RETRACTED: Loss of cell surface TFII-I promotes apoptosis in prostate cancer cells stimulated with activated α2-macroglobulin*
Abstract
Receptor-recognized forms of α2-macroglobulin (α2M*) bind to cell surface-associated GRP78 and initiate pro-proliferative and anti-apoptotic signaling. Ligation of GRP78 with α2M* also upregulates TFII-I, which binds to the GRP78 promoter and enhances GRP78 synthesis. In addition to its transcriptional functions, cytosolic TFII-I regulates agonist-induced Ca2+ entry. In this study we show that down regulation of TFII-I gene expression by RNAi profoundly impairs its cell surface expression and anti-apoptotic signaling as measured by significant reduction of GRP78, Bcl-2, and cyclin D1 in 1-Ln and DU-145 human prostate cancer cells stimulated with α2M*. In contrast, this treatment significantly increases levels of the pro-apoptotic proteins p53, p27, Bax, and Bak and causes DNA fragmentation. Furthermore, down regulation of TFII-I expression activates agonist-induced Ca2+ entry. In plasma membrane lysates p-PLCγ1, TRPC3, GRP78, MTJ1, and caveolin co-immunoprecipitate with TFII-I suggesting multimeric complexes of these proteins. Consistent with this hypothesis, down regulating TFII-I, MTJ1, or GRP78 expression by RNAi greatly attenuates cell surface expression of TFII-I. In conclusion, we demonstrate that not only does cell surface GRP78 regulate apoptosis, but it also regulates Ca2+ homeostasis by controlling cell surface localization of TFII-I. J. Cell. Biochem. 112: 1685–1695, 2011. © 2011 Wiley-Liss, Inc.
Abbreviations used:
α2M*, the activated and receptor-recognized forms of the plasma proteinase inhibitor α2-macroglobulin; IP3, inositol 1,4,5-trisphosphate; DAG, diacylglycerol; SOC, store-activated calcium channels; SOCE, entry by these channels; PLC, phospholipase C; ECF, enhanced chemifluourescence; ACE, agonist-induced calcium entry; [Ca2+]i, intracellular free Ca2+.
TFII-I is a transcription factor which regulates cellular proliferation and apoptosis. Many extracellular signals mediated through cell surface receptors enhance tyrosine phosphorylation of TFII-I thus allowing TFII-I to function as a signaling molecule for the activation of the c-fos promoter by integrating signals from both tyrosine kinases and MAPK pathways [Parker et al., 2001; Roy, 2007; Misra et al., 2009b]. Activated TFII-I is also an important regulator in the endoplasmic reticulum (ER) stress response pathway [Parker et al., 2001; Desgranges et al., 2005; Hong et al., 2005]. During ER stress, TFII-I is recruited to multiple copies of the ER stress response element (ERSE) of the GRP78 promoter, upregulating this primary regulator of the ER stress response pathway [Hong et al., 2005]. Maximal stimulation of ERSE by ATF6 requires its interaction with TFII-I and binding to the conserved GGC sequence motif. TFII-I also modulates transcriptional upregulation of cyclin D1 and enhances cycling of cells and proliferation [Ashworth and Roy, 2007]. In addition to its function as a transcription factor, cytosolic TFII-I regulates calcium homeostasis by modulating agonist-induced extracellular Ca2+ entry [Patterson et al., 2002; Caraveo et al., 2006].
Cancer of the prostate is the most commonly diagnosed malignancy of men [Jemal et al., 2002]. In the development of prostate cancer, dysregulation of cell growth often is accompanied by acquisition of androgen-independence, a poor prognostic indicator [Heinlein and Chang, 2004; Montano and Djamgoz, 2004]. Growth factors play a role in the progression of androgen-independent prostate cancer by binding to their cognate receptors and activating mitogenic cellular responses. GRP78 is constitutively expressed but when its transcriptional upregulation occurs, a small fraction may be expressed on the cell's surface [Misra et al., 2002, 2004]. We have shown that binding of activated forms of the plasma proteinase inhibitor α2-macroglobulin (α2M*) to cells expressing cell surface GRP78 results in the activation of the Ras/MAPK, PI 3-kinase, and PAK-2-dependent signaling pathways [Misra et al., 1993, 1994, 1995, 1997, 2002, 2004; Misra and Pizzo, 1998; Asplin et al., 2000]. Down regulation of GRP78 signaling either by RNAi or by pretreating cells with antibodies directed against the COOH-terminal domain of GRP78 (anti-CTD antibody) nearly abolish these effects of α2M* in 1-LN and DU-145 prostate cancer cells and A375 melanoma cells, but not in PC-3 prostate cancer cells [Misra et al., 2009a; Misra and Pizzo, 2010a,b]. By radioligand binding assays, PC-3 cells express negligible GRP78 on the cell surface [Misra et al., 2009a; Misra and Pizzo, 2010a,b]. Anti-CTD antibody activates pro-apoptotic signaling, including upregulating of p53 [Misra et al., 2010]. About 70% human prostate cancers express high levels of GRP78, which is associated with recurrence, development of castration resistance, and poor survival [Hendershot, 2004; Li and Lee, 2006; Ni and Lee, 2007; Wang et al., 2009]. In addition to ER stress, GRP78 may be upregulated through other ER-stress independent mechanisms during tumor growth, leading to survival advantages to these tumors [Hendershot, 2004; Li and Lee, 2006; Ni and Lee, 2007; Wang et al., 2009]. In a recent report we showed that ligation of cell surface GRP78 in 1-LN prostate cancer cells with α2M* caused transcriptional and translational upregulation of TFII-I [Misra et al., 2009b]. Under these conditions, the synthesis of GRP78 was also upregulated two- to threefold. This upregulation was greatly reduced by silencing TFII-I gene expression.
GRP78 is involved in many cellular processes which include regulation of calcium homeostasis [Lievremont et al., 1997]. Binding affinity of GRP78 for Ca2+ to paired anionic residues is low, but over expression of GRP78 elevates the buffering capacity of Ca2+ and decreases apoptotic cell death [Liu et al., 1998]. Stimulation of various cell types with numerous growth factors results in IP-3-mediated release of Ca2+ from intracellular stores in the ER lumen and Ca2+ influx across the plasma membrane [Berridge et al., 2000; Parekh and Putney, 2005]. This influx of Ca2+ is essential for refilling the ER stores and for many processes involved in regulating cell proliferation, survival, and cell death [Berridge et al., 2000; Parekh and Putney, 2005]. In an earlier publication, we demonstrated that α2M* upregulates TFII-I which then binds to the GRP78 promoter and enhances GRP78 expression [Misra et al., 2009b]. We thus hypothesized that TFII-I, an upstream transcriptional regulator of GRP78, would exert its cell proliferative effects under ER stress conditions via activating cell surface GRP78 anti-apoptotic signaling. We have tested this hypothesis by down regulating the expression of TFII-I by RNAi in 1-LN prostate cancer cells stimulated with α2M*, a physiological agonist of GRP78. We report that under these conditions, that down regulation of TFII-I results in inhibition of its cell surface expression and triggers the onset of apoptotic signaling as evidenced by a significant reduction in levels of anti-apoptotic and prosurvival Bcl-2, cyclin D1, and GRP78 proteins was observed. In contrast, levels of pro-apoptotic proteins p53, p27, Bax, and Bak were significantly increased. Silencing the expression of TFII-I also activated agonist-induced Ca2+ entry in these cells.
