PI3K and ERK/Nrf2 pathways are involved in oleanolic acid-induced heme oxygenase-1 expression in rat vascular smooth muscle cells†
The work was performed at Central laboratory, Southwest Hospital, Third Military Medical University, Chongqing City, PR China.
Abstract
Oleanolic acid (OA), a widely used plant-derived triterpenoid, has been shown to possess potent antiatherosclerotic effects, which may be associated with the induction of heme oxygenase-1 (HO-1). However, the underlying mechanisms involved in the effect of OA on HO-1 expression are unclear. In the current study, primary rat vascular smooth muscle cells (VSMCs) were exposed to OA and we found that it enhanced HO-1 expression in a concentration- and time-dependent manner, accompanied by increased HO-1 activity. VSMCs treated with OA exhibited activation of Akt, p38 and extracellular-signal-regulated kinase (ERK). Wortmannin (a PI3K inhibitor) and PD98059 (an ERK inhibitor) attenuated OA-induced HO-1 expression, whereas SB203580 (a p38 inhibitor) had no effect. The transcription factor NF-E2-related factor 2 (Nrf2) is a key regulator of HO-1 expression. OA treatment increased Nrf2 nuclear translocation, which was also inhibited by wortmannin and PD98059. Furthermore, transfection of VSMCs with the Nrf2 siRNA-expressing lentiviral vector decreased HO-1 expression induced by OA. Finally, pretreatment of VSMCs with OA remarkably reduced hydrogen peroxide-induced cell apoptotic death, and this effect was greatly attenuated in the presence of ZnPP (a HO-1 inhibitor), wortmannin or PD98059. Taken together, these results suggest that activation of Akt and ERK is required for OA-induced activation of Nrf2 followed by upregulation of HO-1 expression in VSMCs, which may confer an adaptive survival response in atherosclerosis. J. Cell. Biochem. 112: 1524–1531, 2011. © 2011 Wiley-Liss, Inc.
Oleanolic acid (OA) is a natural triterpenoid that occurs in many plant foods and some medicinal herbs [Liu, 1995] and has been used as an oral remedy for the treatment of acute and chronic liver disorders in China for many years. Previous pharmacological studies have shown that OA inhibits cancer progression and induces cancer cell apoptosis [Liu, 2005]. OA also has nerve-growth-factor potentiating activity and alleviates oxidative and inflammatory stress in PC12 cells [Li et al., 2003; Tsai and Yin, 2008]. In addition, accumulating evidence has demonstrated a significant vasoprotective effect of OA. For example, OA induces relaxation and activates endothelium-dependent release of nitric oxide [Rodriguez-Rodriguez et al., 2008]. In experimental animals, OA treatment can prevent the development of severe hypertension and atherosclerosis, and improve insulin resistance [Somova et al., 2003]. However, the underlying molecular mechanisms of such protective effects of OA on the vascular system are not fully understood.
A progressive development of oxidative stress is a hallmark of atherosclerosis and it is considered to be responsible for the migration, proliferation and apoptosis of vascular smooth muscle cells (VSMCs), which are vital for the pathogenesis of atherosclerosis and plaque rupture. Heme oxygenase-1 (HO-1) is a novel rate-limiting enzyme degrading heme to biliverdin, free iron, and carbon monoxide. HO-1 and its end products play a key role in protecting cells against oxidative injury. Unlike other HO isoforms, which are constitutively expressed, HO-1 is inducible and can be upregulated by a variety of stimuli, including heme, oxidative stress and some pharmaceutical agents, in various cell types [Deshane et al., 2005]. On the other hand, it has been reported that HO-1 may be involved in the pathophysiology of atherosclerosis and targeting HO-1 may be useful for vascular disease. For example, HO-1 is upregulated in atherosclerotic lesions [Wang et al., 1998]. Overexpression of HO-1 significantly attenuates the development of atherosclerosis in apoE-deficient mice, whereas the absence of HO-1 aggravates atherosclerotic lesion formation compared with wild-type mice [Juan et al., 2001; Yet et al., 2003]. Additionally, HO-1 plays an important role in maintaining vascular homeostasis by regulating migration, proliferation and apoptosis of VSMCs [Guan et al., 2008; Loboda et al., 2008]. Taken together, these findings indicate that HO-1 may be a likely molecule involved in the antiatherosclerotic effects of OA.
