Ailanthoidol suppresses lipopolysaccharide-stimulated inflammatory reactions in RAW264.7 cells and endotoxin shock in mice
Abstract
The biological properties of ailanthoidol, a neolignan from Zanthoxylum ailanthoides or Salvia miltiorrhiza Bunge, which is used in Chinese traditional herbal medicine, have not been evaluated. Here, we report that ailanthoidol inhibits inflammatory reactions in macrophages and protects mice from endotoxin shock. Our in vitro experiments showed that ailanthoidol suppressed the generation of nitric oxide (NO) and prostaglandin E2, as well as the expression of inducible NO synthase (iNOS) and cyclooxygenase (COX)-2 induced by lipopolysaccharide (LPS) in RAW264.7 cells. Similarly, ailanthoidol inhibited the production of inflammatory cytokines induced by LPS in RAW264.7 cells, including interleukin (IL)-1β and IL-6. In an animal model, ailanthoidol protected BALB/c mice from LPS-induced endotoxin shock, possibly through inhibition of the production of inflammatory cytokines and NO. Collectively, ailanthoidol inhibited the production of inflammatory mediators and may be a potential target for treatment of various inflammatory diseases. J. Cell. Biochem. 112: 3816–3823, 2011. © 2011 Wiley Periodicals, Inc.
Inflammation is a complex process mediated by activation of inflammatory and immune cells. During the inflammation process, macrophages play a central role in mediation of many different immunopathological phenomena. Additionally, inflammation is responsible for antigen processing and presentation to antigen-specific T cells. Following activation, macrophages modulate the expression of accessory molecules, such as CD14 and toll-like receptor (TLR) 4 [Hajishengallis and Lambris, 2011]. Stimulation of TLR4 by lipopolysaccharide (LPS) in the presence of the LPS-binding protein, CD14, and the MD2 protein, triggers recruitment of the cytoplasmic adaptor protein MyD88, which culminates in activation of two distinct downstream signaling pathways: the transcription factor nuclear factor-κB (NF-κB) pathway and the mitogen-activated protein kinase (MAPK) pathway [O'Neill and Bowie, 2007]. These two pathways induce the expression of various inflammatory mediators, including nitric oxide (NO), prostaglandins (PGs), and inflammatory cytokines, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α [O'Neill and Bowie, 2007; Medzhitov, 2008; Hajishengallis and Lambris, 2011].
NF-κB is an essential transcription factor that regulates gene expression for various cytokines, chemokines, growth factors, and cell-adhesion molecules [Blackwell and Christman, 1997]. In unstimulated cells, NF-κB is constitutively localized in the cytosol as a heterodimer by physical association with an inhibitory protein known as inhibitor κB (Iκ-B). Many stimuli, such as LPS, cytokines, activators of protein kinase C, oxidants, and viruses activate NF-κB via several signal transduction pathways that all lead to phosphorylation of Iκ-B. Following activation, the NF-κB heterodimer is rapidly translocated to the nucleus, where it activates the transcription of target genes, including genes encoding pro-inflammatory cytokines, adhesion molecules, chemokines, and inducible enzymes such as cyclooxygenase (COX)-2 and inducible NO synthase (iNOS) [Blackwell and Christman, 1997; Medzhitov, 2008]. Because NF-κB plays such a pivotal role in the amplifying loop of the inflammatory response, they have become logical targets for new anti-inflammatory agents [Lang et al., 2006; Kaileh and Sen, 2010; Reuter et al., 2010].
There is currently a strong interest in developing new anti-inflammatory agents from plants used in traditional medicine. Ailanthoidol (3-deformylated 2-arylbenzo[b]furan, Fig. 1A), a neolignan from Zanthoxylum ailanthoides or Salvia miltiorrhiza Bunge, is used in Chinese traditional herbal medicine. Although a method for the synthesis of ailanthoidol has been established [Kao and Chern, 2002; Lin et al., 2003], the physiological and biological functions are largely unknown. Lee et al. [2006] reported that ailanthoidol exhibited a radical quenching property by a 1,1-diphenyl-2-picryl-hydrazyl radical scavenging assay as well as anti-tumor activity using a 12-O-tetradecanoylphorbol-13-acetate-induced skin cancer model. However, the underlying molecular mechanisms of these anti-oxidant and anti-tumor effects have yet to be elucidated. In the present study, we investigated the anti-inflammatory properties of ailanthoidol in vitro and in vivo, as wells as the molecular mechanism.