MATERIALS AND METHODS
Materials
α2M* was prepared as previously described [Misra et al., 1997]. Culture media were purchased from Invitrogen (Carlsbad, CA). Rabbit antibodies against TFII-I, TRPC3, Bcl-2, and Bax were procured from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibodies against PLCγ1, phospho-PLCγ1, Bak, p53, p21, p27, and caveolin were from Cell Signaling Technology (Danvers, MA). Anti-actin antibodies were from Sigma–Aldrich (St. Louis, MO). Antibodies against MTJ1 were raised in rabbits against the sequence beginning at residue 105, NH2-LVAIYEVLKVDERRQRYVDVL-COOH, of MTJ1 (Swiss-Prot primary accession no. Q61712) (Genemed Synthesis, San Antonio, TX) [Misra and Pizzo, 2008]. Other reagents of the highest purity used in this study were procured locally. Antibodies against the COOH-terminal domain of GRP78 (anti-CTD antibody) were purchased from Stressgen (BC).
Cell Culture
The effect of α2M* on cellular proliferation depends on cell surface expression of GRP78, therefore, in the current study we have used 1-LN and DU-145 prostate cancer cells which express high levels of GRP78 on their cell surface [Misra et al., 1993, 1994, 1995, 1997, 2002, 2004, 2009a, 2010; Misra and Pizzo, 1998, 2010a,b; Asplin et al., 2000]. This highly metastatic 1-LN prostate cancer cell line is derived from the less metastatic PC-3 cells and was a kind gift of Dr. Philip Walther (Duke University Medical Center, Durham, NC). These cells were grown in multi-well plates in RPMI 1640 medium containing 10% FBS, 2 mM glutamine, 12.5 U/ml penicillin, 6.5 µg/ml streptomycin, and 10 nM insulin in a humidified CO2 (5%) incubator at 37°C. When cells reached 90% confluency, the media was aspirated, a volume of fresh media was added and cells were used for experiments described in the following sections.
Determining the Effect of Silencing the Expression of TFII-I Gene by RNAi on Protein Levels of GRP78, Bcl-2, Cyclin D1, p53, p27, Bax and Bak in 1-LN, and DU-145 Cells Stimulated With α2M* by Western Blotting
Silencing the expression of TFII-I gene by RNAi in 1-LN and DU-145 prostate cancer cells was done as described previously [Misra et al., 2009b]. Briefly, the sense (5′-UUG AAA GGA AAU AUG CUC AAC-3′) and the anti-sense (5′-UGA GCA AUA UUC CUU UCA AAC-3) oligonucleotides against the target homologous gene sequence nucleotides (5′-GTT TGA AAC GAA ATA TGC TCA-3) (Swiss Prot Entry Name GTF2I Human Primary Accession number P78347) were synthesized and annealed by Ambion (Austin, TX). These cells were grown in six-well plates (3 × 105 cells/well) and transfected with TFII-I dsRNA (50 nM/48 h) or with scrambled dsRNA (50 nM/48 h) as described previously. The magnitude of transfection was routinely determined by estimating mRNA and protein levels of TFII-I by PCR and Western blotting, respectively, as has been described previously. Silencing the expression of TFII-I gene caused a 60–70% reduction in their mRNA and protein levels [Misra et al., 2009b]. Forty-eight hours post-transfection, prostate cancer cells were stimulated with buffer or with α2M* (50 pM/25 min). The reaction was terminated by aspirating the medium and adding a volume of lysis buffer “A” containing 50 mM, Tris–HCl (pH 7.4), 120 mM NaCl, 1% Nonidet p-40, 25 mM sodium fluoride, 1 mM sodium pyrophosphate, 0.1 mM sodium orthovanadate, 1 mM PMSF, 1 mM benzamidine, and 20 µg/ml leupeptin. The cells were lysed over ice for 15 min, scraped into tubes, cell debris removed by centrifugation at 800g for 5 min at 4°C, and protein contents of lysates determined [Bradford, 1976]. An equal amount of lysate protein was electrophoresed on 10 or 12.5% acrylamide gels and proteins transferred to PVDF membranes, which were immunoblotted with antibodies specific for GRP78, Bcl-2, cyclin D1, p53, p27, Bax, and Bak. The immunoblots were reprobed for the protein loading control actin. The specificity of antibodies employed was assessed by reactivity of the blotted antigen with nonimmune serum. Under these conditions no reactivity was observed. The detection and quantification of immunoblots was performed by ECF and phosphorimaging on a Storm phosphorimager® (GE Healthcare, Piscataway, NJ).
Measurement of Bax Expression in 1-LN Cells by Flow Cytometry
α2M*-stimulated cells were washed twice with 8 ml Hanks' Balanced Salt Solution. Staining with antibodies was performed at 4°C. Cells were fixed at 4 × 107 cells/ml in 250 µl Cytofix/Cytoperm solution (BD Biosciences, San Jose, CA) for 20 min at 4°C. Washing and staining were then performed in Perm/Wash buffer (BD Biosciences), with 30 min incubation for both primary and secondary antibodies. Internal staining was performed with Bax (SC-493) antibody (Santa Cruz Biotechnology) or an isotype control (Sigma) at a concentration of 1 µg/106 cells for 30 min. After two washes with Perm/Wash buffer, cells were incubated with FITC-labeled goat anti-rabbit IgG (ABD Serotec, Raleigh, NC) at a concentration of 1 µg/106 cells for 30 min. Cells were washed, then fixed in 1% formalin (Sigma Chemical Co., St. Louis, MO). Samples were analyzed by the Guava EasyCyte Plus system (Millipore, Billerica, MA) collecting 10,000 events per sample.