Several lines of evidence have demonstrated that multiple kinase signaling pathways are involved in natural compound-induced HO-1 expression. Kim et al. [2010] have shown that ERK and phosphatidylinositol 3-kinase (PI3K) are involved in the eckol induction of HO-1 expression. Hwang and Jeong [2008b] demonstrated the involvement of PI3K and p38 pathways in induction of HO-1 by kahweol in human dopaminergic neurons. In human hepatocytes, inhibition of p38 and ERK kinases can prevent the expression of HO-1 induced by quercetin [Yao et al., 2007]. Moreover, treatment of PC-3 cells with isothiocyanates strongly activates ERK and c-Jun N-terminal kinase (JNK), which induce the expression of HO-1 [Xu et al., 2006]. Therefore, signaling mechanisms involved in HO-1 activation may depend on cell types and the chemical inducers. The transcription factor NF-E2-related factor 2 (Nrf2) has been identified as transcriptionally regulating HO-1 expression [Lim et al., 2007; Kim et al., 2008; Minelli et al., 2009]. Under unstimulated conditions, Nrf2 is localized in the cytoplasm by protein Kelch-like ECH-associated protein 1 (Keap1). On stimulation, Nrf2 dissociates from Keap1, translocates into the nucleus and binds to the antioxidant response element (ARE) of the HO-1 promoter [Kobayashi and Yamamoto, 2005]. Although the molecular mechanisms are incompletely understood, previous studies have reported that OA induces HO-1 expression in mouse liver [Reisman et al., 2009] and RAW264.7 cells [Kawahara et al., 2009].
Therefore, the present study was performed to investigate the effect of OA on HO-1 expression and related upstream signaling pathways in VSMCs.
MATERIALS AND METHODS
Cell Culture and Treatment
The investigation is approved by the Animal Research Committee of Third Military Medical University. Rat aortic smooth muscle cells were obtained from the thoracic aorta of 3- to 4-week-old male Sprague–Dawley rats. Isolated VSMCs were cultured in DMEM (Gibco BRL, USA) containing 10% FBS (Gibco), 25 mM HEPES, 100 U/ml penicillin, and 100 µg/ml streptomycin at 37°C in a humidified atmosphere of 95% air/5% CO2. The purity and identity of VSMCs were verified using a monoclonal antibody against smooth muscle α-actin (Beyotime, China). Cells from passages 4–8 were used in all experiments. VSMCs were cultured in a serum-free medium for 24 h. The cells were arrested in G0/G1 phase (85 ± 1%) through the assay of flow cytometry. Before hydrogen peroxide (H2O2) was added to the medium, the cells were pretreated with OA (Sigma, St. Louis, MO). The VSMCs were pretreated with zincprotoporphyrin (J&K Scientific Ltd, China), PD98059 (Beyotime), SB203580 (Beyotime) or wortmannin (Sigma), respectively, at 1 h before OA treatment. OA was dissolved in dimethyl sulfoxide (DMSO) before addition to the culture medium. The final concentration of DMSO in each culture medium was 0.1% or less. The controls were also treated with the same amount of DMSO.
Measurement of Cell Viability
Cells were seeded at density of 2,000 cells/well in 96-well plates, and the cell viability was determined by using the conventional methyl thiazolyl tetrazolium (MTT) reduction assay. After the cells were treated with various indicated chemicals, MTT solutions (final 5 mg/ml) were added for 4 h at 37°C. The dark blue formazan crystals formed in the intact cells were solubilized with DMSO, and then the absorbance of blue color was measured at 490 nm on a microplate reader (BIO-RAD, Hercules, CA).
Hoechst 33342 Staining
As previously described [Xiao et al., 2001], after treatment with various indicated chemicals, the cells were pooled and fixed (4% paraformaldehyde in PBS; 10 min, room temperature), washed by PBS, resuspended in the dye solution (2 µg/ml Hoechst 33342, 10 min), washed again, and spotted onto slides for microscopy. Apoptotic cells with characterized by nuclear condensation of chromatin and/or nuclear fragmentation.
HO Activity Assay
The HO activity was measured as previously described [Kim et al., 2008]. In brief, microsomes from VSMCs were added to a reaction mixture containing hemin (10 µM), NADPH (20 µM), and 1 mg of rat liver cytosol protein which was included as a source of bilirubin reductase. The reaction was incubated for 1 h at 37°C in the dark. After extraction of incubation mixtures with chloroform, the concentration of bilirubin present was calculated by the difference in absorbance between 464 and 530 nm.
Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from cells using Trizol-reagent and quantified by UV absorption at 260 and 280 nm. RT-PCR was performed according to the manufacturer's instructions. The primer pairs of HO-1 were forward 5′-GGGTGACAGAAGAGGCTAAGACC-3′ and reverse 5′-AGATTCTCCCCTGCAGAGAGAAG-3′ [Ohta et al., 2003]. PCR conditions were as follows: 35 cycles of 94°C for 30 s; 55°C for 30 s; and 72°C for 45 s. Amplified products were visualized on 1.5% agarose gel electrophoresis, stained with ethidium bromide, and photographed under ultraviolet light. Densitometric analysis of three different observations was performed using Quantity One Software (BIO-RAD). The quantity of each transcript was normalized to that of GAPDH.
Preparations of Nuclear Proteins
VSMCs were treated with or without reagents. Cells (1 × 107 cells/ml) were harvested, washed with ice-cold PBS, centrifuged, and resuspended in ice-cold isotonic buffer A (10 mM Hepes, pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 0.5 mM DTT, and 0.5 mM PMSF). After incubation in an ice bath for 15 min, cells were centrifuged at 16,000g for 5 min at 4°C, and resuspended in ice-cold buffer C (20 mM Hepes, pH 7.9, 20% glycerol, 0.4 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, and 0.5 mM PMSF) followed by incubation at 4°C for 40 min. After vortex-mixing, the resulting suspension was centrifuged at 16,000g for 10 min at 4°C, and the supernatant was stored at −80°C. Protein content was determined by BCA protein assay reagent (Beyotime).
Western Blot Analysis
The cells were harvested at the indicated times, and washed twice with PBS. The harvested cells were then lysed and 50 µg total protein was separated by using SDS–PAGE on 10% polyacrylamide gels, and transferred to nitrocellulose membranes. After blocking for 1 h with 8% skimmed milk in TBS buffer (10 mM Tis, 150 mM NaCl), the membrane was incubated with primary antibodies at 4°C overnight. Then, the membrane was washed four times for 15 min each with TBST buffer (10 mM Tris, 150 mM NaCl, and 0.1% Tween-20), it was incubated in the appropriate HRP-conjugated secondary antibody at 37°C for 30 min [Wang et al., 2010]. Protein bands were detected using an enhanced chemiluminescene Western blotting detection kit (Amersham, Buckinghamshire, UK). The anti-Akt and anti-pAkt antibodies were purchased from Bioworld Technology, and the other antibodies were all purchased from Santa Cruz Biotechnology.
Electrophoretic Mobility Shift Assay (EMSA)
A synthetic double-stranded oligonucleotide containing the Nrf2-binding domain (ARE) was labeled with [γ-32P]-ATP using T4 polynucleotide kinase, and separated from unincorporated [γ-32P]-ATP by gel filtration using a nick spin column [Kim et al., 2008]. The oligonucleotide was synthesized by Beyotime. Prior to addition of the [γ-32P]-labeled oligonucleotide (100,000 cpm), 10 µg of nuclear extract was incubated on ice for 15 min in the gel-shift assay binding buffer containing 2.5 mM DTT, pH 7.5, 5 mM MgCl2, 20% glycerol, 2.5 mM EDTA, 250 mM NaCl, 50 mM Tris–HCl, and 0.25 µg/ml poly (dI − dC). DNA-protein complexes were subjected to electrophoresis on 6% polyacrylamide gel at 150 V for 2 h, followed by autoradiography.
siRNA Construction, Lentivirus Production, and Transfection
The validated siRNA sequences targeting Nrf2 was 5′-CCGGCATTTCACTGAACACAA-3′ [Cortese et al., 2008]. The oligonucleotides were synthesized, annealed, and inserted into the expression vector pFIV-H1/U6-copGFP (System Biosciences). Lentivirus particles derived from FIV were produced by transient cotransfecting lentiviral expression constructs with pFIV-PACK Lentiviral Packaging Kit (System Biosciences) into 293FT cells, using the Ca3 (PO4)2 transfection method. The lentivirus particles generated were frozen within 48 h and used to transfect VSMCs. VSMCs were infected with 2 × 106 infectious units of virus (IFU) (in 20 µl) in one well of six-well plates for 48 h. Lentiviral particles containing scrambled siRNA sequence was used as a negative control.
