, 20 nM) for 24 h. The fraction of sub-G1 population was determined by FACS. Data are mean values from three independent experiments and bars standard deviations.
BAI Down-Regulates Expression of XIAP and MCL-1 in A549 Cells
We next determined the effect of BAI on intracellular expression levels of Mcl-1 (anti-apoptotic member of the Bcl-2 family) and XIAP (anti-apoptotic protein and cellular inhibitor of some caspases). As shown in Figure 5A, BAI treatment at 10 or 20 nM resulted in significantly decreased expression levels of XIAP and Mcl-1(L) in A549 cells. However, expression levels of HSC70 were not changed by treatment with BAI at the doses tested. We next wanted to know any link between loss of XIAP and Mcl-1(L) proteins and activation of caspases in the BAI-treated A549 cells. As shown in Figure 5B, pretreatment with z-VAD-fmk had no effect on reduction of XIAP and Mcl-1(L) proteins by BAI, implying that the BAI-induced down-regulation of XIAP and Mcl-1(L) proteins is unrelated with the caspases activity. This led us to promptly investigate the effect of BAI on transcriptional regulation of XIAP and Mcl-1(L) in A549 cells. Notably, results of RT-PCR analysis, as shown in Figure 5C, demonstrated a dose-dependent reduction of XIAP and Mcl-1(L) transcripts in the BAI-treated A549 cells, suggesting that BAI down-regulates XIAP and Mcl-1(L) at their transcriptional levels in A549 cells. To evaluate the functional role played by Mcl-1(L) in the BAI-induced apoptosis, we employed Caki renal carcinoma cells engineered for overexpression of Mcl-1(L) (Caki/Mcl-1(L)), and vector-transfected control cells (Caki/Vector) (Fig. 5D). Caki/Vector and Caki/Mcl-1(L) cells were treated with various concentrations of BAI and examined cytotoxicity using FACS analysis. As shown in Figure 5D, overexpression of Mcl-1(L) could not attenuate the apoptosis induced by BAI. Further analysis of early time points revealed overexpression of Mcl-1(L) could not inhibit the apoptosis induced by BAI treatments (12–18 h; Supplementary Fig. 1). Recently, it has been reported that Bcl-2 itself is a pro-survival member of the family and its aberrant expression has been linked to a variety of different cancers, and cancer cells [Shankar et al., 2008]. In our previous data, treatment with BAI led to reduction of the Bcl-2 protein in a dose-dependent manner (data not shown). To investigate whether the decreased expression of Bcl-2 is important to induce apoptosis in BAI treated Caki cells, we established Bcl-2 overexpressing cells. Caki/Bcl-2 cells exhibited an increase in Bcl-2 expression compared with cells containing empty-vector only (Supplementary Fig. 2). Both cells were treated with various concentrations of BAI and examined cytotoxicity using FACS analysis. As shown in Supplementary Figure 2, overexpression of Bcl-2 could not inhibit the apoptosis induced by BAI. To examine the functional significance of BAI-induced XIAP down-regulation, we employed the siRNA duplex against XIAP mRNA. A549 cells were transfected with the XIAP siRNA and treated with or without BAI for 18 h. Immunoblot analysis demonstrated that transfection of siRNA against XIAP resulted in a suppression of XIAP expression in A549 cells as compared to cells transfected with control GFP siRNA (Fig. 5E). Under these conditions, the BAI-induced accumulation of the sub-G1 phase was similar in cells transfected with XIAP siRNA as compared to control siRNA-transfected cells (Fig. 5E). Taken together, these results suggest that down-regulations of Mcl-1(L) and XIAP proteins are not associated with the BAI-induced apoptosis in cancer cells.
