Abstract
Interleukin-6 (IL-6) is a pleiotropic cytokine secreted by macrophages and others and it has been proven to be a potential therapeutic target of RA. Norisoboldine (NOR) is the main isoquinoline alkaloid constituent in the dry roots of Lindera aggregata (Sims) Kosterm. (L. strychnifolia Vill.), which has long been used in traditional Chinese medicine for treating RA and other diseases. Our previous studies indicated that NOR was able to attenuate inflammation and joint destruction in collagen II-induced arthritis of mice. To further recognize the anti-rheumatoid potentials of NOR, the present study addressed whether and how NOR interfered with IL-6 production from fibroblast-like synoviocytes (FLS), key effector cells in the development and progression of RA. FLS, obtained from the synovial tissues of rats with adjuvant arthritis, showed incremental release of IL-6 after stimulated with IL-1β in vitro. NOR (10, 30, and 60 µM) could reduce the production of IL-6 in a concentration-dependent manner. It also down-regulated the phosphorylations of mitogen-activated protein kinases (MAPKs), protein kinase C (PKC), and transcriptional factor nuclear factor-κB (NF-κB)-p65 (ser 276) as well as cAMP response element-binding protein (CREB) in FLS. By using specific inhibitors, PKC was shown to be the upstream protein of MAPKs, and p38 MAPK was at the upstream of CREB. It was concluded that preventing IL-6 release from FLS might be an important mechanism for NOR displaying anti-RA property, and the action of NOR was relative to inhibition of PKC/MAPKs/p65/CREB pathways. J. Cell. Biochem. 113: 2785–2795, 2012. © 2012 Wiley Periodicals, Inc.
Abbreviation:
NOR, norisoboldine; RA, rheumatoid arthritis; IL-6, interleukin-6; FLS, fibroblast-like synoviocytes; AA, adjuvant arthritis; MAPKs, mitogen-activated protein kinases; NF-κB, nuclear factor-κB; IKKα, I-kappa-B kinase alpha; IκBα, Inhibitor of NF-kappa B alpha; cAMP, cyclic adenosine monophosphate; PKC, protein kinase C; CREB, cAMP response element-binding protein.
Rheumatoid arthritis (RA) is a chronic and systemic autoimmune inflammatory disease that primarily attacks synovial joints, resulting in articular destruction and functional disability. It typically presents as a symmetric peripheral polyarthritis, most often involving the small joints of the hands and feet [Silman and Pearson, 2002; Ngian, 2010]. Fibroblast-like synoviocytes (FLS) are key effector cells in the pathogenesis of RA. They produce multiple proinflammatory mediators, cytokines, proteases, matrix metalloproteinases, and growth factors, which help persistent inflammation and ultima joint tissue destruction [Wilkinson et al., 1992; Noss and Brenner, 2008; Bartok and Firestein, 2010]. Interleukin-6 (IL-6), a pleiotropic cytokine originated from FLS and others, plays a particularly important role in local and systemic inflammation of RA [Woodrick and Ruderman, 2010]. It can facilitate production of other cytokines, leukocyte recruitment, FLS differentiation, and osteoclast activation, thus contributing to chronic inflammation, pannus formation, and cartilage and bone destruction [Park and Pillinger, 2007]. Blockade of IL-6 has been proven to be an effective way for RA treatment [Hashizume et al., 2009; Aarat and Larry, 2010]. Tocilizumab, a IL-6 receptor monoclonal antibody, has found its clinical values [Nishimoto et al., 2009].
Radix Linderae, the dry roots of Lindera aggregata, is a commonly used traditional Chinese medicine. Its various extracts were reported to have anti-inflammatory, analgesic, and potential anti-rheumatism properties [Li et al., 1999]. Our previous studies have demonstrated that the alkaloid fraction and the main active constituent norisoboldine (NOR) in Radix Linderae were substantially able to ameliorate systemic inflammation and protect joints from destruction in collagen II-induced arthritis (CIA) of mice [Chou et al., 2005; Wang et al., 2007]. In addition, NOR could prevent the activation of macrophages induced by lipopolysaccharide and resultant production of proinflammatory cytokines via down-regulating mitogen-activated protein kinases (MAPKs) signal pathway [Luo et al., 2009]. To recognize the anti-rheumatoid potential and mechanisms of NOR in more detail, the present study was performed to investigate whether and how NOR interfere with the production of IL-6 from FLS induced by IL-1β.
