SIRT1 and SIRT5 activity expression and behavioral responses to calorie restriction
Abstract
To investigate the effects of calorie restriction (CR) on behavioral performance and expression of SIRT1 and SIRT5 in rat cerebral tissues. Beginning at 18 months of age, 60 rats were randomly divided into a CR group (n = 30) and a group that remained fed ad libitum (AL; n = 30). CR rats were restricted to a diet of 60% of their daily food consumption. After 6 months of CR, CR rats displayed a maximum 50% reduction in escape latency (AL 20 ± 0.3 s vs. CR 10 ± 0.2 s) and a 3.2 s decrease in time and distance to target when evaluated in Morris water maze tests. The levels of SIRT1 and SIRT5 protein in cerebral tissues of CR rats were elevated compared to AL rats (P < 0.05). CR retarded declines in cognitive ability and enhanced the expression of both SIRT1 and SIRT5 proteins in the cerebral tissue of CR rats compared with AL rats. J. Cell. Biochem. 112: 3755–3761, 2011. © 2011 Wiley Periodicals, Inc.
Growing evidence suggests that calorie restriction (CR) is one of the most effective strategies to prolong life span in a variety of organisms, from worms and yeast to rats and fish [Carter et al., 2009]. The mechanisms by which CR exerts its anti-aging effects have been widely studied in many systems. However, those mechanisms involved in an old-aged animal's brain, an organ of maximum relevance to aging, have been only scarcely explored and contradictory findings have been reported. Forster showed an increase in mouse mortality if CR was initiated in late life [Forster et al., 2003]. Yet in rats, life-long food restriction resulted in a significant delay in age-related cognitive decay [Cohen et al., 2009].
Silent information regulator 2 (SIR2) plays a key role in CR. An extra copy of the SIR2 gene can extend life span by nearly 40%, while inactivation of SIR2 shortens life span in yeast, worms, and flies [Tissenbaum and Guarente, 2001; Rogina and Helfand, 2004; Bordone and Guarente, 2005]. In mammals, SIRT1, the homolog of SIR2, is required for CR-mediated extension of life span, and SIRT1 overexpression or deletion, respectively, extends and decreases life span [Yu et al., 2009a, b]. In the central nervous system, overexpression of SIRT1 significantly decreased hippocampal neurodegeneration in Alzheimer's disease (AD) mouse models, and up-regulation of SIRT1 expression can promote α-secretase activity, preventing the generation of Aβ plaques in mammalian neurons [Qin et al., 2006].
Mitochondria are the primary sites of reactive oxygen species (ROS) generation within the cell, and increased oxidative damage is proposed to be one cause of mammalian aging [Wallace, 2005]. On the other hand, failure in mitochondrial function and regulation contribute to aging-related diseases, such as atherosclerosis and Parkinson's disease, likely by increasing cellular levels of ROS [Balaban et al., 2005; Fukae et al., 2007]. Several studies have shown that SIRT5 is a mitochondrial protein [Onyango et al., 2002; Shi et al., 2005]. SIRT5 can deacetylate cytochrome c, a protein of the mitochondrial intermembrane space with a central function in oxidative metabolism, as well as apoptosis [Schlicker et al., 2008]. We demonstrate that, in aged rats, CR is capable of reducing declines in cognitive function, and that this reduction correlates with CR-mediated enhancement of SIRT1 and SIRT5 in cerebral tissue.
MATERIALS AND METHODS
Animals
This study was approved by the Institutional Animal Care and Use Committee of Shantou University Medical College. Eighteen-month-old male Sprague–Dawley rats were housed at 22–24°C in a light-controlled environment with a 12:12-h light–dark cycle. All rats were fed standard lab chow. The rats of the CR group (n = 30) were housed individually. They were given a restricted food allotment consisting of 60% of the initial quantity of food consumption each day. Rats in the control group were fed ad libitum (AL, n = 30). Water was readily available for both groups. All animals in each group were studied for a period of 6 months.
Cell and Cell Culture
PC12 rat pheochromocytoma cells were cultured in high glucose (4.5 g/L) Dulbecco's modified Eagle's medium supplemented with 8% fetal bovine serum, 100 U/ml of penicillin, 100 U/ml streptomycin, and 5% CO2 at 37°C. Caloric restriction was imposed by culturing in low glucose (1 g/L) DMEM supplemented with 8% fetal bovine serum, 100 U/ml of penicillin, 100 U/ml streptomycin. Both groups were incubated in 5% CO2 at 37°C for 48 h. Then the CR culture was maintained in DMEM containing 1 g/L glucose for another 12 h for further analysis.