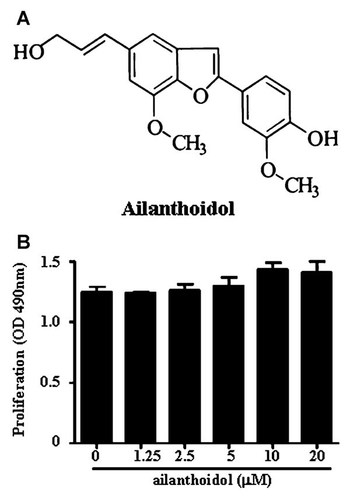
Effects of ailanthoidol on murine macrophage viability. A: The structure of ailanthoidol. B: RAW264.7 cells were treated with 0, 1.25, 2.5, 5, 10, and 20 µM of ailanthoidol for 24 h, after which the proliferation was determined as described in the Materials and Methods Section. The results are reported as the mean ± SEM of four independent experiments in triplicate.
MATERIALS AND METHODS
Reagents
Ailanthoidol was synthesized from vanillin in five steps as previously reported [Hwang et al., 2010]. LPS and fluorescein isothiocyanate (FITC)-conjugated LPS derived from Escherichia coli (0111:B4) were obtained from Sigma–Aldrich (St, Louis, MO). The DMEM, FBS, penicillin, and streptomycin used in this study were obtained from Hyclon (Logan, UT). In addition, the following antibodies were used in this study: anti-iNOS rabbit polyclonal (BD PharMingen, San Diego, CA), anti-COX-2 monoclonal antibody (mAb; BD PharMingen), anti-Iκ-Bα-mAb (BioLegend, San Diego, CA), anti-NF-κB-p65 rabbit polyclonal (Cell Signaling Technology, Danvers, MA), anti-phospho-JNK (pT183, pY185) rabbit polyclonal (Cell Signaling Technology), anti-JNK rabbit polyclonal (Cell Signaling Technology), anti-phospho-pERK1/2 (pT202, pY204) rabbit polyclonal (Cell Signaling Technology), anti-ERK1/2 rabbit polyclonal (Cell Signaling Technology), anti-phospho-p38 (pT180, pY182) rabbit polyclonal (Cell Signaling Technology), anti-p38 rabbit polyclonal (Cell Signaling Technology), anti-laminB rabbit polyclonal (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-β-actin mAb (Sigma–Aldrich).
Cell Culture and Cell Viability Assay
RAW264.7 murine macrophages were obtained from the Korean Cell Bank (Seoul, Korea) and cultured in DMEM containing 10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin at 37°C under 5% CO2. The effect of the ailanthoidol on the cell viability was tested using a CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI). Briefly, RAW264.7 cells were plated at a density of 2 × 104 cells in a 96-well flat-bottom plate, after which ailanthoidol was added to each plate at the indicated concentrations. After a 24 h incubation period, the cell viability was measured according to the manufacturer's instructions. This assay is based on the reduction of a tetrazolium compound, MTS, to formazan, which has an optimum absorption at 490 nm. Thus, the quantity of the product in the cell culture is indicated by the optical density of formazan at 490 nm, which is directly proportional to the number of living cells.
Measurement of Nitrite, PGE2, and Cytokines
The amount of nitrite, PGE2, and cytokines (IL-1β, IL-6, TNF-α) released by the mouse macrophages was determined by measuring the amount of each substance in the RAW264.7 cell culture supernatant. RAW264.7 cells were plated at a density of 5 × 105 cells in a 24-well cell culture plate with 500 µl of culture medium and then incubated for 12 h. The cells were then treated with various doses of ailanthoidol in 1 µg/ml of LPS and incubated for another 24 h. The amount of nitrite and PGE2 production was measured using the Griess reagent system (Promega) and an ELISA kit (R&D Systems, Minneapolis, MN), respectively, according to the manufacturer's instructions. Cytokines were measure using an ELISA kit (eBiosciences, San Diego, CA) according to the manufacturer's instructions.