MATERIALS AND METHODS
Chemicals and Reagents
NOR (purity: 98.7%) was isolated and purified from Radix Linderae by authors, and the structure was established by comparison of its spectral data (UV, IR, MS, 1H-, and 13C-NMR) with the literature data [Chou et al., 2005] (Fig. 1); Mycobacterium butyricum was obtained from Becton Drive Co. Ltd. (New Jersey); rat IL-1beta was purchased from PeproTech Int. (Connecticut); chelerythrine and SB203580 were obtained from Sigma Chemical Co. (St. Louis, MO); rat IL-6 ELISA kits were purchased from Biosourse int. (California); rat cAMP ELISA kits were purchased from R & D systems (Minneapolis); collagenase Type II was purchased from Gibco BRL (Grand Island, NY); 3-[4,5-dimetylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma Chemical Co. (St. Louis, MO); p-ERK, p-p38, p-JNK, ERK, p38, JNK and GAPDH monoclonal antibodies, enhanced chemiluminescent (ECL) plus reagent kits and the peroxidase-conjugated secondary antibody were purchased from KangChen Bio-tech (Shanghai, China); antibodies for IκBα, p-IκBα, p-IKKα, p-NF-κB p65 (ser 276), PKCα, p-PKCα/βII, PKCδ, p-PKCδ, PKCζ, p-PKCζ, and p-CREB were purchased from Bioworld (Georgia). The other chemicals and reagents used were of analytical grade.
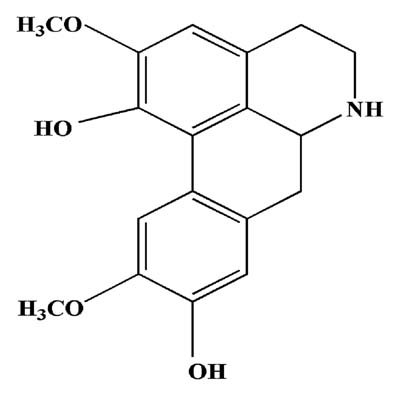
Chemical structure of norisoboldine.
Induction of Adjuvant Arthritis (AA) in Rats
Male Sprague–Dawley rats, weighing 180–220 g, were purchased from the experimental animal center of China Pharmaceutical University and kept under climate-controlled conditions. Studies were conducted under the guidelines of current ethical regulations for institutional animal care and use at China Pharmaceutical University. Arthritis was induced by an intradermal injection of 100 µl of a 10 mg/ml Mycobacterium butyricum paraffin oil suspension into the bases of right hind paws and the tails of rats [Carter et al., 2011].
Cell Culture
On day 14 after AA induction when secondary hindpaw responses appeared significantly, rats were killed by etherization. Fresh synovial membrane tissues of knee joints were obtained, and washed twice with PBS. The synovium was minced, and digested in 2 ml DMEM maintained 0.4% collagenase Type II, 10% newborn calf serum (NCS), 100 U/ml penicillin, and 100 µg/ml streptomycin in a humidified 5% CO2-containing atmosphere at 37°C for 2 h. Then, synovium was centrifugated for 10 min at a speed of 2000 rpm. The precipitation was digested in 0.25% trypsin for 20 min, and centrifugated for 10 min at a speed of 1200 rpm. After filtration, the precipitations were incubated in DMEM with 10% NCS, 100 U/ml penicillin, 100 µg/ml streptomycin and maintained at 37°C in 5% CO2 humidified air. After 24 h, the adherent cells (FLS) were cultured in the fresh medium, and non-adherent cells were removed [Zhu et al., 2005; Wang et al., 2011]. All experiments were performed using cells within passage 3–6.
Cell Viability Assay
MTT assay was used to detect the cell viability of FLS [Distler et al., 2005]. Briefly, FLS (1 × 105 cells/ml, 200 µl per well) were plated into 96-well plates, and then incubated for 12 h at 37°C and 5% CO2. After treated with various concentrations of NOR (3, 10, 30, and 60 µM) in the absence or presence of IL-1β (10 ng/ml) for 20 h, cells were added with 20 µl of MTT (5 mg/ml) each well and incubated for an additional 4 h. Subsequently, the supernatants were removed and the formazone crystals were dissolved using 150 µl of DMSO. The optical absorbance at 570 nm was read with a Model 1500 Multiskan spectrum microplate Reader (Thermo, Waltham, MA).
Measurement of IL-6 and cAMP
FLS (1 × 105 cells/ml, 200 µl per well) were seeded into 96-well plates and then treated with different concentrations of NOR (3, 10, 30, and 60 µM) and IL-1β (10 ng/ml) for 24 h. After that, the culture supernatants were collected, and the levels of IL-6 and cAMP were measured using ELISA kits according to the manufacturer's instructions.
Western Blot Analysis
FLS (1 × 105 cells/ml) were cultured in flasks until 90% confluence. They were incubated with serum-free DMEM for 2 h to allow cells to achieve quiescence. After pretreated with different concentrations of NOR (3, 10, 30, and 60 µM) for 24 h, cells were stimulated with 10 ng/ml of IL-1β for appropriate periods. Then, cells were washed twice with ice-cold PBS buffer (pH 7.2). Proteins were extracted with lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 0.02% NaN3, 1% NP-40) for 30 min on ice and determined by Bradford assay. Samples were fractionated on a 10% SDS–PAGE and transferred to PVDF membranes. Membranes were blocked for 1 h at room temperature with 5% nonfat milk and then incubated with different antibodies. Then, they were incubated with secondary antibodies for 1 h. The bands were visualized using film exposure with ECL reagent.