Statistical Analysis
All the measurements were presented as means ± SEM. Data were analyzed for statistical significance by Student's t-test. A value of P < 0.05 was considered significant.
RESULTS
Effect of OA on HO-1 Expression in VSMCs
To test whether OA can induce HO-1, VSMCs were treated with different concentrations of OA for 12 h. OA treatment concentration-dependently increased HO-1 protein expression, and the induction of HO-1 expression by OA was further shown by RT-PCR analysis (Fig. 1A). When VSMCs were treated with OA at 50 µM, HO-1 protein was increased up to 6 h, and then it decreased thereafter (Fig. 1B). We also investigated HO-1 activity in OA-treated VSMCs. OA at 25 and 50 µM greatly increased HO-1 activity (Fig. 1C). These results suggest that OA increases the levels of HO-1 mRNA and protein, accompanied by enhanced HO-1 activity. In the range of the doses of OA used in this study, cells maintained a good viability within the planned time frame (Fig. 1D). As shown in Figure 1E, OA did not mediate the expression of HO-2 protein, suggesting that HO-2 might be not involved in the effect of OA on VSMCs.
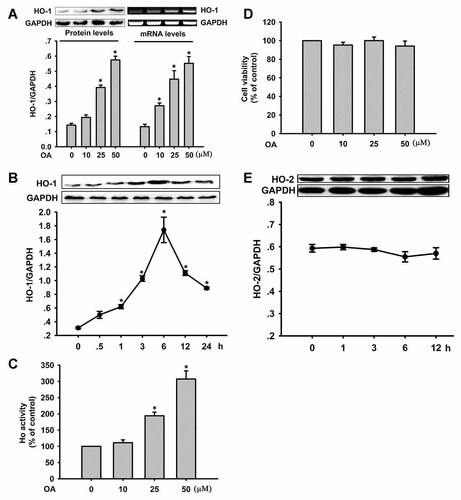
Effect of oleanolic acid (OA) on HO-1 expression in VSMCs. A: Cells were incubated with various concentrations of OA (10, 25, and 50 µM) for 12 h and HO-1 expression was determined by Western blot analysis and RT-PCR. GAPDH was used as a control for equal loading. *P < 0.05 versus 0 µM OA. B: VSMCs were incubated with 50 µM OA for the indicated times to detect HO-1 by Western blot. *P < 0.05 versus the 0 h time point. C: HO-1 activity was increased in OA-treated VSMCs for 6 h. *P < 0.05 versus 0 µM OA. D: Effect of oleanolic acid on cell viability. Cells were treated with various concentrations of OA for 24 h. E: VSMCs were incubated with 50 µM OA for the indicated times to detect HO-2 by Western blot. Data are expressed as the mean ± SEM of at least three independent experiments.
Effects of PI3K/Akt and Mitogen-Activated Protein Kinases (MAPK) Signaling Pathways on OA-Induced HO-1 Expression in VSMCs
To determine the upstream signaling pathways responsible for upregulation of HO-1 expression, we examined the effect of OA on the phosphorylation of Akt and MAPKs. Early activation of Akt, p38, and ERK was observed in OA-treated VSMCs, whereas the expression of total Akt, p38, and ERK proteins remained unchanged (Fig. 2A). Wortmannin (a PI3K inhibitor), SB203580 (a p38 inhibitor) and PD98059 (an ERK inhibitor) suppressed OA-induced activation of Akt, p38, and ERK (Fig. 2B). We then examined the effects of wortmannin, SB203580 and PD98059 on HO-1 expression. Pretreatment of VSMCs with wortmannin and PD98059 effectively inhibited the OA-mediated upregulation of HO-1 expression, whereas SB203580 had no effect (Fig. 2C). These data suggest that PI3K/Akt and ERK are involved in OA-induced upregulation of HO-1 in VSMCs.