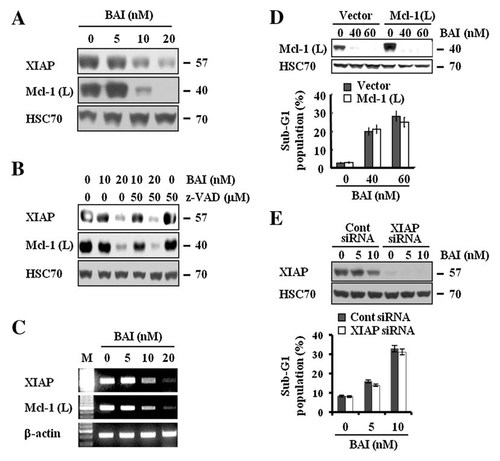
Effect of BAI on apoptosis-related proteins. A: A549 cells were treated for 24 h with the indicated concentrations of BAI. Whole cell lysates were then isolated and analyzed by Western blot with respective antibody. HSC70 was used as a protein loading control. B: A549 cells were pretreated with z-VAD-fmk or vehicle for 1 h and then treated with BAI (10, 20 nM) for 24 h. Whole cell lysates were then isolated and analyzed by Western blot with respective antibody. HSC70 was used as a protein loading control. C: A549 cells were treated with the indicated concentrations of BAI for 24 h. Total mRNA was isolated and analyzed by RT-PCR with respective primer. Expression levels of β-actin mRNA were used to compare the relative expression of Mcl-1(L) and XIAP mRNA. D: Caki/Vector and Caki/Mcl-1(L) cells were treated with the indicated concentrations of BAI for 24 h. The fraction of sub-G1 population was determined by FACS. Whole cell lysates obtained from Caki cells stably transfected with a Mcl-1(L) expression vector or the empty vector was subjected to SDS–PAGE, transferred to membranes, and immunoblotted using Mcl-1 and HSC70 antibodies as indicated. HSC70 was used as a protein loading control. E: Caki cells were transfected with XIAP siRNA or GFP siRNA. Thirty hours after transfection, cells were treated with BAI (5, 10 nM) for 18 h. The fraction of sub-G1 population was determined by FACS. Whole cell lysates were then isolated and analyzed by Western blot with XIAP and HSC70 antibodies. HSC70 was used as a protein loading control. Data are mean values from three independent experiments and bars standard deviations.
BAI-Induced Apoptosis Is Correlated With Inhibition of AKT
Protein kinase B (PKB/Akt) is a serine–threonine kinase that has been established as an important intracellular signaling in regulating cell survival [Datta et al., 1999]. Here, we investigated whether activation of Akt protein is altered during the BAI-induced apoptosis in A549 cells. Notably, as shown in Figure 6A, data of kinetic analysis showed that short time treatments (0.5–8 h) with BAI led to strong inactivation of Akt without affecting the protein's total expression levels, but long time treatments (12–24 h) with BAI strongly suppressed Akt at the protein expression levels in A549 cells. Moreover, short time treatment (0.5–4 h) with BAI led to significant down-regulation of Mcl-1(L) (Fig. 6A). However, the expression levels of XIAP were not changed by treatment with BAI until 12 h (Fig. 6A). We next tested whether Akt protein down-regulation by BAI at long-term treatments is linked to activities of caspases. Profoundly, pretreatment with z-VAD-fmk largely blocked Akt protein down-regulation induced by long-term treatments (12, 16, or 20 h) with BAI in A549 cells (Fig. 6B). These results suggest that caspases are responsible for the down-regulation of Akt in A549 cells after long-term treatments with BAI. However, in presence of z-VAD-fmk, though Akt protein is normally expressed in A549 cells treated with long-term treatments (12, 16, or 20 h) with BAI, there was no detection of phosphorylated form of Akt protein. Next, to directly examine the role of Akt inhibition in the BAI-induced apoptosis, we transiently transfected A549 cells with the cDNA of Myc-tagged form of Akt (myr-Akt), which encodes constitutively active form of Akt and measured the effect of BAI on expression (and activity) of caspase-3 and induction of apoptosis in the myc-Akt-transfected cells. As shown in Figure 6C, there was strong activation of Akt in the myc-Akt-transfected cells compared with the vector-transfected ones, confirming the transfection efficiency. Profoundly, though BAI treatment (10 or 20 nM) for 24 h led to decreased expression levels of pro-caspase-3, increased activity of the DEVDase, and increased accumulation of the sub-G1 phase in the vector-transfected A549 cells, the same treatment showed much less effect of BAI treatment (10 or 20 nM) for 24 h on the expression levels of