Statistical Analysis
Data were presented as means ± S.D. Statistical differences were assessed by one-way analysis of variance (ANOVA) followed by a post-hoc Tukey's test. P-values less than 0.05 (P < 0.05) were accepted as a significant difference.
RESULTS
Effects of NOR on the Viability of FLS from AA Rats
On day 14 after AA induction, rat synovial membrane tissues were obtained, and FLS were prepared. MTT assay was performed to identify the effects of NOR on the viability of FLS. As shown in Figure 2, NOR (3, 10, 30, and 60 µM) in the presence or absence of IL-1β (10 ng/ml) did not display remarkable cytotoxicity on FLS. In the consequent experiments, 3, 10, 30, and 60 µM were used as the test concentrations of NOR.
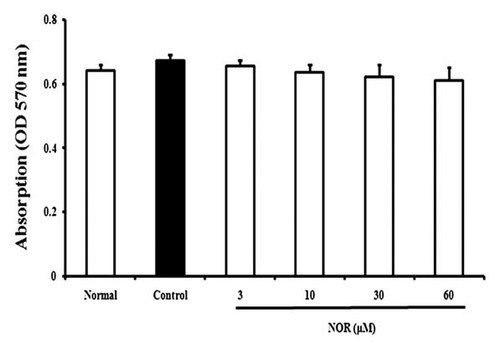
Effects of norisoboldine (NOR) on the viability of fibroblast-like synoviocytes (FLS) from adjuvant arthritis rats. FLS were treated with NOR (3, 10, 30, and 60 µM) in the presence or absence of IL-1β (10 ng/ml) for 24 h. Cell viability was determined by MTT assay. Data were expressed as means ± S.D. of three independent experiments.
Effects of NOR on IL-1β-Induced Production of IL-6 in FLS From AA Rats
IL-6 in joint synovial fluids is mainly originated from FLS, which plays an extremely important role in the onset and maintenance of synovial inflammation. In the present study, FLS from AA rats produced high levels of IL-6 after stimulated with IL-1β (10 ng/ml). NOR (10, 30, 60 µM) showed concentration-dependent inhibition of IL-6 production. The inhibitory percentages were 11.3, 27.9, and 39.5%, respectively (Fig. 3).
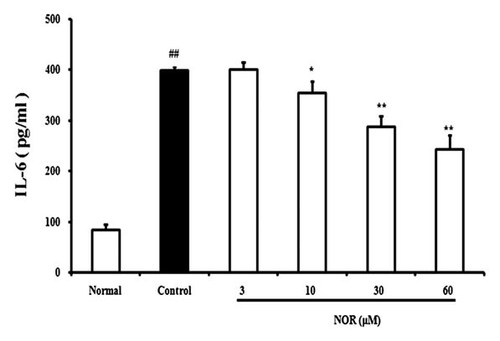
Effects of norisoboldine (NOR) on IL-1β-induced production of IL-6 in fibroblast-like synoviocytes (FLS) from adjuvant arthritis rats. FLS were treated with NOR (3, 10, 30, and 60 µM) in the presence or absence of IL-1β (10 ng/ml) for 24 h. IL-6 levels in the supernatants were measured using ELISA kits. Data were expressed as means ± S.D. of three independent experiments. ##P < 0.01 versus normal; *P < 0.05, **P < 0.01 versus control.
Effects of NOR on IL-1β-Induced Phosphorylation of MAPK in FLS From AA Rats
IL-1β acts by binding to its receptor IL-1R, and resulting in cascade activation of downstream signaling molecules. MAPKs (mainly including ERK1/2, JNK, and p38 MAPK), at the downstream of TRAF6 signaling complex, play an important role in IL-1β-induced activation of FLS and resultant expressions of proinflammatory cytokines by triggering a cascade reaction. As Figure 4A shows, IL-1β stimulation leads to evident phosphorylations of both ERK1/2 and p38 MAPK in FLS from AA rats, and they reach peaks at 10–20 min. NOR (10, 30, 60 µM) pretreatments for 24 h inhibited the phosphorylation of p38 MAPK by 17.8, 41.5, and 49.3%, and that of ERK by 13.6, 20.2, and 30.0%, respectively (Fig. 4B). Of note, at the concentration of 60 µM, NOR almost completely reversed the increased phosphorylation of p38 MAPK.