Assay of DNA Fragmentation in 1-LN Cells Transfected With TFII-I dsRNA and Treated With α2M*
The measurement of cytoplasmic histone-associated DNA fragments (mononucleosomes and oligonucleosomes) after treatment was performed with a Cell Death Detection Elisa kit (Roche). Details of 1-LN cell transfection with dsTFII-I RNA and treatment with α2M* were the same as described in the preceding sections. DNA fragmentation under these treatments was measured according to the manufacturer's instructions. The DNA fragmentation is expressed as the specific enrichment of cytosol with mononucleosomes and oligonucleosomes [Misra et al., 2009a].
Measurements of Intracellular Ca2+
Changes in levels of intracellular Ca2+ in 1-LN and DU-145 cells were determined as previously described [Misra et al., 1993, 1994, 1995, 1997, 2002, 2004; Misra and Pizzo, 1998; Asplin et al., 2000]. Briefly, 1-LN cells incubated overnight in the above RPMI medium were plated at a cell density of 3.0 × 105 on glass coverslips kept in 35 mm Petri dish, and incubated for 3 h at 37°C in a humidified CO2 (5%) incubator. The cells were transfected with dsTFII-I RNA as described above. Forty-eight hours post-transfection, Fura-2/AM (2.5 µM) was added to each dish and dishes were incubated at room temperature for 30 min in the dark. The monolayers on glass coverslips were washed twice with Hanks' Balanced Salt Solution containing 75 µM Ca2+, mounted on fluorescent microscope stage in this buffer, and changes in intracellular Ca2+ consequent to the addition of α2M* were measured by digital video imaging employing a Carl Zeiss, Inc. (Thornwood, NY) model IN35 microscope with a 100× NA 1.4 objective (Nikon, Inc., Garden City, NY). Routinely, excitation intensity was attenuated 100–1,000-fold before reaching the cell and background from images were obtained. Intracellular free Ca2+ ([Ca2+]i) was measured by subtracting the background from images on a pixel basis. To obtain [Ca2+]i for an individual cell, the mean value of the pixel ratio for the cells was compared with values obtained with the same equipment using Fura-2 containing EGTA-Ca2+ buffers.
Western Blotting of TFII-I, TRPC3, and p-PLCγ1 in 1-LN and DU-145 Cells Treated With α2M*
These cancer cells in six-well plates (3 × 106 cells/well) were treated with α2M* (50 pM) and incubated for various periods of time as above. The reaction was terminated by adding a volume of lysis buffer “A.” The cells were lysed for 10 min over ice, scraped into tubes, and centrifuged at 800g for 5 min at 4°C. Supernatants were collected, their protein contents determined [Bradford, 1976], and equal amounts of protein electrophoresed according to Laemmli (10% gel). The immunoblotting of membranes with antibodies specific for p-PLCγ1, TFII-I, and TRPC3 was performed according to the manufacturer's instructions. The specificity of the antibodies was assessed by reactivity of the blotted antigens with nonimmune serum. Under these conditions, no reactivity was observed. The detection and quantification of immunoblots was performed by ECF and phosphorimaging. The membranes were reprobed for the protein loading control actin or PLCγ1.
Determination of Co-Localization of TFII-I With TRPC3, p-PLCγ1, PLCγ1, GRP78, MTJ1, and Caveolin in Plasma Membranes of 1-LN and DU-145 Prostate Cancer Cells
These cells incubated overnight in six-well plates (3 × 106 cells/well) as above were stimulated with α2M* (50 pM/25 min). The reactions were stopped by aspirating the medium, and a volume of buffer containing 20 mM Tris–HCl (pH 7.4), 10 mM KCl, 2 mM MgCl2, 1 mM PMSF, 20 µg/ml leupeptin, 0.3 mM CaCl2, 1 mM NaF, and 1 mM sodium orthovanadate was added. Cells were allowed to swell for 10 min over ice, followed by addition of three volumes of “buffer B” containing 50 mM Tris–HCl (pH 7.4), 25 mM KCl, 5 mM MgCl2, 0.25 M sucrose, 1 mM PMSF, 20 µg/ml leupeptin, 0.2 mM EGTA, 0.2 mM CaCl2, 1 mM NaF, 1 mM sodium orthovanadate, and 100 mM benzamidine. The plasma membrane-enriched fraction of cell homogenates was prepared as described previously [Misra and Pizzo, 1999, 2002; Misra et al., 2006]. Briefly, the cells were scraped into chilled glass tubes and homogenized in a Potter–Elvehjem homogenizer by 15 up-and-down strokes at 4°C. The homogenates were centrifuged for 10 min at 800g at 4°C. The supernatant was carefully removed and layered on a 30–70% sucrose gradient and centrifuged at 200,000g for 60 min at 4°C. The membrane-enriched fraction at the sucrose interphase was collected and pelleted at 200,000g, and the pellet containing plasma membrane was suspended in a volume of “buffer B.” The purity of plasma membrane enriched fraction was evaluated by electron microscopy as described previously [Misra and Pizzo, 1999, 2002, 2006]. The pellet was lysed in a volume of lysis buffer “A” and membrane lysates containing equal amount of protein [Bradford, 1976] and protein A agarose were immunoprecipitated with TFII-I antibodies (1:50) overnight at 4°C. The immunoprecipitates were washed thrice with lysis buffer “A” by centrifugation at 2,000 rpm for 5 min at 4°C. A volume of 4× sample buffer was added and the tube boiled for 5 min, centrifuged and supernatant electrophoresed as above, protein bands on the gel transferred to membrane and membranes immunoblotted with antibodies against TFII-I, p-PLCγ1, TRPC3, GRP78, MTJ1, and caveolin, respectively. The detection and quantification of immunoblots was performed by ECF and phosphorimaging.