Immunobloting Analysis
Whole cell, cytoplasmic, and nuclear extracts (30 µg protein/lane) were separated on 10% SDS–PAGE. The separated proteins were then electrophoretically transferred onto nitrocellulose membranes, which were incubated with the indicated antibodies. Finally, specific bands were visualized using an ECL kit (Amersham Biosciences, Piscataway, NJ).
AP-1 and NF-kB Activity
RAW264.7 cells were placed in a 24-well cell culture plate at a density of 5 × 105 cells/0.5 ml of culture medium and incubated for 12 h. The cells were then exposed to 0–20 µM ailanthoidol and 1 µg/ml of LPS, after which they were incubated for 4 h. The transcriptional activities of AP-1 and NF-κB were measured using TransAM (Active Motif, Carlsbad, CA) according to the manufacturer's instructions.
Immunofluorescent Staining and Flow Cytometry
RAW264.7 cells were incubated with FITC-conjugated LPS (10 µg/ml) for 20 min in the presence or absence of ailanthoidol. The cells were then fixed with 4% formaldehyde and 3% sucrose for 20 min. The stained cells were subsequently analyzed using a Guava Easycyte cytometer (Guava Technologies, Hayward, CA) or AxioImager M1 fluorescence microscope (Carl Zeiss, Gottingen, Germany) with the Axiovision software.
Survival Study
All studies were approved by the Institutional Animal Care and Use Committee of Hallym University. Eight-week-old male BALB/c mice were purchased from KOATECH (Pyeongtak, Korea) and divided into five groups of 10 mice each. Ailanthoidol was administered orally through an esophageal catheter at doses of 0, 0.1, 1, 10, and 20 mg/kg at 24 and 1 h before LPS exposure. LPS was then injected intraperitoneally at a dose of 20 mg/kg, after which the mice were monitored for 5 days to determine their survival rate. To measure the plasma levels of TNF-α, IL-1β, and IL-6, whole blood samples were collected by orbital puncture 1 or 6 h after LPS injection.
Statistical Analysis
Values are expressed as the mean ± SEM of the results of at least three experiments. The values were then evaluated by one-way analysis of variance (ANOVA) with Bonferroni multiple comparison post-tests using the GraphPad Prism 4.0 software (GraphPad Software Inc., San Diego, CA). The log-rank test was used to compare the survival data. Differences with P-values <0.05 were considered statistically significant.
RESULTS
Ailanthoidol Inhibits the Release of Inflammatory Mediators
To evaluate the ailanthoidol-induced anti-inflammatory effects, we used an in vitro model with the murine RAW264.7 macrophage cell line. Since ailanthoidol showed no cytotoxicity toward RAW264.7 macrophages at concentrations up to 20 µM for 24, 48, and 72 h treatment (Fig. 1B and data not shown), we used up to 20 µM ailanthoidol for the rest of the in vitro experiments. We first sought to determine the effect of ailanthoidol on the LPS-stimulated release of the inflammatory mediators NO and PGE2 by RAW264.7 cells. As shown in Figure 2A and 2B, ailanthoidol inhibited LPS-induced NO and PGE2 secretion in a dose-dependent manner. The nitrite concentrations in LPS-stimulated RAW264.7 cells and in cells exposed to 20 µM ailanthoidol were 24.5 ± 1.1 and 4.6 ± 0.7 µM, respectively. The inhibitory effects of ailanthoidol against PGE2 production in LPS-exposed cells were similar to their effects on NO production (Fig. 2B). Consistent with the findings related to NO and PGE2 production, immunoblotting analysis showed that LPS-stimulated iNOS and COX-2 induction in RAW264.7 cells was also reduced by ailanthoidol treatment in a dose-dependent manner (Fig. 2C). These findings indicate that the ailanthoidol-mediated reduction in iNOS and COX-2 expression was responsible for the inhibition of NO and PGE2 production.