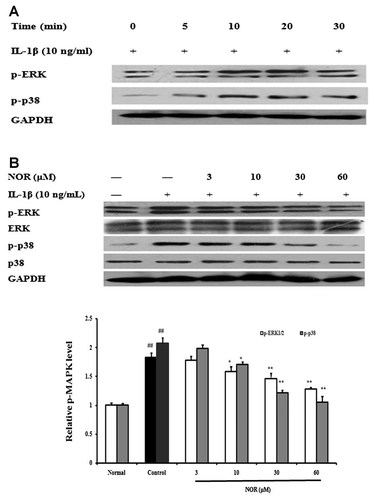
Effects of norisoboldine (NOR) on IL-1β-induced phosphorylation of MAPK in fibroblast-like synoviocytes (FLS) from adjuvant arthritis rats. A: Time course of the phosphorylations of ERK1/2 and p38 MAPK in IL-1β-stimulated FLS. Cells were treated with IL-1β (10 ng/ml), and the cell lysates were collected at the time points indicated. Phosphorylations of ERK1/2 and p38 were detected by western blot using specific antibodies. B: Effects of NOR on MAPK phosphorylation. FLS were pretreated with various concentrations of NOR (3, 10, 30, and 60 µM) for 24 h, and stimulated with IL-1β (10 ng/ml) for 15 min. Then, ERK, p38, p-ERK1/2, and p-p38 were analyzed. GAPDH was used as the internal control. Data were expressed as means ± S.D. of three independent experiments. ##P < 0.01 versus normal; *P < 0.05, **P < 0.01 versus control.
Effects of NOR on IL-1β-Induced Activation of NF-κB in FLS From AA Rats
In addition to MAPKs, NF-κB also regulates the expressions of multiple proinflammatory cytokines. The activation of NF-κB is linked to a sequential cascade, including IKK-dependent IκBα phosphorylation, ubiquitination, and proteolytic degradation, as well as translocation of cytosolic NF-κB-p65 to nucleus. As shown in Figure 5A, IL-1β (10 ng/ml) stimulation resulted in a rapid and evident phosphorylation of both IKKα and IκBα in FLS from AA rats with peaks at 10-20 and 30 min, respectively. NOR (60 µM) pretreatment for 24 h only slightly reduced the phosphorylation of IKKα induced by IL-1β stimulation for 15 min (Fig. 5B), and it showed little effect on the phosphorylation and degradation of IκBα (Fig. 5C). However, NOR (30, 60 µM) markedly inhibited the phosphorylation of p65 (ser 276), and the inhibitory percentages were 15.6% and 26.3%, respectively (Fig. 5D).
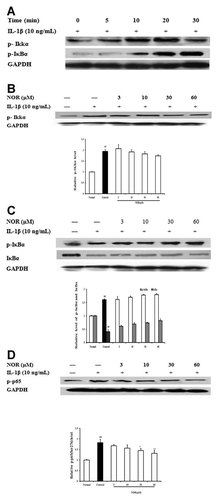
Effects of norisoboldine (NOR) on IL-1β-induced activation of NF-κB in fibroblast-like synoviocytes (FLS) from adjuvant arthritis rats. A: Time course of the phosphorylations of IKKα and IκBα. FLS were treated with IL-1β (10 ng/ml), and the cell lysates were collected at the time points indicated. Phosphorylations of IKKα and IκBα were detected by western blot using specific antibodies. B: Effects of NOR on IKKα phosphorylation. FLS were pretreated with NOR (3, 10, 30, and 60 µM) for 24 h, then stimulated with IL-1β (10 ng/ml) for 15 min. p-IKKα was analyzed by western blot using specific antibody. C, D: Effects of NOR on the phosphorylation of IκBα and p65. FLS were pretreated with NOR (3, 10, 30, and 60 µM) for 24 h, then stimulated with IL-1β (10 ng/ml) for 30 min. IκBα, p-IκBα, and p-NF-κB-p65 (ser 276) were analyzed by western blot using specific antibodies. GAPDH was used as the internal control. Data were expressed as means ± S.D. of three independent experiments. ##P < 0.01 versus normal; *P < 0.05, **P < 0.01 versus control.
Effect of NOR on Intracytoplasmic cAMP Level Induced by IL-1β in FLS From AA Rats
There was a report suggesting that IL-1β up-regulated the expression of IL-6 in H19-7/IGF-IR neuronal cells by activating cAMP-PKA pathway [Bergamaschi et al., 2006]. We therefore investigated whether IL-6 production induced by IL-1β in FLS was mediated by cAMP and the effect of NOR on cAMP production. As shown in Figure 6, IL-1β stimulation for 30 min indeed led to a significant increase of cytoplasmic cAMP level in FLS from AA rats. But, NOR (3, 10, 30, 60 µM) pretreatment for 24 h failed to affect it.
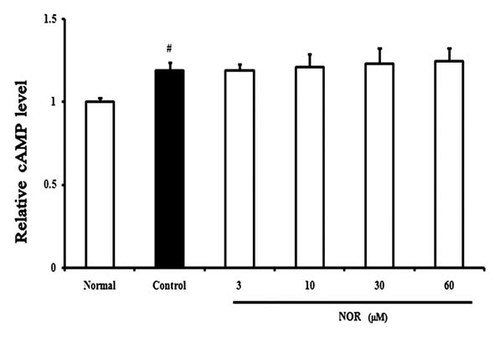
Effects of norisoboldine (NOR) on intracytoplasmic cAMP level in fibroblast-like synoviocytes (FLS) from adjuvant arthritis rats. FLS were pretreated with NOR (3, 10, 30, 60 µM) for 24 h, then stimulated with IL-1β (10 ng/ml) for 30 min. cAMP levels were determined by ELISA kits. Data were the means ± S.D. of three independent experiments. #P < 0.05 versus normal.