pro-caspase-3, the activity of DEVDase, and the sub-G1 phase in the myc-Akt-transfected cells (Fig. 6C). It has been previously shown that overexpression of Akt has an anti-apoptotic effect in many cancer cells, resulting in a resistance to chemotherapy [Fresno Vara et al., 2004]. Thus, we examined the anti-apoptotic potency of Akt on the BAI-induced apoptosis. Against our expectation, overexpression of Akt could not attenuate the apoptosis induced by BAI treatment (Supplementary Fig. 3). Interestingly, the phosphorylated forms of Akt in Akt overexpressed cells were increased more than A549/Vector cells, however, treatment with BAI led to inactivation of Akt in A549/WT-Akt cells as much as in A549/Vector cells (Supplementary Fig. 3). Because of this, maybe overexpression of Akt could not attenuate the apoptosis induced by BAI. To further evaluate the effect of Akt signaling on the BAI-induced apoptosis, we employed Caki/Vector and Caki/DA-Akt cells. As shown in Figure 6D, ectopic expression of constitutive active Akt did not restore the down-regulation of Mcl-1(L) proteins induced by BAI in Caki/DA-Akt cells. However, constitutive active Akt rescue BAI mediated down-regulation of XIAP (Fig. 6D). Collectively these results indicate that inactivation of Akt is critical for the BAI-induced apoptosis in cancer cells. Next, we examined whether XIAP plays an anti-apoptotic functional role in the reduction of the BAI-induced apoptosis in Akt overexpressing Caki cells. As shown in Figure 6E, transfection of Caki/DA-Akt cells with siRNA against XIAP resulted in a complete suppression of XIAP expression compared with cells transfected with control siRNA. However, interestingly, the accumulation of sub-G1 phase induced by BAI was rarely different between cells transfected with control siRNA and XIAP siRNA (Fig. 6E). In addition, the expression of cleaved caspase-3 was not detected. Taken together, these data suggest that XIAP is not associated with Akt-mediated the inhibition of the BAI-induced apoptosis.
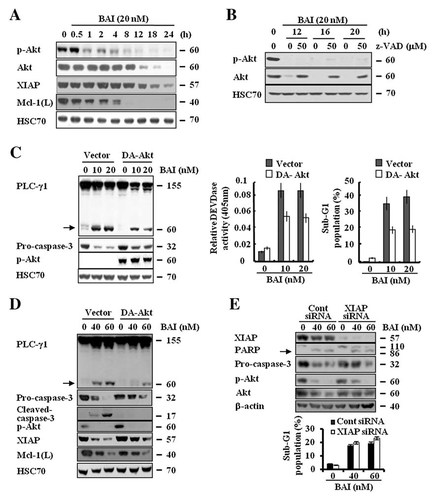
Effect of BAI on expression and activity of Akt in A549 cells. A: A549 cells were treated for 24 h with the indicated concentrations of BAI. Whole cell lysates were then isolated and analyzed by Western blot with respective antibody. HSC70 was used as a protein loading control. B: A549 cells were pretreated with z-VAD-fmk or vehicle for 1 h and then treated with BAI (20 nM) for the indicated times. Whole cell lysates were then isolated and analyzed by Western blot with respective antibody. HSC70 was used as a protein loading control. C: A549 cells were transiently transfected with vector or myc-tagged Akt cDNA encoding constitutively active Akt protein for 24 h. The transfected cells were then treated with the indicated concentrations of BAI for 24 h. Whole cell lysates were isolated and analyzed by Western blot with respective antibody. Cleaved form of PLC-γ1 is indicated by arrow. HSC70 was used as a protein loading control. An aliquot of the same whole cell lysates was analyzed by an in vitro DEVDase activity using 200 µM DEVD-pNA. The release of chromophore pNA was monitored spectrophotometrically at 405 nm. The fraction of sub-G1 population in the transfected cells was analyzed by FACS. D: Caki/Vector and Caki/DA-Akt cells were treated with the indicated concentrations of BAI for 18 h. Whole cell lysates were then isolated and analyzed by Western blot with respective antibody. Cleaved form of PLC-γ1 is indicated by arrow. HSC70 was used as a protein loading control. E: Caki/DA-Akt cells were transfected with XIAP siRNA or GFP siRNA. Thirty hours after transfection, cells were treated with BAI (40, 60 nM) for 18 h. The fraction of sub-G1 population was determined by FACS. Whole cell lysates were then isolated and analyzed by Western blot with respective antibodies. Cleaved form of PARP is indicated by arrow. β-actin was used as a protein loading control. Data are mean values from three independent experiments and bars represent standard deviations.