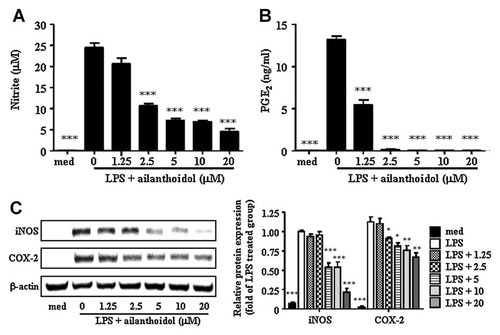
Effects of ailanthoidol on LPS-induced NO and PGE2 production, as well as on iNOS and COX-2 expression. RAW264.7 cells were treated with 0–20 µM ailanthoidol in the presence of 1 µg/ml of LPS or with LPS alone for 24 h. (A) NO and (B) PGE2 release were determined. C: RAW264.7 cells treated the same as in (A). A total of 30 µg of protein obtained from each cell lysate was resolved on 10% SDS–PAGE for iNOS and COX-2 determination. β-Actin expression is shown as a loading control. The bands were quantified using NIH image analysis software and their relative intensity was expressed as fold against the image of the LPS-stimulated RAW264.7 cells. The results are reported as mean ± SEM of three independent experiments. Statistical significance is based on the difference when compared with LPS-stimulated cells, *P < 0.05, **P < 0.01, ***P < 0.001.
We next investigated whether ailanthoidol reduced the release of inflammatory cytokines in LPS-stimulated RAW264.7 cells. The release of IL-1β, IL-6, and TNF-α was dramatically reduced by ailanthoidol co-treatment of LPS-exposed RAW264.7 cells (Fig. 3). These results demonstrate that ailanthoidol inhibits LPS-induced inflammatory reactions in RAW264.7 cells through the inhibition of inflammatory mediators, such as NO, PGE2, and inflammatory cytokines.
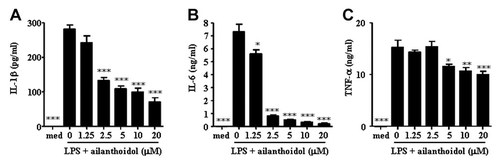
Effects of ailanthoidol on LPS-induced inflammatory cytokine production in murine macrophages. RAW264.7 cells were treated with 0–20 µM of ailanthoidol in the presence of 1 µg/ml LPS or with LPS alone for 24 h. The cell culture media were then collected, and the amount of (A) IL-1β, (B) IL-6, and (C) TNF-α released within them was measured as described in the Materials and Methods Section. The results are reported as the mean ± SEM of three independent experiments conducted in triplicate. Statistical significance is based on the difference when compared with LPS-stimulated cells, *P < 0.05, **P < 0.01, ***P < 0.001.
Ailanthoidol-Reduced Inflammatory Mediator Release Is Regulated by NF-κB
We further investigated the mechanism by which ailanthoidol inhibited the secretion of inflammatory mediators, such as NO and PGE2, as well as inflammatory cytokines. NF-κB is a major transcription factor involved in the release of proteins that mediate the inflammatory response. In addition, the degradation of Iκ-Bα is necessary to release NF-κB from the cytoplasmic NF-κB/Iκ-Bα complex and allow its subsequent translocation to the cell nucleus [Blackwell and Christman, 1997; Medzhitov, 2008]. We evaluated the effect of ailanthoidol on NF-κB activation to determine if it is mediated by Iκ-Bα degradation. As shown in Figure 4A, the Iκ-Bα degradation was hardly detected in resting RAW264.7 cells, but upon treatment of LPS, Iκ-Bα degradation was strongly initiated. Incubation of cultures with ailanthoidol (2.5–20 µM) significantly inhibited the degradation of Iκ-Bα, suggesting that ailanthoidol suppresses NF-κB activation by blocking Iκ-Bα degradation. Since nuclear translocation of NF-κB (p50/p65) is required for gene transcription of various inflammatory mediators, the effect of ailanthoidol on nuclear translocation of NF-κB was investigated using anti-p65 antibody. Consistent with Iκ-Bα degradation, immunoblot analysis showed that translocation of the p65 subunit of NF-κB from the cytoplasm to the nucleus was blocked by ailanthoidol treatment (Fig. 4B).