Effect of NOR on IL-1β-Induced Phosphorylation of CREB in FLS From AA Rats
CREB, one nuclear factor that can be activated by PKA, PKC, and p38 MAPK, is an important regulator for IL-6 transcription. In the present study, the impacts of NOR on the activation of CREB in FLS from AA rats were observed. As Figure 7A showed, IL-1β (10 ng/ml) stimulation resulted in significant phosphorylation of CREB with a peak time of 30 min. NOR (10, 30, 60 µM) concentration-dependently reversed the phosphorylation by 17.6, 26.5, and 41.3%, respectively (Fig. 7B).
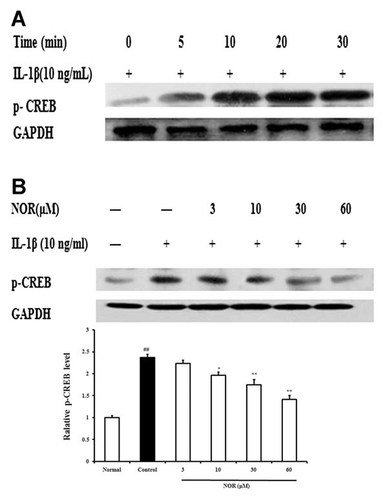
Effects of norisoboldine (NOR) on the phosphorylation of CREB in fibroblast-like synoviocytes (FLS) from adjuvant arthritis rats. A: Time course of CREB phosphorylation. FLS were treated with IL-1β (10 ng/ml), and the cell lysates were collected at the time points indicated. CREB phosphorylation was detected by western blot using specific antibody. B: Effect of NOR on CREB phosphorylation. FLS were pretreated with NOR (3, 10, 30, 60 µM) for 24 h, then stimulated with IL-1β (10 ng/ml) for 30 min. The p-CREB was analyzed by western blot. GAPDH was used as the internal control. Data were expressed as means ± S.D. of three independent experiments. ##P < 0.01 versus normal; *P < 0.05, **P < 0.01 versus control.
Participation of PKC in IL-1β-Induced Production of IL-6 in FLS From AA rats and Effects of NOR on PKC Phosphorylation
Participation of PKC
Growing evidence has showed the importance of PKC in IL-6 expression in various cells, such as human skin fibroblasts and mouse embryonic stem cells [Lipsky, 2006; Kim et al., 2009; Koyama and Aizawa, 2002]. But whether it participates in IL-6 expression in FLS remains a problem. To solve this, chelerythrine, a specific PKC inhibitor, was used in the present study.
FLS from AA rats were treated with different concentrations of chelerythrine for 24 h, and cell viability was tested by MTT assay. It was shown that chelerythrine at concentrations of 1, 2, and 4 µM did not affect the cell viability, but it exhibited significant cytotoxic effect when the concentration increased to 8 µM (Fig. 8A). In FLS stimulated with IL-1β (10 ng/ml), chelerythrine (1, 2, 4 µM) concentration-dependently reduced the levels of IL-6 in cell supernatants (Fig. 8B), and the inhibitory percentages were 8.6, 35.1, and 53.8%, respectively. The data indicated the participation of PKC in the regulation of IL-6 expression in FLS induced by IL-1β.
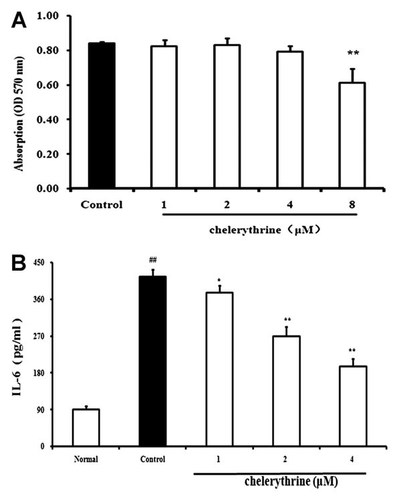
Participation of PKC in IL-1β-induced production of IL-6 in fibroblast-like synoviocytes (FLS) from adjuvant arthritis rats. A: Effect of chelerythrine on the viability of FLS. FLS were treated with chelerythrine for 24 h. Cell viability was tested with MTT assay. B: Effect of chelerythrine on IL-6 production. FLS were treated with chelerythrine (1, 2, 4 µM) in the presence or absence of IL-1β (10 ng/ml) for 24 h. IL-6 levels in the cell supernatants were measured using ELISA kits. Data were expressed as means ± S.D. of three independent experiments. ##P < 0.01 versus normal; *P < 0.05, **P < 0.01 versus control.