Determination of Cell Surface Expression of TFII-I in 1-LN Prostate Cancer Cells Transfected With dsRNA Directed Against TFII-I, MTJ1, or GRP78
Cell surface expression of TFII-I in 1-LN cells was determined by “on cell Western” as previously described [Misra et al., 2009a]. Silencing the expression of TFII-I gene was done as described in preceding sections. For silencing expression of MTJ1 [Misra and Pizzo, 2008] or GRP78 genes [Misra et al., 2004, 2005, 2006], RNAi was performed as described previously. For silencing MTJ1 gene expression, the sense (5′-AAG ACA AGC ACC AGA AUG GTT-3′) and the anti-sense (5′-CCA UUC UGG UGC UUG UCU UTT-3′) oligonucleotides against the target homologous gene sequence nucleotide (5′-AAA AGA CAA GCA CCA GAA TGG-3′) (Swiss Prot Primary accession number Q61712) were chemically synthesized and annealed by Ambion. For silencing expression of the GRP78 gene, the sense (5′-GAA UAA AAU AAC AAU AAC ATT-3′) and the anti-sense (5′-UGU UAU UGU UAU UUU AUU CTT-3′) oligonucleotides against the target homologous gene sequence nucleotide (5′-AAG AAT AAA ATA ACA ATA ACA-3′) (Swiss-Prot Primary accession number P11021) were chemically synthesized by Ambion. 1-LN cells were grown in 48-well plates (3 × 105 cells/well). Other details of transfection with TFII-I dsRNA (50 nM/48 h), MTJ1 dsRNA (50 nM/48 h), or GRP78 dsRNA (50 nM/48 h) and evaluation of the magnitude of transfection by estimating mRNA levels of TFII-I, MTJ1, or GRP78 by qualitative PCR and their protein levels by Western blotting were as described previously [Misra et al., 2004, 2005, 2006, 2009a]. Silencing the expression of these genes caused a 60–70% reduction in their mRNA and protein levels. Forty-eight hours post-transfection, cells transfected were stimulated with buffer or with α2M* (50 pM/25 min) and incubated as above. The reactions were terminated by aspirating the medium and monolayers were washed twice with cold PBS. Cells were fixed by adding a volume of 2% formaldehyde in PBS and cells were incubated for 20 min at room temperature. Then the formaldehyde was aspirated and cells were washed twice with PBS containing 0.05% Tween 20 (PBST). A volume of blocking buffer containing 3% bovine serum albumin and 5% nonimmune goat serum in PBST was added to each well, and cells were incubated at room temperature with gentle shaking for 2 h. The blocking buffer was removed, and cells were incubated with TFII-I antibody (1:50) in PBST at 4°C overnight with gentle rotation. The reaction was terminated by aspirating the buffer and washing the cells thrice with PBST. The cells were incubated with the IRDye-800 DX-conjugated affinity-purified anti-rabbit IgG (goat) in PBST for 60 min with shaking at room temperature. Cells were washed thrice with PBST and once with PBS, dried, and imaged. Cell surface expression of TFII-I was quantified on an Odyssey® Infared Imaging System (LiCOR®, Lincoln, NE) according to the manufacturer's instructions [Misra et al., 2009a]. The specificity of the antibodies employed was assessed by reactivity of the blotted antigen with rabbit IgG. Under these conditions, the reactivity of IgG with blotted antigen was comparable to or lower than the buffer-treated cells (data not shown).
RESULTS
Regulation of Anti-Apoptotic Bcl-2 and Pro-apoptotic Bax and Bak Expression by TFII-I in α2M*-Stimulated 1-LN and DU-145 Prostate Cancer Cells
Evading pro-apoptotic cellular signals is one of the hallmarks of human malignancies, and over expression of anti-apoptotic Bcl-2 family members or dysregulation of BH3-only proteins is commonly observed in these cells [Orrenius et al., 2003; Kim et al., 2008; Pinton et al., 2008; Szegedi et al., 2009]. Apoptotic cell death is triggered by extrinsic, receptor-mediated or intrinsic mitochondria-mediated signaling pathways that induce death-associated proteolytic and/or nucleolytic activities [Orrenius et al., 2003; Kim et al., 2008; Pinton et al., 2008; Szegedi et al., 2009]. In response to apoptotic stimuli, Bax translocates to mitochondrial outer membrane, where Bax and Bak homodimerize into pores facilitating the release of cytochrome c from the mitochondrial intramembrane space into the cytosol [Scorrano et al., 2003; Oakes et al., 2005]. Bcl-2 protects mitochondrial integrity by complexing with Bax and Bak. Bcl-2, Bax, and Bak also co-localize in the ER and regulate Ca2+ homeostasis [Orrenius et al., 2003; Scorrano et al., 2003]. In our earlier reports we have shown that ligation of cell surface GRP78 in 1-LN prostate cancer cells with α2M* upregulated TFII-I and GRP78 expression and activated pro-proliferative and anti-apoptotic signaling [Misra et al., 2009b]. Down regulation of GRP78 either by RNAi or anti-CTD antibody directed against GRP78 reversed this pattern [Misra et al., 2002, 2004, 2005, 2006, 2009a, 2010; Misra and Pizzo, 2010a,b]. To discern the involvement of TFII-I in GRP78-induced down regulation of apoptosis we first examined the effect of down regulating TFII-I on the expression of Bcl-2, Bax, and Bak in 1-LN and DU-145 cancer cells stimulated with α2M*. In untransfected cells, as expected, α2M* induced an approximately twofold increase in Bcl-2 (Fig. 1A) without affecting Bax or Bak expression (Fig. 1A,B). In contrast, transfection of cells with TFII-I dsRNA, greatly attentuated α2M*-induced expression of Bcl-2 in both 1-LN and DU-145 cancer cells (Fig. 1A), and caused an approximately two- to threefold increase of Bax and Bak expression (Fig. 1A,B). Furthermore, cells transfected with TFII-I dsRNA showed significant DNA fragmentation, indicative of apoptosis, under our experimental conditions (Fig. 2).
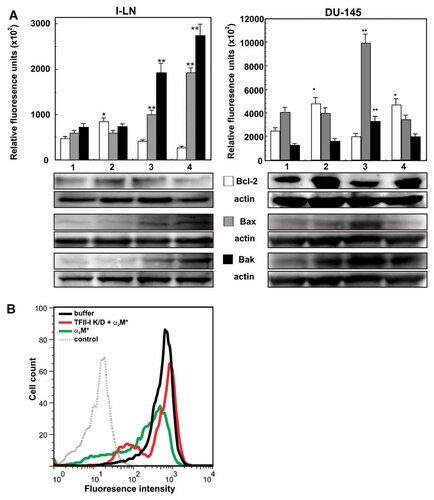
Down regulation of TFII-I expression by RNAi induces upregulation of pro-apoptotic proteins and down regulation of anti-apoptotic proteins in 1-LN and DU-145 cells stimulated with α2M*. Panel A: The changes in the expression of Bcl-2 (□), Bax ( ), and Bak (▪) proteins in cells treated with: (1) lipofectamine + buffer; (2) lipofectamine + α2M*; (3) TFII-I dsRNA, and (4) TFII-I dsRNA + α2M* are shown in relative fluorescence units normalized to actin and are the mean ± SE from three to four individual experiments. Changes in the expression of Bcl-2 (□) Bax (
), and Bak (▪) proteins in cells treated with: (1) lipofectamine + buffer; (2) lipofectamine + α2M*; (3) TFII-I dsRNA, and (4) TFII-I dsRNA + α2M* are shown in relative fluorescence units normalized to actin and are the mean ± SE from three to four individual experiments. Changes in the expression of Bcl-2 (□) Bax ( ) and Bak (▪) in DU-145 cells treated with: (1) lipofectamine + buffer; (2) lipofectamine + α2M*; (3) TFII dsRNA + α2M*; and (4) scrambled dsRNA + α2M* are shown in relative fluorescence units normalized to actin and are the mean ± SE from three individual experiments. A representative immunoblot of Bcl-2, Bax, and Bak along with their respective protein loading control actin is shown below the respective bar diagrams. Values significantly different from buffer-treated cells at the 5% levels are marked with asterisk (*) and from α2M*-treated cells at 5% level are marked with two asterisks (**). Panel B: Detection of Bax in 1-LN cells. FACS analysis was performed on 1-LN cells stained with either Bax-specific rabbit polyclonal antibody or nonimmune isotype control. Bax staining was detected, as indicated by the shift relative to isotype control. Knockdown of TFII-I was associated with increased Bax expression in α2M*-stimulated cells relative to untreated cells or cells stimulated in the absence of TFII-I knockdown. Gray dotted line: isotype control. Green line: α2M*-stimulated. Red line: TFII-I knockdown + α2M*-stimulated. Black line: unstimulated.