Morris Water Maze (MWM) Navigation
Rats were tested in an MWM according to standard procedures [Valentinuzzi et al., 2004; Nisoli et al., 2005; Ishida et al., 2007] that typically included 4.5 days of training. Briefly, a circular pool of water (180 cm in diameter) was divided into four quadrants (I, II, III, and IV) as the starting point for training the rats. A dark escape platform (9.5 cm in diameter) was submerged 1.0–2.0 cm below the surface of the pool water at a fixed location in the center of quadrant III. Temperatures of the room and pool water were maintained at 16 ± 2°C. Animals were not allowed to access food or water until the end of each test in each block.
All rats were trained at the same time for the navigation trials. Each training period consisted of four trials. Each trial lasted for 60 s or ended as soon as the rat reached the submerged platform. If any rat failed to find the platform, he was guided gently and remained on the platform for 10 s before being returned to a holding cage. Starting points were randomized across trials, but the same sequence was used for each rat. During the training period, escape latency, swimming speed, and the swimming path were recorded by a computerized tracking system.
Morris Water Maze (Probe Trial)
On the afternoon of the 5th day, after the navigation trial, the escape platform was removed. Each rat was released in a fixed quarter (I), which was opposite to the target quarter (III). Rats were allowed to swim inside the pool for 2 min, during which time we recorded, using a computerized tracking system, the number of times it takes for the rat to cross the former goal annulus, the trace, the percentage of time, the speed, and the distance spent in each quarter.
Immunofluorescence Labeling of PC12 Cells
For detection of SIRT1 and SIRT5 proteins, cells were fixed with 4% paraformaldehyde for 15 min, treated with 0.2% Triton X-100 in PBS for 15 min, incubated in 2% bovine serum albumin in PBS at room temperature for 1 h, and incubated overnight at 4°C with the primary antibody. Cells were then incubated with a secondary antibody for 1 h at room temperature, and counterstained with 4′,6-diamidino-2-phenyl-indol dihydrochloride (DAPI). Images were captured with a fluorescence microscope.
Immunofluorescence Staining of Rat Brains
For histology, 60 rats were killed by overanesthetization with Nembutal, and subsequently perfused with saline containing 4% paraformaldehyde. Brain sections were then prepared on a cryostat. Sections were incubated with primary antibody against SIRT1, SIRT5, or NeuN monoclonal antibody overnight at 4°C, then incubated with Alexa Fluor 555-conjugated anti-rabbit IgG, Alexa Fluor 488-conjugated anti-goat IgG, or Alexa Fluor 555-conjugated anti-mouse IgG for 1 h at room temperature, and counterstained with DAPI. Images were captured under a fluorescence microscope.
Immunohistochemical Labeling of CR and AL Rat Brains
Deparaffinized CR and AL rat cerebral hemisphere sections, taken from rats at 24 months of age, were blocked in 2% BSA in PBS, incubated with primary antibodies (anti-SIRT1, anti-SIRT5, anti-NeuN) overnight at 4°C, and with a biotinylated secondary antibody for 1 h at RT. Finally, the sections were incubated with Avidin–Biotin Complex reagent for 30 min at RT. The reaction was developed in PBS containing 3,3-diaminobenzidine tetrahydrochloride (DAB, 0.5 mg/ml). Sections were then lightly counterstained with hematoxylin and analyzed by light microscopy.
Western Blot Analysis
Total protein of brain tissues or PC12 cells was extracted with RIPA buffer, and equal amounts of protein were separated on 12% SDS–PAGE and transferred onto nitrocellulose membranes. Membranes were probed with primary antibodies against β-actin, SIRT1, or SIRT5, followed by incubation with the appropriate horseradish peroxidase-conjugated secondary antibody. The immune complexes were visualized by enhanced chemiluminescence methods, and quantified using Image Quant software.
RT-PCR
Total RNA was isolated from PC12 cells using the RNA Simple Total RNA Kit. First-strand cDNAs were generated by reverse transcription from RNA samples using a first-strand cDNA synthesis kit. Primer sequences are shown below. After cDNA synthesis, the PCR reaction consisted of 29 cycles of denaturing at 94°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 1 min, and extension for a further 10 min at 72°C at the end of last cycle. PCR products were subjected to 1% agarose gel electrophoresis and visualized with ethidium bromide. The relative expression was quantified densitometrically using the Gel Image Ver. 3.74 System.