Effects of NOR on PKC Phosphorylation
In FLS from AA rats, IL-1β (10 ng/ml) stimulation resulted in significant phosphorylations of various PKC isoforms, including PKCδ, PKCα, and PKCζ, and the peak times of phosphorylation were between 10 and 20 min (Fig. 9A). NOR (10, 30, 60 µM) pretreatment for 24 h remarkably prevented the phosphorylations of both PKCα and PKCζ. The inhibitory percentages against PKCα were 38.8%, 52.3%, and 64.2%, respectively. The inhibitory percentages against PKCζ were 19.0%, 25.8%, and 45.9%, respectively. In contrast, NOR did not affect the phosphorylation of PKCδ (Fig. 9B). These findings suggested that NOR down-regulated IL-1β-induced expression of IL-6 in FLS probably through PKC-dependent manner.
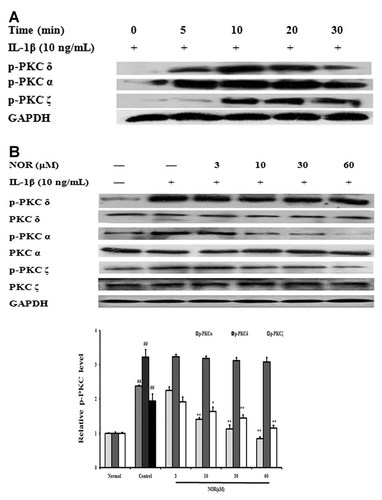
Effects of norisoboldine (NOR) on PKC phosphorylation in fibroblast-like synoviocytes (FLS) from adjuvant arthritis rats. A: Time course of the phosphorylations of PKCδ, PKCα, and PKCζ. FLS were stimulated with IL-1β (10 ng/ml), and the cell lysates were collected at the time points indicated. Phosphorylations of PKCδ, PKCα, and PKCζ were detected western blot using specific antibodies. B: Effects of NOR on PKC phosphorylation. FLS were pretreated with NOR (3, 10, 30, 60 µM) for 24 h, then stimulated with IL-1β (10 ng/ml) for 15 min. PKCδ, PKCα, PKCζ, p-PKCδ, p-PKCα, and p-PKCζ were analyzed by western blot. GAPDH was used as the internal control. Data were expressed as means ± S.D. of three independent experiments. ##P < 0.01 versus normal; *P < 0.05, **P < 0.01 versus control.
Interplay of Signal Pathways in IL-1β-Induced Production of IL-6 in FLS From AA Rats
As mentioned above, multiple signal pathways, such as p38 MAPK, NF-κB, and PKC, might participate in IL-1β-induced expression of IL-6 in FLS from AA rats. Their interplay was probed in the following experiments by using relative inhibitors.
Effects of PKC on MAPK and CREB activation
FLS from AA rats were pretreated with PKC inhibitor chelerythrine for 24 h, and then stimulated with IL-1β (10 ng/ml). The stimulation intervals were 15 min for MAPK assay and 30 min for CREB assay, respectively. As shown in Figure 10A, chelerythrine (2, 4 µM) reduced the phosphorylation of p38 MAPK by 25.6 and 35.2%, respectively, but it poorly affect the phosphorylation of ERK1/2. On the other hand, chelerythrine (2, 4 µM) also inhibited the phosphorylation of CREB in FLS with inhibitory percenages of 28 and 49.2%, respectively (Fig. 10B).
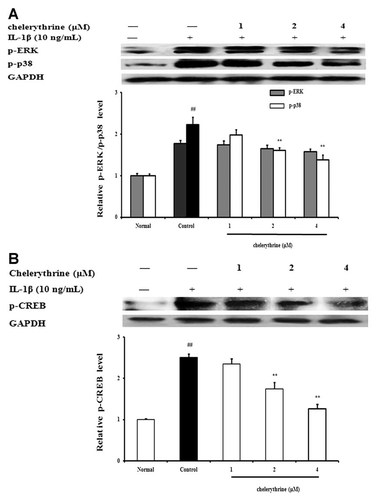
Effects of PKC on MAPK and CREB activation in fibroblast-like synoviocytes (FLS) from adjuvant arthritis rats. A: Effects of PKC on MAPK activation. FLS were pretreated with chelerythrine (1, 2, 4 µM) for 24 h, then stimulated with IL-1β (10 ng/ml) for 15 min. The p-ERK1/2 and p-p38 were analyzed by western blot using specific antibodies. B: Effects of PKC on CREB activation FLS were pretreated with chelerythrine (1, 2, 4 µM) for 24 h, then stimulated with IL-1β (10 ng/ml) for 30 min. The p-CREB was analyzed by western blot. GAPDH was used as the internal control. Data were expressed as means ± S.D. of three independent experiments. ##P < 0.01 versus normal; *P < 0.05, **P < 0.01 versus control.
Effects of p38 MAPK on PKC and CREB activation
FLS from AA rats were pretreated with SB203580 (a specific inhibitor of p38 MAPK) for 24 h, and then stimulated with IL-1β (10 ng/ml) for 15 min. The activations of PKC and CREB were detected by western blot assay. The results showed that SB203580 (5, 10, 20 µM) exhibited little effect on IL-1β-induced phospphorylation of PKC in FLS (Fig. 11A). But it concentration-dependently inhibited the phosphorylation of CREB. At concentrations of 10 and 20 µM, the inhibitory percentages were 35.7 and 54.5%, respectively (Fig. 11B).