) and Bak (▪) in DU-145 cells treated with: (1) lipofectamine + buffer; (2) lipofectamine + α2M*; (3) TFII dsRNA + α2M*; and (4) scrambled dsRNA + α2M* are shown in relative fluorescence units normalized to actin and are the mean ± SE from three individual experiments. A representative immunoblot of Bcl-2, Bax, and Bak along with their respective protein loading control actin is shown below the respective bar diagrams. Values significantly different from buffer-treated cells at the 5% levels are marked with asterisk (*) and from α2M*-treated cells at 5% level are marked with two asterisks (**). Panel B: Detection of Bax in 1-LN cells. FACS analysis was performed on 1-LN cells stained with either Bax-specific rabbit polyclonal antibody or nonimmune isotype control. Bax staining was detected, as indicated by the shift relative to isotype control. Knockdown of TFII-I was associated with increased Bax expression in α2M*-stimulated cells relative to untreated cells or cells stimulated in the absence of TFII-I knockdown. Gray dotted line: isotype control. Green line: α2M*-stimulated. Red line: TFII-I knockdown + α2M*-stimulated. Black line: unstimulated.
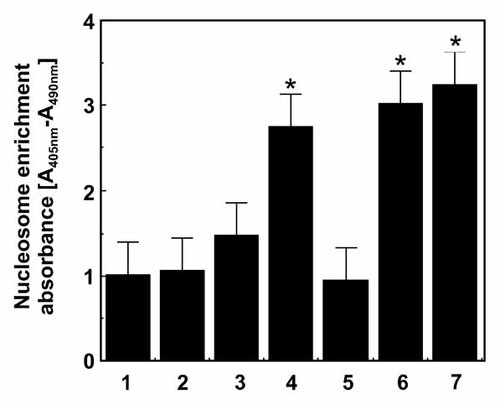
Down regulation of TFII-I expression by RNAi triggers apoptosis as measured by DNA fragmentation. See “Materials and Methods” Section for details. The bars are: (1) lipofectamine + buffer; (2) lipofectamine + α2M*; (3) TFII-I dsRNA; (4) TFII-I dsRNA + α2M*; (5) scrambled dsRNA control; (6) staurosporine, 5 µM; and (7) positive control as supplied by the manufacturer. Changes in nucleosome enrichment is shown as the mean ± SE from two experiments performed in quadruplicate. Values significantly different at 5% level from controls are marked with asterisk (*).
Transfection of Prostate Cancer Cells With TFII-I dsRNA Upregulates p53 and Down Regulates Cyclin D1
TFII-I modulates transcriptional upregulation of cyclin D1 by binding to its promoter, thus regulating cell cycle progression and proliferation [Desgranges et al., 2005; Hong et al., 2005]. Down regulation of TFII-I by dsRNA in both 1-LN and DU-145 prostate cancer cells stimulated with α2M* suppresses GRP78 and cyclin D1 expression and upregulates the pro-apoptotic proteins p53 and p27KIP (Fig. 3).
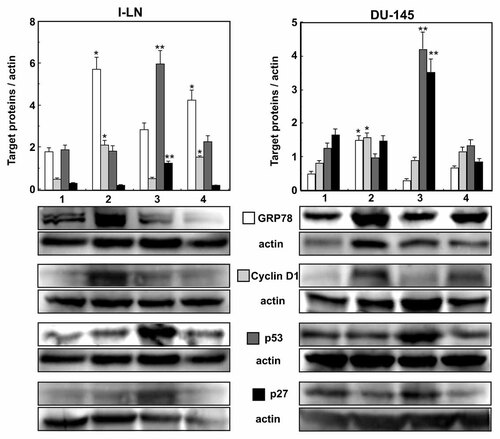
Effect of silencing TFII-I expression by dsRNAi on expression of GRP78, cyclin D1, p53, and p27kip in 1-LN and DU-145 cancer cells stimulated with α2M*. Changes in the levels of expression of GRP78 (□), cyclin D1 ( ), p53 (
), p53 ( ), and p27kip (▪) are shown as ratios of target protein/actin and are mean ± SE from two experiments done in duplicate. A representative immunoblot of GRP78, cyclin D1, p53, and p27kip along with their respective protein loading control actin is shown below the respective bar diagrams. Values significantly different from buffer treated cells at the 5% level are marked with asterisk (*) and from α2M*-treated cells at 5% level are marked with two asterisks (**). The lanes in both graphs: are (1) lipofectamine + buffer; (2) lipofectamine + α2M* (50 pM); (3) TFII-I dsRNA + α2M*, and (4) scrambled dsRNA + α2M*.
), and p27kip (▪) are shown as ratios of target protein/actin and are mean ± SE from two experiments done in duplicate. A representative immunoblot of GRP78, cyclin D1, p53, and p27kip along with their respective protein loading control actin is shown below the respective bar diagrams. Values significantly different from buffer treated cells at the 5% level are marked with asterisk (*) and from α2M*-treated cells at 5% level are marked with two asterisks (**). The lanes in both graphs: are (1) lipofectamine + buffer; (2) lipofectamine + α2M* (50 pM); (3) TFII-I dsRNA + α2M*, and (4) scrambled dsRNA + α2M*.