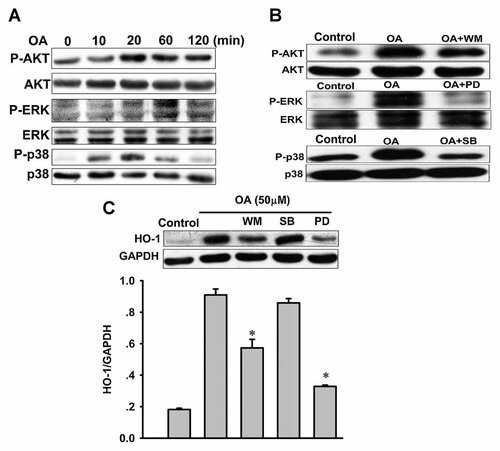
Roles of the PI3K/Akt and MAPKs pathways in oleanolic acid (OA)-induced upregulation of HO-1 in VSMCs. A: Effect of OA on the phosphorylation of Akt, ERK, and p38. VSMCs were treated with OA (50 µM) for the indicated times. Western blots were analyzed for p-Akt, Akt, p-ERK, ERK, p-p38, and p38. B: Effects of signaling inhibitors on signaling protein phosphorylation by OA. Cells were treated with 50 µM OA in the presence or absence of 10 µM wortmannin (WM), 50 µM PD98059 (PD), or 10 µM SB203580 (SB). Control indicates normal VSMCs without any treatment. C: Effects of PI3K and MAPKs inhibitors on OA-induced HO-1 expression. Cells were treated with 50 µM OA in the presence or absence of WM, PD, or SB for 6 h. Western blots were analyzed for HO-1 protein. Data are expressed as the mean ± SEM of three independent experiments. *P < 0.05 versus OA alone.
Involvement of Nrf2 in OA-Induced HO-1 Expression in VSMCs
Nrf2 has been reported to play a central role in the induction of several cytoprotective phase II detoxifying enzymes, including HO-1. Therefore, we first examined whether OA can activate Nrf2 in VSMCs. As shown by Western blotting using nuclear extract fraction, when we treated cells with OA for 4 h, Nrf2 translocation to the nucleus increased in a dose-dependent manner (Fig. 3A). The OA-induced increase in Nrf2-ARE DNA binding activity was attenuated by wortmannin or PD98059 (Fig. 3B), further suggesting that PI3K/Akt and ERK are also upstream activators of Nrf2. Furthermore, to determine whether OA activates Nrf2 leading to HO-1 upregulation, VSMCs were transiently transfected with either control or Nrf2 siRNA. Nrf2 siRNA significantly inhibited Nrf2 protein expression compared with the controls (Fig. 3C), and the OA-induced upregulation of HO-1 was significantly reduced by transfection with Nrf2-specific siRNA, whereas there was no change in VSMCs transfected with control siRNA (Fig. 3D). These findings further support the notion that transcriptional activation of HO-1 elicited by OA is mainly mediated by Nrf2.
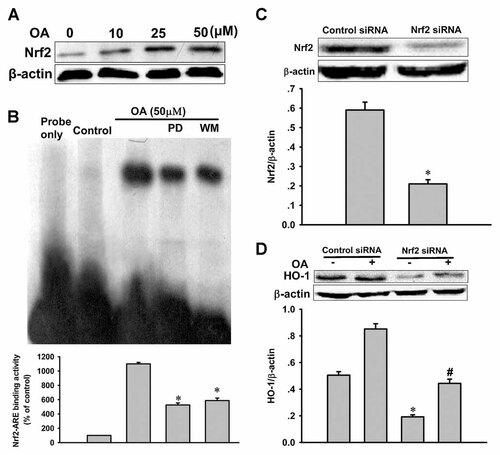
Effect of Nrf2 siRNA on oleanolic acid (OA)-upregulated HO-1 expression. A: Nuclear extracts were prepared from VSMCs treated with OA for 4 h to detect Nrf2 proteins by Western blot. B: Effects of inhibitors of PI3K and ERK on OA-induced Nrf2-ARE binding. Nuclear extracts were prepared from VSMCs treated with OA in the presence or absence of wortmannin (WM) or PD98059 (PD) for 4 h and subjected to EMSA for the measurement of Nrf2-ARE binding. *P < 0.05 versus OA alone. Control indicates normal VSMCs without any treatment. C: Cells were transfected with Nrf2 siRNA or control siRNA for 48 h, and then cells were harvested to detect Nrf2 protein levels by Western blot. *P < 0.05 versus control siRNA. D: Cells were transfected with Nrf2 siRNA or control siRNA for 48 h, and then cells were treated with 50 µM OA for an additional 6 h to detect the expression of HO-1 by Western blot. Data are expressed as the mean ± SEM of three independent experiments. *P < 0.05 versus control; #P < 0.05 versus control siRNA.