Data Analysis
Results are presented as the mean ± standard deviation for a given number of observations. All data were analyzed using SPSS 11.0 software. Statistical significance between each group was calculated by a two-way analysis of variance (ANOVA) for repeated measures that estimate escape latency and swimming speed in the MWM training task. For the open-field test, including the percentage of time and path length, and histochemistry staining, data were compared using independent sample t-tests. The data were then analyzed using a two-way ANOVA and the results interpreted as the statistical significance of differences in the mean latencies between each group.
RESULTS
Effect of CR on Spatial Learning in Rats Tested in Navigation Trails
The effects of CR on brain function were initially evaluated by comparing CR rats (n = 30) and AL rats (n = 30) in navigation trials by measuring the differences of escape latencies in a Morris water maze test, that is, the time (s) each animal required to reach a platform located in the pool (Fig. 1A,B). Prior to caloric restriction, rats were randomly divided into CR and AL groups at 18 months of age. The latencies of both groups were identical (P = 0.92; Fig. 2). However, following a 6-month experimental period, AL rats showed an increased escape latency period when compared to that of the CR rats (P = 0.003). This difference accounted for between-subjects effects (P = 0.038), while within-subjects, latency periods showed no difference (P = 0.281). After 6 months of CR, CR rats displayed a maximum 50% reduction in escape latency time (AL 20 ± 0.3 s vs. CR 10 ± 0.2 s) and a 3.2 s decrease in time and distance to target when evaluated in Morris water maze tests (Fig. 2). In other words, the variations in latency periods resulted mainly from the different diets between the two groups (CR vs. AL).
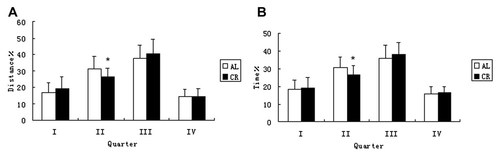
Time ratio (A) and distance ratio (B) spent in the probe trials. CR rats showed better performance in quarter II. *P < 0.01 versus AL rats. Time % = (time in each quarter/total time) × 100; Distance % = (distance in each quarter/total distance) × 100.
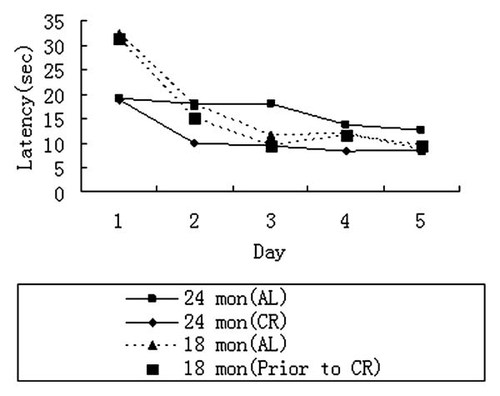
The effect of CR on escape latency tested in navigation trials. CR rats showed shorter escape latency than AL rats.
Effect of CR on Reference Memory of Rats Tested in Probe Trials
Reference memory was evaluated in the Morris water maze by measuring several parameters, including duration, path length, and speed following removal of the platform. CR rats spent less time (P = 0.027) and had a shorter path length (P = 0.029) in quadrant II than AL rats (Fig. 1A,B). No statistical differences were observed between groups in crossing the former goal annulus (P = 0.232). Although the CR rats swam for longer time periods in target quadrant III, the differences between the two groups was not statistically significant when we measured either the distance (P = 0.324) or time (P = 0.367).
Localization of SIRT1 and SIRT5 in Rat Cerebral Tissues
In sagittal sections of cerebral tissue, immunohistochemical staining for SIRT5 revealed a predominant distribution in nerve fibers of the cerebral medullary substance and the boundary regions between the cerebral cortex and the cerebral medullary substance (Fig. 3A,B). The level of expression in the cerebral medullary substance was much higher than that in the cerebral cortex. In addition, hippocampal SIRT5 staining mainly co-localized with neurons (Fig. 3D), where staining was predominantly nuclear (Fig. 3E). Immunohistochemical results in the cerebral cortex also showed that SIRT5 protein could be found in additional cell types besides neurons (Fig. 3C). Double-immunofluorescence labeling showed that SIRT5 was localized in the cytoplasm of neurons in the cerebral cortex and cerebral medullary substance (Fig. 4). In the cerebellar cortex, SIRT5 could be found in Purkinje cells, and was mainly distributed in the cytoplasm, but was not markedly expressed in the molecular layer or granular layer cells (Fig. 3F). Immunohistochemical staining and double-immunofluorescence labeling showed that SIRT1 was located in the nucleus of cerebral cortex neurons (Fig. 3I).