TFII-I Regulates Agonist-Induced Calcium Influx in Prostate Cancer Cells Stimulated With α2M*
Ca2+ is central to both proliferation and cell death phenomena, functioning as a major signaling agent with spatial localization, magnitude, and temporal characteristics of Ca2+ signals ultimately determining cell fate [Orrenius et al., 2003; Kim et al., 2008; Pinton et al., 2008; Szegedi et al., 2009]. Ca2+-dependent signaling mechanisms are frequently upregulated in cancer cells [Orrenius et al., 2003; Kim et al., 2008; Pinton et al., 2008; Szegedi et al., 2009]. Many apoptotic stimuli alter cytosolic Ca2+ levels, its storage in the ER and/or uptake into mitochondria. Cell stimulation with various agonists including α2M* causes PLCγ activation, hydrolysis of PIP2, and IP3-mediated release of Ca2+ from ER Ca2+ stores [Misra et al., 1993; Asplin et al., 2000; Berridge et al., 2000; Parekh and Putney, 2005]. Emptying of ER stores triggers store operated calcium entry (SOCE) and Ca2+ influx across the plasma membrane [Berridge et al., 2000; Parekh and Putney, 2008]. Down regulation of SOCE is involved in prostate cancer progression to androgen independence and apoptotic resistance [Li et al., 2008]. To initiate apoptosis in prostate cancer, sustained SOCE is required in addition to IP3-mediated Ca2+ release from ER stores. Over expression of Bax synergizes with Ca2+ to initiate apoptosis in prostate cancer cells [Li et al., 2008]. Down regulation of endogenous TFII-I by RNAi causes induction of apoptosis in α2M*-stimulated 1-LN prostate cancer cells (Figs. 1-3). In view of the role of SOCE in the regulation of cell survival in prostate cancer cells [Li et al., 2008], we next determined the effect of TFII-I knockdown on intracellular Ca2+ in 1-LN prostate cancer cells stimulated with α2M* (Fig. 4). Addition of α2M* to untransfected cells caused an approximately twofold transient increase in IP3-mediated Ca2+ release from ER stores (Fig. 4). To determine the functioning of SOCE in untransfected cells, we added 1 mM Ca2+ at 6 min after α2M* addition (Fig. 4). This treatment caused no further increase in intracellular Ca2+, showing thereby SOCE was down regulated or aberrantly functioning (Fig. 4). As in untransfected cells, addition of α2M* to 1-LN cells transfected with TFII-I dsRNA also caused a similar increase in IP3-mediated Ca2+ release from ER Ca2+ stores (Fig. 4). However, unlike untransfected cells addition of 1 mM Ca2+ 6 min after α2M* addition to transfected cells caused an increase in Ca2+ influx (Fig. 4). These results suggest that TFII-I expression prevents agonist-induced Ca2+ influx. Transfection with TFII-I dsRNA reverses this effect, potentially contributing to induction of apoptosis.
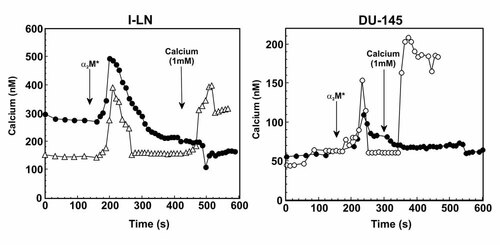
Agonist-induced Ca2+ entry in 1-LN and DU-145 cells stimulated with α2M* before and after transfection with dsTFII-I RNA. See “Materials and Methods” Section for details. Changes in [Ca2+]i in cells treated with α2M* (●) or TFII-I dsRNA + α2M* (Δ). Representative graphs showing changes in [Ca2+]i in a single 1-LN or DU-145 cell from three experiments employing 20–25 cells each is shown. The arrows indicate the time point of addition of α2M* and Ca2+.
Effect of Down Regulating TFII-I Gene Expression by RNAi on the Assembly of Signaling Complexes Involved in SOCE in Prostate Cancer Cells Stimulated With α2M*
In addition to its transcriptional role, cytosolic TFII-I inhibits agonist-induced Ca2+ entry (ACE) in several cell types stimulated with growth factors [Patterson et al., 2002; Caraveo et al., 2006]. As shown above, silencing the expression of TFII-I promotes ACE (Fig. 4). TFII-I binds to PLCγ, promoting TFII-I translocation to the cell surface. TFII-I modulation of Ca2+ entry is hypothesized to occur through competition with TRPC3 for binding to PLCγ, which is required for ACE. In the next series of experiments we analyzed the plausible mechanism(s) by which endogenous TFII-I is involved in modulating ACE in 1-LN cells stimulated with α2M*. Stimulation of 1-LN cells with various concentrations of α2M* caused a two- to threefold increase in the expression of TFII-I, PLCγ1, and TRPC3 (Fig. 5). The α2M*-induced transient increase in PLCγ1 occurred prior to the increase in TFII-I and TRPC3 but declined at higher expression of TFII-I (Fig. 5).
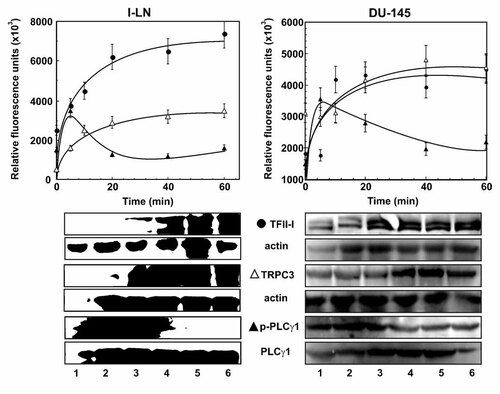
Binding of α2M* to cell surface GRP78 upregulates the expression of levels of p-PLCγ1, TFII-I, and TRPC3 in 1-LN and DU-145 prostate cancer cells. Changes with time in levels of p-PLCγ1 (▴); TFII-I (●); and TRPC3 (Δ) are expressed in relative fluorescence units and are the mean ± SE from three to four individual experiments. Representative immunoblots of three experiments along with their respective loading controls is shown below the respective graphs.
TRPC3, p-PLCγ and Caveolin-1 Co-Immunoprecipitate With TFII-I From the Plasma Membranes of Stimulated 1-LN and DU-145 Cells
TRPC channels are assembled into lipid rafts/caveolin microdomains where they interact with caveolin [Pani et al., 2008; Pani and Singh, 2009]. This interaction is critical for channel assembly, function, and plasma membrane retention. In α2M*-stimulated both 1-LN and DU-145 cells p-PLCγ1, TRPC3, GRP78, MTJ1, and caveolin co-immunoprecipitated with TFII-I in plasma membrane lysates suggesting the assembly of SOC macromolecule complex or signalplex (Fig. 6A). Silencing TFII-I gene expression by RNAi reduced levels of TFII-I protein by about 70%, but the cell surface levels of TRPC3, and p-PLCγ1 either remained the same or were increased compared with untransfected cells (Fig. 6B). This would suggest that in untransfected 1-LN prostate cells, TFII-I prevents cell surface recruitment of TRPC3 by binding to PLCγ1. This interaction would also inhibit PLCγ1-mediated production of DAG, which is required for TRPC3 activation. TFII-I-induced inhibition of ACE, therefore, most likely involves increased TRPC3 activation and cell surface expression.