Effect of OA on H2O2-Induced Apoptotic Death
H2O2 is considered as an important reactive oxidant species and causes cell death either via apoptosis or necrosis. As shown in Figure 4A, a significant decline in cell viability was observed after 24 h of 400 µM H2O2 incubation. We used the Hoechst 33342 assay to observe nuclear condensation after treatment with OA or H2O2. OA reversed apoptotic death induced by H2O2 (Fig. 4B). To determine whether the OA-induced upregulation of HO activity confers an adaptive survival response against H2O2-induced cell death, VSMCs were treated with zinc protoporphyrin (ZnPP), an inhibitor of HO activity. As illustrated in Figure 4C, ZnPP mitigated the cytoprotection of OA against H2O2-induced cell death. When the effect of OA was further evaluated in the presence of wortmannin or PD98059, cell death was greatly increased, suggesting the involvement of PI3K/Akt and ERK signaling in OA-mediated cytoprotection.
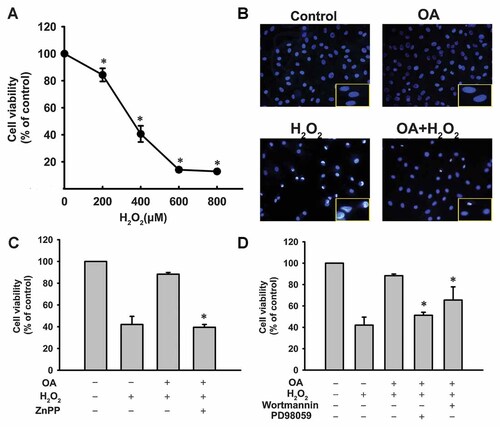
Effects of oleanolic acid (OA) or various inhibitors on H2O2-induced cellular injury. A: Dose dependent effect of H2O2 on cell viability as assessed by MTT assay. *P < 0.05 versus the control. B: VSMCs were treated with 50 µM OA for 6 h with or without 400 µM H2O2 for a further 24 h to detect apoptosis by Hoechst 33342 assay. Cells with condensed nuclei or fragmented nuclei were defined as apoptotic cells. Control indicates normal VSMCs without any treatment. C,D: VSMCs were treated with 50 µM OA in the presence or absence of ZnPP (10 µM), wortmannin or PD98059 for 6 h followed by addition of H2O2 to the cells for a further 24 h. Cell viability was detected by MTT assay. *P < 0.05 versus OA alone.
DISCUSSION
OA supplementation has been reported to be useful for vascular diseases; however, the mechanism by which OA contributes to this protective effect is not entirely clear. HO-1 is an important intracellular defense against oxidative stress and inflammation associated with vascular diseases [Ogborne et al., 2005]. In the current study, we have shown that OA may have a beneficial effect on the vascular system by upregulation of HO-1 via the PI3K/Akt and ERK pathways. To our knowledge, we have shown for the first time that the PI3K/Akt and ERK pathways are involved in OA-induced HO-1 expression through activation of Nrf2 in VSMCs.
The effect of OA on the activation of members of MAPKs, including ERK, JNK, and p38 kinase, remains controversial. A recent study showed that OA-induced cyclooxygenase-2 expression is dependent on activation of p38 and ERK, but not JNK, in human vascular smooth muscle cells [Martínez-González et al., 2008]. However, Wang et al. [2010] have shown that phosphorylation of JNK and ERK, but not p38, is involved in the antioxidant activity of OA in QZG cells. These inconsistent observations may be due to different experimental conditions and cell types. In the present study, we found that both the ERK and p38 pathways, but not the JNK pathway, were transiently phosphorylated by OA, which is consistent with a previous report [Martínez-González et al., 2008]. However, the ERK and p38 pathways did not contribute equally to HO-1 expression induced by OA in VSMCs. Treatment of VSMCs with inhibitors specific for ERK significantly reduced the effect of OA on the expression of HO-1, whereas SB203580, a p38 inhibitor, did not inhibit OA-induced HO-1 expression. These results demonstrated that the ERK pathway is involved in OA-induced HO-1 expression, but not through p38. However, Anwar et al. [2005] found that p38 and JNK are involved in oxidized low-density lipoprotein-induced HO-1 expression. Members of the MAPKs may differ in mediating the induction of HO-1 in VSMCs according to the nature of the stimulus.