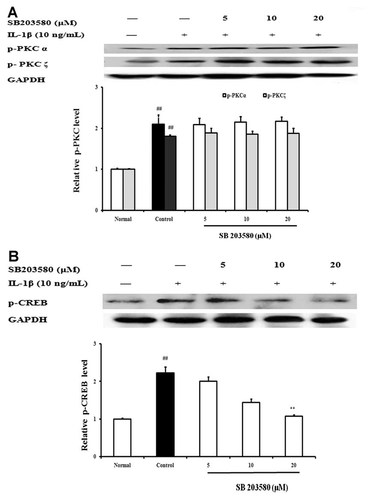
Effects of p38 MAPK on the activation of PKC (A) and CREB (B) in fibroblast-like synoviocytes (FLS) from adjuvant arthritis rats. FLS were pretreated with SB203580 (5, 10, 20 µM) for 24 h, then stimulated with IL-1β (10 ng/ml) for 15 min. The PKC and p-CREB were analyzed by western blot using specific antibodies. Data were expressed as means ± S.D. of three independent experiments. ##P < 0.01 versus normal; *P < 0.05, **P < 0.01 versus control.
Taken together, the signal pathways that participate in IL-6 expression induced by IL-1β in FLS can be ranked as follows: PKC-p38 MAPK-CREB.
DISCUSSION
Various kinds of cytokines, growth factors, and proteases, originating from T lymphocytes, B lymphocytes, macrophages and FLS and other cells, and their complex interactions are involved in the development and progression of RA. They are directly responsible for abnormal autoimmune responses, local and systemic inflammation, and eventual joint cartilage destruction. Among them, IL-6 is an important proinflammatory cytokine in addition to IL-1β and TNF-α. Growing evidence has proven its importances in the pathogenesis of RA [Flannery et al., 2000; Lipsky, 2006]: (1) In the synovial fluids and serum of RA patients, IL-6 levels increase significantly; (2) IL-6 acts in the activation of T and B lymphocytes, and the induction of acute phase reactants; (3) IL-6 expressed at local could stimulate leukocyte recruitment to the joints, enhance osteoclast differentiation and activation, and stimulate synovial proliferation, eventually lead to joint damage; (4) tocilizumab, a specific IL-6 inhibitor, has been successfully applied in clinical trials for RA treatment. Intervention of either production or action of IL-6 has become a promising strategy for RA management.
NOR, the highest content of isoquinoline alkaloid compound in Radix Linderae, has previously been demonstrated to be the major anti-rheumatoid constituent of the herb drug [Chou et al., 2005; Luo et al., 2009]. In the present study, NOR was shown to be able to prevent IL-1β-induced release of IL-6 from rat FLS, key producers of IL-6 in synovial membranes of joints. The underlying mechanisms of action were addressed in views of interfering with intracellular signal transduction pathways triggered by IL-1β.
IL-1β belongs to IL-1 family that consists of three members. Of which, IL-1α and IL-1β possess biological activities. In contrast, IL-1Ra as IL-1 receptor antagonist is an endogenous inhibitor that blocks the action of the other two members. After secreted, IL-1β acts on other cells to produce its biological activities through binding with IL-1RI [Kay and Calabrese, 2004]. It can cause the recruitment and oligomerization of tumor necrosis factor-associated factor (TRAF) 6, activate MEKK and IKK, consequently trigger the activation of MAPK and NF-κB pathways, and then mediate the expressions of genes encoding IL-6 and other cytokines. PKC and cAMP-PKA pathways may also work as the downstream of IL-1 receptor and participate in the expression of IL-6 [Dinarello, 1994; Weber et al., 2010].
MAPKs consist of extracellular signal-regulated kinases (ERKs) and two stress-activated protein kinase (SAPKs) families, c-jun N-terminal kinase (JNK) and p38 in mammals. Accumulative findings demonstrate their extensively regulatory roles in RA-related events, especially in the expressions of proinflammatory cytokines and proteases. For instance, ERK1/2 inhibitor PD-98059 can reduce nociceptive responses in an adjuvant-induced monoarthritis in rats, and ERK is involved in the regulation of the synthesis of IL-6, IL-12, IL-23, and TNF-α [Cruz et al., 2005]. p38 MAPK is able to regulate IL-1-stimulated production of IL-6 and IL-8 in FLS, and the inhibitors have shown significant inhibition of various arthritis animal models. At present, p38 MAPK has been accepted as one of most promising therapeutic targets for RA. Moreover, JNK appears to play a major role in the regulation of collagenase production by FLS [Thalhamer et al., 2008]. Our present studies showed that IL-1β stimulation led to significant phosphorylations of both ERK1/2 and p38 MAPK in FLS from AA rats, and NOR concentration-dependently reduced the phosphorylations. Of note, it almost completely reversed the phosphorylation of p38 at a concentration of 60 µM.