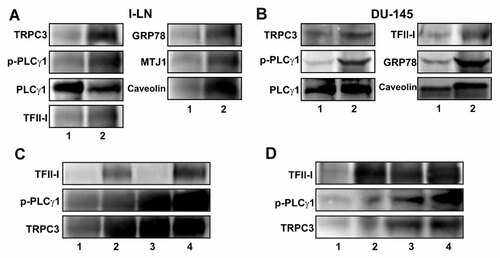
Co-localization of proteins with TFII-I in plasma membranes of 1-LN and DU-145 cancer cells. Panel A: Co-immunoprecipitation of TRPC3, p-PLCγ1, GRP78, MTJ1, and caveolin with TFII-I in plasma membranes of 1-LN and DU-145 cells stimulated with buffer (1) or α2M* (2). Panel B: Effect of silencing TFII-I gene expression by RNAi on TFII-I, p-PLCγ1, and TRPC3 in cell lysates of 1-LN and DU-145 cells stimulated with α2M*. The lanes in Panel B are: (1) buffer + lipofectamine; (2) α2M* + lipofectamine; (3) TFII-I dsRNA + α2M*; and (4) scrambled dsRNA + α2M*. See “Materials and Methods” Section for details of Panel A and B. A representative immunoblot of three experiments is shown in Panel A and B. A control rabbit IgG fraction resulted in minimal precipitation of the components studied (data not shown).
Silencing TFII-I, GRP78, or MTJ1 Gene Expression by RNAi Inhibits Cell Surface Localization of TFII-I
To further analyze regulation of plasma membrane association of TFII-I, we silenced TFII-I, GRP78, or MTJ1 expression. We then studied its cell surface localization by “on cell Western” in control versus α2M*-treated cells (Fig. 7). As expected, knockdown of TFII-I resulted in a significant reduction in cell surface localization of TFII-I of the stimulated cells (Fig. 7). More importantly, similar inhibition of cell surface expression of TFII-I was observed in 1-LN cells transfected with GRP78 dsRNA or MTJ1 dsRNA (Fig. 7). We have previously demonstrated that MTJ1 is essential for localization of GRP78 on the plasma membrane. Here we demonstrate that in addition to the previously reported TRPC3 and PLCγ, components of SOC complexes [Parekh and Putney, 2005], TFII-I in conjunction with GRP78 and MTJ1 is also involved in α2M*-induced ACE in 1-LN prostate cancer cells, regulating calcium homeostasis and thus cell proliferation and survival.
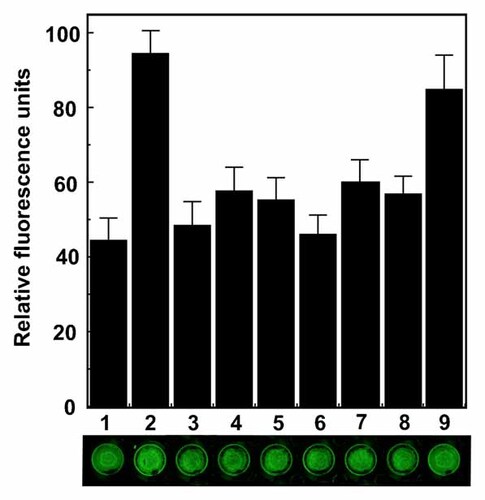
Cell surface expression of TFII-I in 1-LN cancer cells transfected with TFII-I dsRNA, MTJ1 dsRNA, and GRP78 dsRNA as determined by “on cell Western.” See “Materials and Methods” Section for details. The bars show the treatment with: (1) lipofectamine + buffer; (2) lipofectamine + α2M*; (3) TFII-I dsRNA, (4) TFII-I dsRNA + α2M*; (5) MTJ1 dsRNA; (6) MTJ1 dsRNA + α2M*; (7) GRP78 dsRNA; (8) GRP78 dsRNA + α2M*; and (9) scrambled dsRNA + α2M*. The changes in cell surface expression of TFII-I are shown in relative fluorescence units and are expressed as the mean ± SE from four to six individual experiments.
DISCUSSION
In this report, we show that cytosolic TFII-I regulates α2M*-induced survival of 1-LN and DU-145 prostate cancer cells by regulating the expression of anti- and pro-apoptotic proteins as well as α2M*-induced Ca2+ entry. The salient observations of this study are as follows: (1) Stimulation of these cancer cells with α2M* caused an approximately twofold increase in GRP78, Bcl-2, and cyclin D1 without affecting pro-apoptotic p53, p27kip, Bax, or Bak. Silencing TFII-I gene expression by RNAi, reversed α2M*-induced effects and induced DNA fragmentation. (2) α2M*-induced inhibition of ACE was reversed in cells transfected with TFII-I dsRNA. However, IP3-mediated Ca2+ release from the ER was comparable in both untransfected and transfected cells. (3) In α2M*-stimulated cells a profound increase in the p-PLCγ, TFII-I, and TRPC3 was observed. In plasma membranes, TFII-I co-immunoprecipitated with TRPC3, GRP78, MTJ1, and caveolin-1. Thus, in addition to its role in the transcriptional upregulation of various genes, cytosolic TFII-I controls cell proliferation, and survival by regulating the expression of anti- and pro-apoptotic proteins and agonist-induced calcium entry. These effects are mediated at least in part by cell surface GRP78, a physiological receptor for α2M*. The plausible mechanisms involved in the impairment of cellular proliferative and survival functions in TFII-I-down regulated 1-LN prostate cells stimulated with α2M* are as follows: (1) Down regulation of TFII-I causes decreased GRP78 expression, resulting in the deregulation of its cell survival functions and its Ca2+ regulatory capacity. (2) Down regulated cellular levels of TFII-I and GRP78 inhibit the expression of the anti-apoptotic protein Bcl-2 and increase expression of pro-apoptotic p53, p27, Bax, and Bak causing induction of apoptosis. (3) Reduced cellular levels of TFII-I limit its binding to PLCγ, thus freeing more PLCγ for binding to TRPC3 and the formation of functional SOC. This would also cause enhanced activation of PLCγ, making more DAG available for TRPC activation. The resulting increased levels of intracellular Ca2+ would also activate pro-apoptotic signaling pathways.