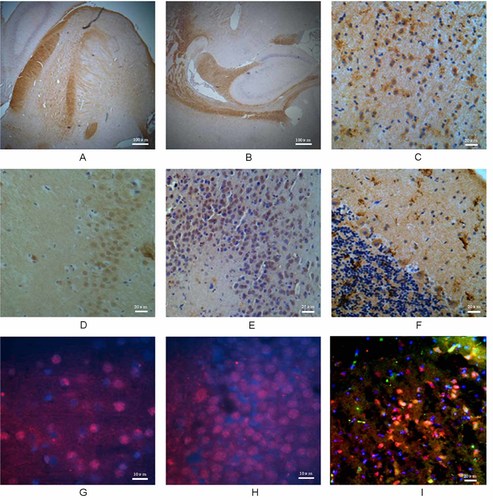
The distribution and localization of SIRT1 and SIRT5 in rat cerebral tissue. A: distribution of SIRT5 in the cerebral medullary substance. B: Expression of SIRT5 in the boundary regions of the cerebral cortex and cerebral medullary substance, and surrounding the hippocampus. C: SIRT5 distribution in the cerebral cortex. D: Hippocampal neuron staining. E: Expression of SIRT5 in hippocampal neurons. F: Purkinje cell staining in the cerebellum cortex. G: Expression of SIRT1 in the cerebral cortex. H: Expression of SIRT1 in the hippocampus. I: Double-immunofluorescent labeling of SIRT1 expression in cerebral cortex neurons.
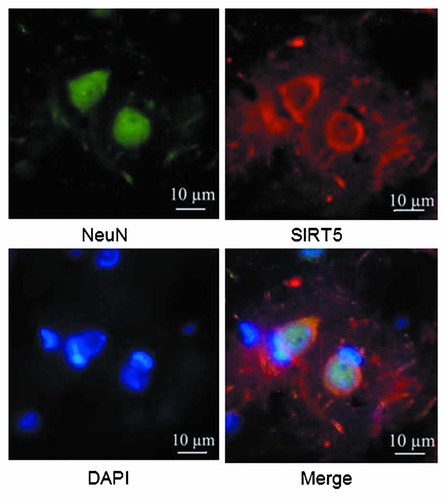
Double-immunofluorescence labeling of SIRT5 and a neuronal marker in the cerebral cortex and cerebral medullary substance of CR rats.
Western Blot Analysis of SIRT1 and SIRT5 Protein and mRNA Expression
In PC12 cells, the expression levels of SIRT1 and SIRT5 proteins (Fig. 5A,C) and mRNA (Fig. 5B,D) also increased following caloric restriction (*P < 0.05). These results show that CR results in increased expression of both SIRT1 and SIRT5 in both CR rats and PC12 cells.
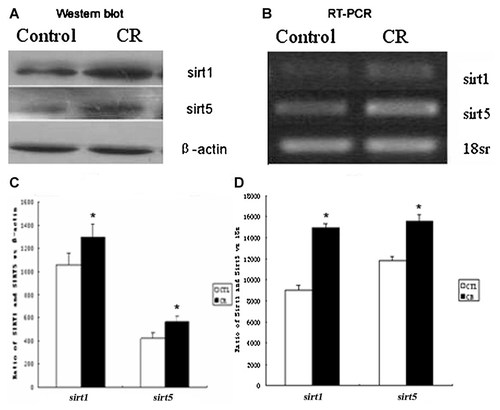
The expression levels of SIRT1 and SIRT5 proteins (A,C) and mRNA (B,D) in PC12 cells. *P < 0.05 CR versus control.
DISCUSSION
Although several studies have shown the benefits of CR on pathological neurodegeneration, few studies have observed a similar effect of CR, on normal aging, particularly at the molecular level. In a previous study, life-long food restriction resulted in a significant delay in age-related cognitive decay [Cohen et al., 2009]. However, the early age at which CR was initiated complicated interpretation of the effects of CR directly on cognitive function. Impairment of behavior in their study may result, in part, from low blood glucose levels. Intriguingly, in probe trials, the AL rats unexpectedly spent more time in the “wrong” quarter instead of the target quarter (III). This suggested that AL rats failed to develop efficient spatial search strategies, and as a result, failed to find the target precisely [Chen et al., 2005].