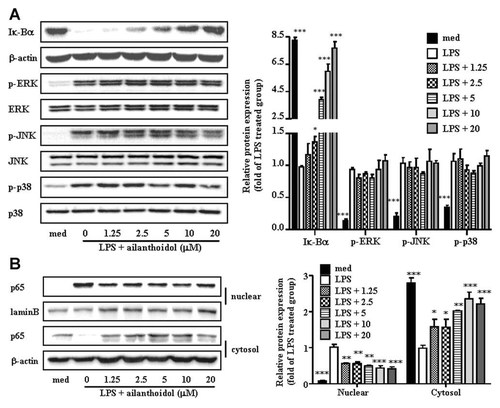
Effects of ailanthoidol on LPS-induced Iκ-Bα degradation and MAPK phosphorylation in murine macrophages. A: RAW264.7 cells were plated in 100-mm dishes. After 12 h of seeding, cells were treated with different doses of ailanthoidol for 1 h, followed by stimulation with 1 µg/ml of LPS for 30 min. Whole cell extracts were immunoblotted with the indicated antibodies. β-Actin was used as a control. B: RAW264.7 cells treated with LPS (1 µg/ml) alone or with various concentrations of ailanthoidol in the presence of LPS for 60 min. Nuclear and cytoplasmic fractions were obtained for detection of the p65 subunit of NF-κB. The bands were quantified using NIH image analysis software and their relative intensity was expressed as fold against the image of the LPS-stimulated RAW264.7 cells. The results are reported as mean ± SEM of three independent experiments. Statistical significance is based on the difference when compared with LPS-stimulated cells, *P < 0.05, **P < 0.01, ***P < 0.001.
Since LPS also activates MAPK family members, such as ERK, p38, and JNK, which play a crucial role in mediating the induction of inflammatory cytokines, we sought to determine if ailanthoidol modulated the MAPK signal transduction pathway. As shown in Figure 4A, the activation of MAPK via LPS stimulation in RAW264.7 cells was not altered by ailanthoidol.
Since the production of various inflammatory mediators is regulated by transcription factors NF-κB and AP-1 [Lang et al., 2006; Medzhitov, 2008], we investigated the effect of ailanthoidol on the nuclear translocation and DNA binding of NF-κB and AP-1 by conducting a transcription factor ELISA assay. The results revealed that LPS-induced DNA binding of NF-κB, but not AP-1, was significantly inhibited in LPS-stimulated RAW264.7 cells exposed to ailanthoidol, and these effects occurred in a dose-dependent manner (Fig. 5).
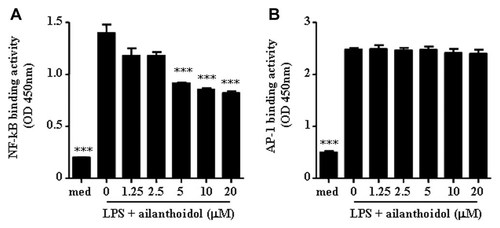
Effects of ailanthoidol on nuclear translocation and DNA-binding of NF-κB and AP-1 in LPS-stimulated murine macrophages. RAW264.7 cells were treated with various concentration of ailanthoidol in the presence of 1 µg/ml LPS or with LPS alone for 4 h. Nuclear proteins were extracted, and 2 µg of nuclear protein were assayed for the ability to bind with the immobilized (A) NF-κB consensus site and (B) AP-1 consensus site, as described in the Materials and Methods Section. The results are reported as the mean ± SEM of three independent experiments conducted in triplicate. Statistical significance is based on the difference when compared with LPS-stimulated cells, ***P < 0.001.
Ailanthoidol Suppressed LPS-Induced Endotoxin Shock
To assess the anti-inflammatory effects of ailanthoidol in vivo, a well-established experimental animal model for endotoxin shock, the most severe form of LPS-mediated inflammatory disease, was used. Endotoxin shock BALB/c mice injected intraperitoneally with a lethal dose LPS (25 mg/kg) nearly died within 48 h of challenge (Fig. 6). To determine if ailanthoidol could improve survival in these mice, animals were administered orally with vehicle alone or varying doses of ailanthoidol at 24 and 1 h before LPS challenge, after which they were monitored for 5 days. Ailanthoidol significantly improved the mortality of these mice in a concentration-dependent manner and no mice were killed in the 20 mg/kg group (Fig. 6).
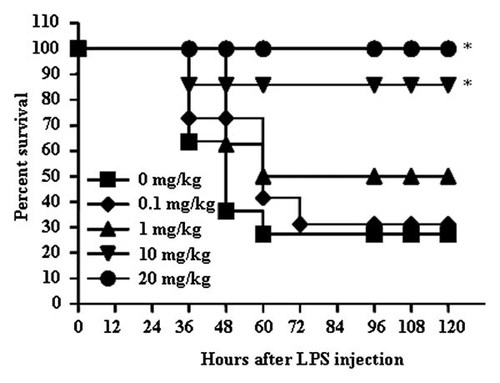
Effects of ailanthoidol on survival rate in LPS-treated mice. BALB/c mice (10/group) were injected intraperitoneally with 25 mg/kg of LPS. Ailanthoidol doses of 0, 0.1, 1, 10, and 10 mg/kg were administered orally through an esophageal catheter at 24 and 1 h before LPS was injected. The survival rate was monitored for 5 days after LPS injection. Statistical significance is based on the differences when compared with LPS-treated mice, *P < 0.05.