Co-Treatment With LY294002 and BAI Enhances Apoptosis in A549 Cells
Considering that BAI inhibits Akt and Akt inhibition is critical for the BAI-induced apoptosis in A549 cells (Fig. 6C), we next investigated any synergistic effect between BAI and LY294002 (LY), a PI3K/Akt inhibitor on induction of apoptosis in A549 cells. Since BAI at 20 nM has a strong apoptotic effect on A549 cells herein, we used a subcytotoxic concentration of BAI (4 nM) to see the apoptotic synergism with LY. A549 cells were exposed to BAI (4 nM) alone or BAI (4 nM) plus LY (25 µM) for 24 h, measurement of apoptotic response in the conditioned cells. As shown in Figure 7A,B, single treatment of BAI at 4 nM had no effect on expression levels of pro-caspase-3 and PLC-γ1, phosphorylated forms of Akt, total Akt protein expression, and did not induce apoptosis in A549 cells. Moreover, single treatment with LY that inhibited Akt did not influence the expression levels of pro-caspase-3 and PLC-γ1 and had little effect on apoptosis in A549 cells. However, profoundly, co-administration of LY with BAI caused strong induction of apoptosis in A549 cells in association with reduction of the expression levels of pro-caspase-3, increase in the cleavage of PLC-γ1, enhancement of Akt inhibition. These findings indicate that LY and BAI have a synergistic effect on induction of apoptosis in A549 cells.
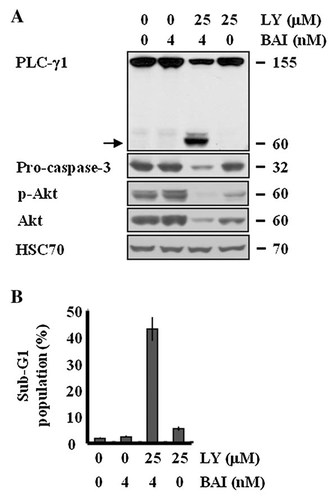
Effect of BAI and/or LY on the expression and/or activity of pro-caspase-3, PLC-γ1, or Akt and induction of apoptosis in A549 cells. A: A549 cells were treated with a sub-cytotoxic concentration of BAI in absence or presence of LY294002 (LY), a PI3K/Akt inhibitor for 24 h. Whole cell lysates were then isolated and analyzed by Western blot with respective antibody. Cleaved form of PLC-γ1 is indicated by arrow. HSC70 was used as a protein loading control. B: A549 cells were treated with a subcytotoxic concentration of BAI in absence or presence of LY for 24 h. The fraction of sub-G1 population in the conditioned cells was analyzed by FACS. Data are mean values from three independent experiments and bars represent standard deviations.