NF-κB is a family of inducible dimeric transcription factors, that recognizes a common consensus DNA sequence and regulates large numbers of target genes, especially the genes concerned with inflammation, injury, and stress. The activation of NF-κB refers to IκB (the inhibitor of NF-κB) phosphorylation, which depends on the regulation of IKK (the kinase of IκB). IKK/IκB/NF-κB constitute an organism system. IL-1 stimulation results in IKK activation, and causes IκBα phosphorylation. Then, polyubiquitinated IκBα is degradated by proteasomes, and the dissociative NF-κB-p65 subunit should enter into nucleolus, bind with the particular position of DNA, and then start the target protein expression [Karin et al., 2004]. In this study, NOR showed little effect on the phosphorylation of IKKα, and the phosphorylation and degradation of IκBα induced by IL-1β in FLS from AA rats. In contrast, it showed remarkable inhibition of p65 phosphorylation at serine 276 locus. The findings suggested that NOR might interfere with NF-κB pathway at downstream location. Furthermore, considering that there is a close relationship between the phosphorylation and transcription of p65 and the activation of p38 MAPK, the action of NOR against p65 phosphorylation in FLS might achieve through preventing p38 activation [Vanden Berghe et al., 1998; Olson et al., 2007; Twait et al., 2010].
In the 5′-flanking region of genes encoding human IL-6, there are various putative responsive elements, such as NF-κB, CREB, NF-IL-6, and activator protein-1 (AP-1). Among them, both NF-IL-6 and AP-1 have been proven to be not associated with IL-6 expression in FLS [Georganas et al., 2000]. In addition to NF-κB, CREB is also a critical transcription factor implicated in IL-6 production. Moreover, either NF-κB or CREB functions by binding with CREB binding protein (CBP). They cooperate to recruit CBP at the IL-6 promoter, thus enable synergistic gene activation. In consistent with previous reports [Miyazawa et al., 1999; Bergamaschi et al., 2006; Ishizu et al., 2010; Spooren et al., 2010], IL-1β stimulation led to evident phosphorylation of CREB in FLS from AA rats, NOR showed notable inhibition of it. On the other hand, CREB is a phosphorylation substrate for diverse protein kinases (PKs), such as PKA, PKC, p38 MAPK, and others. If intracellular cAMP level increases, PKA will be activated, and then CREB phosphorylation will be up-regulated [Pigazzi et al., 2007; Lee et al., 2010]. In this study, IL-1β stimulation only led to a slight increase of cAMP content in FLS from AA rats, and NOR showed little effect on cAMP level. These findings suggested that NOR prevented CREB phosphorylation in FLS by pathways independent on cAMP-PKA.
PKC pathway also participates in the expressions of various proinflammatory cytokines in cells. Of which, PKCδ, PKCα, and PKCζ were reported to play important roles in the production of IL-6 and other cytokines of IL-6 family [Newton, 2010]. PKC inhibitor calphostin C could prevent phorbol-12-myristate-13-acetate (PMA)- or lipopolysaccharide-induced IL-6 expression at protein and mRNA levels in human peripheral blood monouclear cells [Kontny et al., 1999; Kandere-Grzybowska et al., 2006]. IL-1β, an inducer used in the present study, leads to PKC activation probably by the following steps: IL-1β/IL-1R complex triggers phospholipase D-1 (PLD-1), induces the generation of diacylglycerol (DAG), and then activates PKC [Dinarello, 1994]. Our data showed that PKC inhibitor chelerythrine concentration-dependently prevented IL-1β-induced IL-6 production in FLS from AA rats, which confirmed for the first time the participation of PKC in IL-6 production in FLS. Furthermore, it was shown that IL-1β stimulation resulted in rapid phosphorylations of PKCδ, PKCα, and PKCζ in FLS, NOR (10, 30, 60 µM) pretreatment remarkably inhibited the phosphorylations of PKCα and PKCζ but not PKCδ. Of which, NOR (60 µM) almost completely reversed the phosphorylation of PKCα.
As mentioned above, there were multiple signal pathways probably participating in the downregulation of NOR against IL-1β-induced IL-6 expression in FLS from AA rats. Their relationships were addressed by using relative inhibitors. Chelerythrine, a PKC inhibitor, showed significant inhibition of p38 MAPK phosphorylation induced by IL-1β in FLS. In contrast, SB203580, a p38 inhibitor, could not affect the activation of PKC. However, both chelerythrine and SB203580 showed remarkable inhibition of the phosphorylation of CREB induced by IL-1β. Of which, SB203580 made CREB phosphorylation down to base line level. These findings suggested that NOR reduced IL-6 production induced by IL-1β in FLS through interfering with PKC/p38 MAPK/CREB pathways.
In conclusion, the present study confirmed that NOR was indeed able to inhibit the production of IL-6 induced by IL-1β in FLS from AA rats, which provided a new explanation for anti-RA action of NOR and its original herb Radix Linderae. The underlying mechanisms of NOR seemed to be down-regulating activation of PKC/MAPK/NF-κB-p65/CREB pathways. The precise action and mechanisms of NOR against other effector cells of RA need to be studied in future.