The aging of replicating cells is determined by the SIR2 gene [Lin et al., 2000]. In yeast, SIR2 protein regulates gene silencing, apoptosis, and lifespan [Lamming et al., 2005]. As mentioned above, there are seven SIR2 homologs in mammals, SIRTs 1–7. In addition to histones, SIRT1 also deacetylates other proteins, including FoxO, MyoD, P53, and PGC-1α [Vaziri et al., 2001; Fulco et al., 2003; Motta et al., 2004; Rodgers et al., 2005]. Initial studies showed that SIRT1 localized in the nucleus [Cohen et al., 2004], consistent with our findings in rat neurons. In addition, we further show that SIRT1 is expressed more in the cerebral medullary substance than cerebral cortex, and mostly in nerve fibers. However, SIRT1 appeared in both nucleus and cytoplasm in PC12 cells. These results show SIRT1 is distributed in different regions according to different tissues. In addition, SIRT1 is distributed extensively throughout cerebral tissue, suggesting that SIRT1 could play an important role in regulating organism function.
Axonal degeneration is a major morphological characteristic observed in both peripheral neuropathies and neurodegenerative diseases, such as AD and amyotrophic lateral sclerosis [Cohen et al., 2004]. It is well known that CR protects neurons from degeneration in mouse models of AD and Parkinson's disease, and SIRT1 might facilitate neuronal survival [Luchsinger et al., 2002; Patel et al., 2005].
SIRT1 is important for many cellular processes, including gene silencing, cell cycle regulation, and life span extension [Hisahara et al., 2005]. SIRT1 overexpression enhances the expression of Bcl-2 protein and further inhibits Bax translocation from cytoplasm to nucleus. SIRT1 subsequently increases mitochondrial function and decreases cell damage, suggesting that the mitochondrial function triggered by CR is mediated by the SIRT1 activation (Han et al., 2008). Prior reports demonstrate that SIRT1 is up-regulated by CR in many tissues and cell lines (Cohen et al., 2004). We extend these results to show that similar to SIRT1, CR increases SIRT5 expression in rat cerebral tissues and PC12 cells.
Unlike SIRT1, little information about mammalian SIRT5 is known at present. SIRT5 has been described as a mitochondrial protein (Onyango et al., 2002; Shi et al., 2005) with weak deacetylase activity [North et al., 2003], but no ADP-ribosyltransferase activity (Haigis et al., 2006). Up to now, no other target for SIRT5 has been reported except for cytochrome c, which has a central function in oxidative metabolism and apoptosis. SIRT5 can indeed be translocated into both the mitochondrial intermembrane space and matrix, indicating that the localization might contribute to SIRT5 function and substrate selection (Schlicker et al., 2008). Failures in mitochondrial function and regulation contribute to aging-related diseases, such as atherosclerosis [Davidson and Duchen, 2007] and Parkinson's disease [Fukae et al., 2007], likely by increasing cellular levels of ROS and consequent damage [Balaban et al., 2005]. In our study, SIRT5 localized in PC12 cell mitochondria, but appeared in the nucleus of neurons in the hippocampus and cytoplasm of neurons in the cerebral medullary substance and cerebral cortex. A recent report showed that SIRT5 was distributed in the nucleus, cytoplasm, and mitochondria in cerebellar granule neuron cells [Pfister et al., 2008]. SIRT5 localization studies have correlated nuclear and cytoplasm with resistance to cell death, whereas translocation of SIRT5 to mitochondria results in enhanced cell death [Pfister et al., 2008]. In the present study, like SIRT1, SIRT5 was enhanced in CR neurons both in vivo and in vitro, and SIRT5 is homologous with SIRT1, so we may presume that SIRT5 may be a novel anti-aging regulation factor that counteracts aging-related neurodegenerative diseases. SIRT5 is present in the nucleus of neurons in the rat hippocampus and is expressed more in the cerebral medullary substance than cerebral cortex, and mostly in nerve fibers. SIRT1 can delay the neurodegenerative diseases. Therefore, we might also infer that SIRT5 protein might play an important role in neurodegenerative or axonal degeneration diseases, and have a profound function in neurons. These results provide useful information for the further studies on SIRT5 function in neuron aging.
In summary, this is the first time the relationship between CR and Sirtuin proteins have been analyzed in early aged rats. Our findings showed that early aged rats maintained on a CR diet showed much better behavioral performance than their AL counterparts, as demonstrated by elevated behavioral tests.
Acknowledgements
PC12 cells were kindly supplied by the Pathophysiology Department of Sun Yat-sen University. This work is supported by the Foundation for Distinguished Young Talents in Higher Education of Guangdong, China (LYM08057) and the Medical Scientific Research Foundation of Guangdong Province, China (B2008140). We thank Professor Qingjun Huang for professional assistance in the behavior tests at Shantou University Mental Health Center in China, and Professor Jianjun Zhang for statistical analyses at the Department of Statistics of Shantou University Medical College. The authors would like to thank Dr. Stanley Lin for the English language editing.