DISCUSSION
The cell cycle is governed by the regulations of cyclins and the activities of CDKs. However, in cancer, these components are commonly deregulated, contributing to tumorigenesis and poor patient survival rates. Therefore, in an effort to generate a new class of CDK inhibitor with anti-cancer property, the regulation of cellular proliferation through the modulation of CDK activity has become the attractive issues as a critical therapeutic method [Cai et al., 2006; Payton et al., 2006]. Previously, we reported a novel CDK inhibitor, BAI, which is recently synthesized by our groups and demonstrated that BAI specifically inhibits the activities of CDK1 and CDK2 and also has potent anti-cancer effect on several types of human cancer cells [Lee et al., 2008]. We here report for the first time that BAI at nanomolar concentrations largely inhibits growth of multiple human cancer cells, including A549 (lung), HCT116 (colon), and Caki (kidney). Importantly, we provide evidence that BAI at 20 nM induces apoptosis in A549 cells through modulating the expression and/or activity of caspases and Akt.
In the initial experiments, we have demonstrated that though BAI at 20–60 nM shows strong growth inhibitory effects on A549, HCT116, and Caki cells (Fig. 1B,C), this small molecule at such doses has very low or no toxicity in HSF and mesangial cells applied as untransformed cell lines in present study (Fig. 3A). These data may thus imply that BAI may have preferential growth inhibitory effect on cancer cells. Importantly, further biochemical analyses for apoptotic markers such as accumulation of sub-G1 phase and DNA fragmentation illustrate that BAI at 20 nM strongly induces apoptosis in A549 cells (Fig. 2A–C).
Induction of apoptosis is influenced by a variety of proteins and/or factors. Among those, caspase-3 is the valuable candidates for cell death-inducing proteases that cleave PARP, PLC-γ1, and other vital proteins [Lazebnik et al., 1994; Emoto et al., 1995; Bae et al., 2000]. Notably, the present study demonstrates significance of the caspases activity in the BAI-mediated apoptotic response to A549 cells, which is deduced from the experimental data that the pan-caspase inhibitor z-VAD-fmk effectively blocks activation of caspases (herein caspase-3), cleavage of PLC-γ1, and apoptosis induced by BAI in A549 cells (Fig. 4C–E). Evidence suggests involvement of the member(s) of IAP and/or Bcl-2 family in induction of apoptosis in cancer cells. XIAP is a member of the IAP family and plays a key role in cell survival by modulating death-signaling pathways at the post-mitochondrial level [Schimmer et al., 2006]. It is shown that down-regulation of XIAP is an important mechanism for caspase activation in response to various apoptotic stimuli [Shi et al., 2005]. Mcl-1(L) is a member of the Bcl-2 family and recognized as an anti-apoptotic protein [Bae et al., 2003]. In present study, we have clearly shown that there is strong reduction of XIAP and Mcl-1(L) proteins in the BAI-treated A549 cells (Fig. 5A). Importantly, further analysis has shown that XIAP and Mcl-1(L) protein down-regulation induced by BAI in A549 cells is independent of the caspases activity (Fig. 5B) but due to the down-regulation of XIAP and Mcl-1(L) in transcriptional levels (Fig. 5C). Nonetheless, assuming the anti-apoptotic roles of XIAP and Mcl-1(L), it is conceivable that decreased cellular levels of XIAP and Mcl-1(L) may facilitate the BAI-induced apoptosis in A549 cells. Thus, in the present study, here we have examined the functional roles of Mcl-1(L) and XIAP in the BAI-induced apoptosis of Caki cells. Previously, we confirmed the anti-apoptotic effect of Caki/Mcl-1(L) (FLAG-tagged) in compound C plus TRAIL-induced apoptosis [Jang et al., 2010]. Nevertheless, the BAI-induced apoptosis was not blocked by exogenously overexpressed Mcl-1(L) in Caki cells (Fig. 5D). Moreover, the BAI-induced apoptosis was not enhanced by suppression of XIAP expression using XIAP-targeted siRNA (Fig. 5E). Therefore, these results suggest that the down-regulations of Mcl-1(L) and XIAP proteins induced by BAI may not be involved in the BAI-induced apoptosis.