Endotoxin shock is mediated by the excessive production of inflammatory cytokines, such as TNF-α, IL-1β, and IL-6. To demonstrate the effect of ailanthoidol on the LPS-induced release of inflammatory cytokines, serum levels of these cytokines following ailanthoidol administration in LPS-challenged mice were analyzed. High concentrations of inflammatory cytokines including IL-1β, IL-6, and TNF-α were detected in sera in LPS-injected mice (Fig. 7A–C). However, the levels of these cytokines were significantly reduced by ailanthoidol treatment in a dose-dependent manner (Fig. 7A–C). Moreover, the levels of NO, which is also critically involved in LPS shock [Alvarez et al., 2009; Cauwels and Brouckaert, 2011] decreased in response to ailanthoidol administration (Fig. 7D).
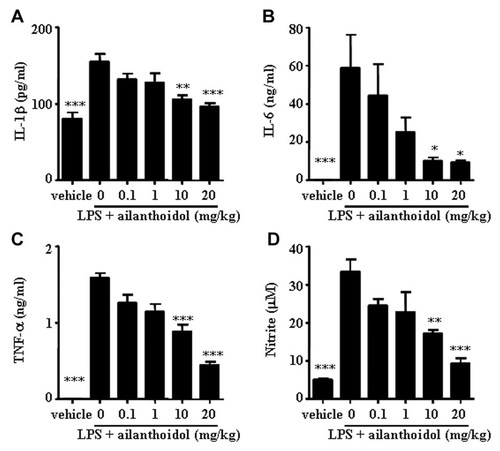
Effects of ailanthoidol on the production of inflammatory mediators in mice. BALB/c mice were injected intraperitoneally with LPS 25 mg/kg. Ailanthoidol doses of 0, 0.1, 1, 10, and 10 mg/kg were administered orally through an esophageal catheter at 24 and 1 h before LPS was injected. Serum was collected 1 and 6 h after LPS injection for measurement of (A) TNF-α, (B) IL-1β, (C) IL-6, and (D) NO, respectively. Statistical significance is based on the differences when compared with LPS-treated mice, *P < 0.05, **P < 0.01, ***P < 0.001.
Ailanthoidol Inhibits LPS Binding to RAW Inhibits LPS Binding to Cells
To identify the mechanism through which the inhibitory effect of ailanthoidol on inflammatory response against LPS occurs, we tested whether ailanthoidol inhibits the interactions between LPS and RAW264.7 cells. As shown in Figure 8A and 8B, ailanthoidol can inhibit LPS binding to RAW264.7 cells. The mean fluorescence intensity (MFI) of FITC–LPS-treated RAW264.7 cells was 6.2 ± 0.2, whereas that of ailanthoidol-treated (20 µM) cells was 4.4 ± 0.3 (Fig. 8C).
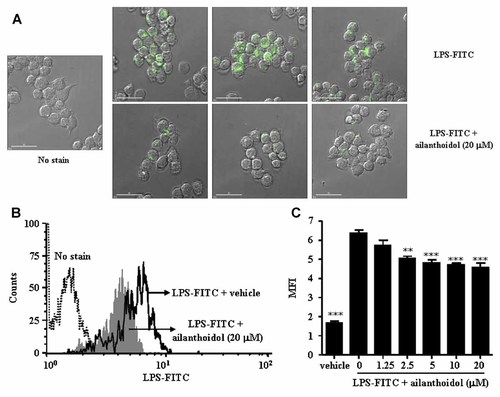
Inhibitory effects of ailanthoidol on interaction between LPS and RAW264.7 cells. RAW264.7 cells were incubated with FITC-conjugated LPS for 30 min in the absence or presence of the indicated concentration of ailanthoidol. A: Interaction between FITC-conjugated LPS and RAW264.7 cells was detected by fluorescence microscopy and (B,C) flow cytometry. Statistical significance is based on the differences when compared with FITC-conjugated LPS treated cells, **P < 0.01, ***P < 0.001.