In addition to MAPKs, PI3K/Akt also serves as an important signaling pathway in the induction of HO-1. The PI3K inhibitor, wortmannin, can reduce HO-1 expression induced by a carbazole analogue in VSMCs [Ho et al., 2007]. It has been previously reported that an increase in HO-1 promoter activity is preceded by an increase in Akt phosphorylation, while Akt knockdown with siRNA nearly suppresses HO-1 expression in response to H2O2 in VSMCs [Brunt et al., 2006]. It has also been reported that inhibition of the PI3K/Akt pathway can significantly reduce puerarin-induced HO-1 expression and transcription activity of HO-1 [Hwang and Jeong, 2008a]. In the present study, OA-induced activation of Akt was observed in VSMCs. As expected, we found that wortmannin suppressed the OA-upregulated HO-1 expression. These data suggest that OA induces the expression of HO-1 through PI3K/Akt pathways.
The HO-1 gene can be transcriptionally activated by Nrf2, nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1) [Kim et al., 2008]. Among them, Nrf2 is considered to play a major role in HO-1 expression. In this study, we showed that OA increased translocation of Nrf2 into the nucleus, and treatment of VSMCs with PI3K and ERK inhibitors significantly inhibited the nuclear translocation of Nrf2, indicating that the PI3K/Akt and ERK pathways are involved in OA-induced Nrf2 activation. Moreover, increased HO-1 protein expression induced by OA was suppressed by Nrf2 siRNA. These findings further suggest that HO-1 expression is induced by OA via the PI3K and ERK/Nrf2 pathways. Notably, the increased HO-1 protein expression induced by OA was not completely abolished by Nrf2 siRNA in our study. The cause of this phenomenon may be attributed to the finding that expression of HO-1 can be induced through an Nrf2-independent signaling pathway. It has been reported that OA not only serves as an Nrf2 activator, but it also activates Nrf2-independent pathways [Reisman et al., 2009].
Dysfunction of VSMCs, including migration, proliferation, and apoptosis in pathological states, is considered to play an important role in development of atherosclerosis. In this experiment, we focused on the effect of OA on the apoptosis of VSMCs induced by oxidative stress. Vascular smooth muscle cells are the only cells in atherosclerotic plaques capable of synthesizing structurally important collagen isoforms [Rudijanto, 2007], and the apoptosis of VSMCs might result in the formation of a necrotic core, plaque rupture, and subsequent thrombotic events [Clarke et al., 2006]. Treatment of VSMCs for 6 h with OA resulted in enhanced resistance to oxidative injury. This result is in agreement with a recent study demonstrating that OA protects against cerebral ischemic damage and attenuates H2O2-induced PC12 cell death by improving endogenous antioxidants as well as mitochondrial function [Rong et al., 2011]. Among the various endogenous antioxidants, HO-1 is considered as a “therapeutic funnel” against diseases associated with oxidative stress based on its potent physiological regulating properties [Yao et al., 2007]. In this study, we found that OA upregulated the levels of HO-1 mRNA and protein expression and enhanced enzyme activity in VSMCs. An inhibitor of HO-1, ZnPP, significantly attenuated the cytoprotective activity of OA, indicating that the cytoprotection of OA was partially mediated by HO-1. Moreover, when we treated VSMCs with a PI3K or ERK inhibitor, cell death was also increased. This result further confirmed the involvement of PI3K and ERK in the OA-induced protective effect. We also tested HO-2 expression after OA treatment in VSMCs. Our results showed that OA had no effect on HO-2 expression. HO-2 is constitutively expressed and is almost unaffected by agents that induce HO-1 [Loboda et al., 2008]. Since it is not inducible, HO-2 is unlikely to participate in the protective effect of OA in VSMCs.
In addition, although the present study was conducted in cell culture, these findings should provide some useful molecular mechanisms for the beneficial effects of OA on oxidative stress-associated diseases. In the future, we will confirm these observations in in vivo experiments.
In summary, our results demonstrated that OA leads to the activation of Akt and ERK, which are involved in Nrf2 nuclear localization and subsequent upregulation of HO-1 expression. Additionally, enhanced expression and activity of HO-1 by OA may protect VSMCs from cellular injury in response to oxidative stress. Further investigation is required to focus on the emerging issues from this study.