Identification of the specific cell cycle events responsible for CDK inhibitor-mediated lethality remains an elusive goal. An attractive possibility is that the induction of cell death by CDK inhibitors like flavopiridol is regulated by the activity of one or more signal transduction pathways, which are known to play critical roles in cell survival decisions [Pepper et al., 2003; Mitchell et al., 2007]. Of the various possibilities, activation of the PI3K signaling pathway, which is intimately involved in promoting cell survival [Cantley, 2002], represent a plausible candidate. In line of this, it is interesting to address the present findings that BAI negatively regulates Akt, a PI3K down-stream effector in A549 cells by suppressing activity and expression of the signaling protein in a time-dependent manner (Fig. 6 A). It is supported by that short-term treatment with BAI (1–8 h) only reduces the phosphorylated forms of Akt, but long-term BAI treatments (12–24 h) down-regulates expression levels of total Akt protein. Notably, the present findings that z-VAD-fmk strongly blocks reduction of total Akt protein expression induced by long-term treatments with BAI (Fig. 6B) indicate involvement of the caspases in this process. Evidence indicates that Akt involves in regulation of the expression of anti-apoptotic proteins including XIAP [Leverrier et al., 1999]. Importantly, results of Akt cDNA transfection experiments have demonstrated that BAI shows less apoptotic effect on the myr-Akt-transfected A549 and Caki cells, addressing importance of Akt inhibition in the BAI-induced apoptosis (Fig. 6C,D). Moreover, ectopic expression of constitutive active Akt restored the down-regulation of XIAP proteins induced by BAI in Caki/DA-Akt cells (Fig. 6D). Recently, there is strong evidence for a functional relationship between Akt and XIAP in cancer cells: XIAP expression is regulated by PI3K and Akt activity in cancer cells [Liu et al., 2006; Deeb et al., 2007]. In the present study, we evaluated the functional role of XIAP in association with the survival effect of Akt in the BAI-induced apoptosis. However, the BAI-induced apoptosis was not enhanced by XIAP siRNA in Caki/DA-Akt cells. These data thus demonstrate that XIAP is not associated with Akt-mediated the inhibition of the BAI-induced apoptosis.
It is shown that roscovitine, a well-known CDK inhibitor has the anti-proliferative activity in cancer cells at micromolar concentrations [Mgbonyebi et al., 1999]. In the present study, we have shown much higher apoptotic potency of BAI than roscovitine in inducing apoptosis in A549 cells (Fig. 3C). Previously, it has been reported that PI3K/Akt pathway plays a major role in regulating the apoptotic response of human leukemia cells to pharmacological CDK inhibitors, including flavopiridol and roscovitine, and raised the possibility that combined interruption of CDK- and PI3K-related pathways may represent a novel therapeutic strategy in hematological malignancies [Yu et al., 2003]. In agreement with these previous findings, in present study, we have demonstrated that there is also a synergistic effect between blockage of the PI3K/Akt pathway (by LY) and inhibition of CDK pathway (by BAI, particularly at a subcytotoxic concentration) on induction of apoptosis in A549 cells. This may, therefore, extend the possibility that targeting both pathways may be a useful therapeutic method in lung cancer. At present, however, the underlined mechanism by which co-administration of LY with BAI at a subcytotoxic dose enhances apoptotic response to A549 cells remains unclear. There is a recent study addressing that co-treatment of CDK inhibitors and LY diminishes Bad phosphorylation, which leads to protection cells from mitochondrial injury [Yu et al., 2003]. Considering that BAI leads to both Akt inhibition and XIAP and Mcl-1(L) expression in A549 cells herein, it may be conceivable that the potentiation of BAI-mediated apoptosis in A549 cells by LY may be attributed to down-regulation of XIAP and Mcl-1(L) expression by LY and/or reduction of Bad phosphorylation by BAI, which may need to be further clarified.
Taken together, the findings presented herein demonstrate for the first time the potency of BAI to inhibit multiple cancer cell growth, including A549, HCT116, and Caki. The BAI-induced apoptosis in A549 cells appears to be mediated through activation of caspases and inactivation of Akt. It is suggested that BAI may be useful for developing as an anti-cancer drug against lung and kidney malignancies, which may deserve further clinical application.