Over expression of GRP78 promotes tumor proliferation, metastasis, and drug resistance [Li and Lee, 2006; Ni and Lee, 2007]. Thus upregulation of GRP78 correlates with an aggressive malignant phenotype and poor prognosis post-treatment. Binding of α2M* cell surface GRP78 results in the activation of the Ras/MAPK, PI 3-kinase, and PAK2-dependent signaling pathways [Misra et al., 1993, 1994, 1995, 1997, 2002, 2004, 2005, 2006, 2009a, 2010; Misra and Pizzo, 1998, 1999, 2002, 2008, 2010a,b; Asplin et al., 2000]. Thus activated α2M* is a pro-proliferative, anti-apoptotic, and pro-migratory growth factor-like molecule. Down regulation of GRP78 expression either by RNAi or by pretreating cells with antibodies directed against the COOH-terminal domain of GRP78 nearly abolishes these effects of α2M* [Misra et al., 2009a, 2010; Misra and Pizzo, 2010a,b; Li and Lee, 2006]. In addition, binding of α2M* to GRP78 upregulates the synthesis and expression of GRP78 as well as that of TFII-I [Misra et al., 2009b], which causes further transcriptional upregulation of GRP78. TFII-I also promotes cell cycle progression, which may synergize with GRP78 to potentiate survival and pro-proliferative responses of cancer cells during cellular ER stress in a variety of pathophysiological condition [Hendershot, 2004; Li and Lee, 2006; Ni and Lee, 2007; Wang et al., 2009].
Cytosolic TFII-I plays a role in agonist-induced Ca2+ entry in several types of cells [Misra and Pizzo, 1999, 2002] stimulated with a variety of growth factors [Patterson et al., 2002; Caraveo et al., 2006]. Excess Ca2+ or perturbations of intracellular Ca2+ stores may lead to cytotoxic stress and cell death. By inhibiting ACE, TFII-I regulates Ca2+ homeostasis and protects prostate cancer cells from Ca2+ overload [Orrenius et al., 2003; Scorrano et al., 2003; Oakes et al., 2005; Kim et al., 2008; Li et al., 2008; Pinton et al., 2008; Szegedi et al., 2009]. Inhibition of extracellular Ca2+ influx also guards against mitochondrial uptake of Ca2+ from the cytosol, thus protecting mitochondrial integrity. In either cells untransfected or transfected with dsTFII-I RNA, α2M* caused IP3-mediated Ca2+ release from ER Ca2+ stores and presumably also increased levels of DAG, an activator of TRPC3 channels. However, entry of external Ca2+ after this initial Ca2+ release is inhibited in untransfected cells. The likely mechanism of the inhibition of ACE in untransfected cells is unavailability of TRPC3 channels on the cell surface due to TFII-I binding to PLCγ. Down regulating TFII-I inhibits competition between TFII-I and TRPC3 for PLCγ1, facilitating PLCγ1-mediated recruitment of TRPC3 to the cell surface and activation by DAG to allow Ca2+ entry.
TFII-I is an antagonist to agonist-induced Ca2+ entry in a variety of cells [Patterson et al., 2002; Caraveo et al., 2006]. In this report we have studied 1-LN prostate cancer cells stimulated with α2M* which binds to cell surface GRP78. We show that upregulation of endogenous TFII-I proteins by α2M* inhibits ACE and promotes cell proliferation, growth, and survival of these cells by regulating Ca2+ homeostasis in intracellular organelles [Orrenius et al., 2003; Scorrano et al., 2003; Oakes et al., 2005; Kim et al., 2008; Li et al., 2008; Pinton et al., 2008; Szegedi et al., 2009]. Stimulation also caused elevated levels of anti-apoptotic Bcl-2 protein and reduced levels of pro-apoptotic Bax and Bak proteins. Ca2+ can induce apoptosis via mitochondrial Ca2+ overload by upregulating Bim and disrupting mitochondrial integrity through insertion of Bax and Bak into mitochondria [Orrenius et al., 2003; Scorrano et al., 2003; Oakes et al., 2005; Kim et al., 2008; Pinton et al., 2008; Szegedi et al., 2009]. Over expression of Bcl-2 reduces ER Ca2+ stores and diminish Ca2+ entry after ER Ca2+ release. Over expression of Bax and Bak promotes Ca2+ mobilization from the ER to the mitochondria during apoptosis [Orrenius et al., 2003; Scorrano et al., 2003; Oakes et al., 2005; Kim et al., 2008; Pinton et al., 2008; Szegedi et al., 2009]. Ectopic and stable expression of a wild type TFII-I leads to enhanced cell cycle entry, cyclin D1 transcriptional upregulation and thus cell proliferation [Roy, 2007; Ashworth and Roy, 2007, 2009; Enkhmandakh et al., 2009]. The upregulation of p53 and transcription factor E2F1 [Rasek et al., 2008] and their contributions to apoptotic events is not ruled out in 1-LN cells transfected with TFII-I dsRNA. In B cells post-translational silencing of TFII-I upon stimulation with anti-IgM or TGFβ caused proliferation which was absent in control cells [Ashworth and Roy, 2009; Enkhmandakh et al., 2009]. Restoration of α2M*-induced influx of extracellular Ca2+ upon down regulating TFII-I protein appears to initiate pro-apoptotic events in 1-LN prostate cancer cells. In conclusion, we show that α2M* signaling through GRP78 plays a permissive role in inhibiting ACE through upregulation of TFII-I, thus protecting cells against Ca2+ overload. Once this homeostasis is perturbed by ACE, as seen in cells transfected with TFII-I dsRNA, Ca2+-mediated apoptotic events are initiated.
Down regulation of SOC is a general phenomenon during progression of androgen-independent prostate cancer [Haverstich et al., 2000; Li et al., 2008]. Over expression of Bcl-2 in LnCap prostate cancer cells converts these cells from an androgen-dependent to androgen-independent phenotype by reducing functioning of SOC [Li et al., 2008]. By contrast, Bax over expression activates SOC and apoptotic signaling [Li et al., 2008]. Proliferation in some cancer cell lines can be slowed or stopped at specific cell cycle points by removal of extracellular Ca2+ and drugs that block Ca2+ entry can impair metastases [Li et al., 2008].
In conclusion, we show that stimulation of castration-resistant 1-LN and DU-145 prostate cancer cells with α2M* causes transcriptional upregulation of TFII-I which in turn increases transcription of GRP78, a protein previously shown to promote cell proliferation and survival. Down regulation of TFII-I is associated with decreased cell surface expression of GRP78 and the induction of pro-apoptotic signaling. Thus TFII-I-GRP78 interactions play a critical role in regulating prostate cancer cell proliferation and survival by regulating ACE. The disruption of this TFII-I GRP78 synergy could be a powerful tool in arresting/inhibiting prostate cancer growth, survival, and metastases.