DISCUSSION
A great deal of attention has been devoted to identifying phytochemicals of dietary and medicinal origin that have preventive and/or therapeutic effects against various diseases. It has been reported that phenolic compounds, which are ubiquitous in fruits and vegetables, possess various biological and physiological functions [Boudet 2007]. Conversely, lignans and neolignans are other groups in nature that have been found to exhibit a broad range of biological activity, such as anti-cancer, anti-viral, and anti-platelet activities [Chen et al., 2007; Vaid et al., 2010; Yang et al., 2010]. Ailanthoidol is a neolignan that is isolated from the chloroform-soluble fraction of stems of Zanthoxylum ailanthoides and the aqueous extracts of Salvia miltiorrhiza Bunge. Although the anti-cancer effect of ailanthoidol was demonstrated in a 12-O-tetradecanoyl-phorbol-13-acetate-induced tumor promotion model [Lee et al., 2006], the other biological properties and the details regarding the mechanism by which these effects occur are not known. In the present study, we demonstrated that ailanthoidol inhibits inflammatory reactions via inhibition of LPS binding to the surface of RAW264.7 cells (Fig. 8) and DNA binding of NF-κB transcription factor (Fig. 5). Additionally, the anti-inflammatory properties of ailanthoidol were evident in vivo in a LPS-induced endotoxin shock model (Figs. 6 and 7).
LPS stimulation activates two major intracellular signaling pathways via TLR4, myeloid differentiation factor 88 (MyD88)-dependent [Takeda and Akira, 2004] and MyD88-independent pathways [Takeda and Akira, 2004; Yamamoto et al., 2004]. Activation of the MyD88-dependent pathway results in Iκ-B kinase and MAPK activation. This activation subsequently triggers activation of Rel family transcription factor NF-κB and several members of AP-1 transcription factors, which finally regulate the expression of proinflammatory genes. In the MyD88-independent pathway, Toll/Interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon (IFN)-β (TRIF) mediates activation of IFN regulatory factor 3 and the late-phase activation of NF-κB, which subsequently activates a large set of antiviral genes, including IFN-α and IFN-β. We found that ailanthoidol could inhibit LPS binding to RAW264.7 cells in a dose-dependent manner and that the inhibition of binding by ailanthoidol could attenuate the binding of LPS to TLR-4. These findings indicate that ailanthoidol might exert its anti-inflammatory actions via the inhibition of LPS and its receptor, TLR-4. However, further study is required to determine how ailanthoidol inhibits LPS binding to macrophages.
NF-κB has been shown to be essential for the expression of iNOS and COX-2, as well as the release of various inflammatory cytokines [Medzhitov, 2008; Kaileh and Sen, 2010; Reuter et al., 2010]. We demonstrated that ailanthoidol impairs the degradation of Iκ-Bα, but not MAPK in LPS-stimulated RAW264.7 cells. Moreover, ailanthoidol had no inhibitory effect on LPS-induced AP-1 activity, but it significantly inhibited LPS-induced NF-κB in macrophages (Fig. 5). Hence, ailanthoidol may exert at least part of its anti-inflammatory effects via a reduction in NF-κB signaling.
The results of the present study also demonstrated that ailanthoidol exerts a potent protective effect against endotoxemia mortality in mice that reaches up to 80–90%. This effect is apparently related to the reduced concentration of various pro-inflammatory cytokines and NO. Among pro-inflammatory cytokines, TNF-α is involved in the development of endotoxin shock and tissue injury during overwhelming bacterial infection or challenge of lethal doses of LPS [Mookherjee and Hancock, 2007]. Therefore, the protective effect of ailanthoidol on endotoxin shock in mice challenged with LPS was associated with inhibition of TNF-α production, and an obvious dose–effect was observed.
In conclusion, our observations suggest that ailanthoidol strongly inhibits the production of inflammation-associated mediators (IL-1β, IL-6, TNF-α, iNOS, and COX-2) by suppressing NF-κB activation. In addition, we established that ailanthoidol can modulate inflammation responses in vivo. These findings suggest that ailanthoidol may be a potential source for the treatment of inflammatory diseases in humans.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0004728).




