Transforming growth factor-β induces epithelial to mesenchymal transition and suppresses the proliferation and transdifferentiation of cultured human pancreatic duct cells†‡
Jeong-Ah Shin and Oak-Kee Hong contributed equally to this work.
The authors have declared no conflict of interest.
Abstract
Pancreatic duct cells are considered a potential source of β-cell regeneration, and transforming growth factor-β (TGF-β) has been suggested to perform an important role in these processes, but the underlying mechanism of the signal pathways, especially in humans, remains poorly understood. To evaluate the role of TGF-β1, pancreatic duct cells were isolated from three brain-dead organ donors. Pancreatic cell clusters harvested after islet isolation were dispersed to single cells and cultured in monolayers, then treated with TGF-β1. We analyzed the characteristics of the cultured cells, the TGF-β1 intracellular signaling pathway, the proliferation, and transdifferentiation rates of the duct cells. We also evaluated the genes and protein expression patterns after TGF-β1 treatment. After TGF-β1 treatment, typical morphologic changes representative of EMT were observed and Erk1/2, JNK, and AKT phosphorylation, Ras downstream effectors, were increased. β cell-specific transcription factors including PDX-1, Beta2/NeuroD, Ist-1, and NGN3 were markedly suppressed and the rate of transdifferentiation into β cells was also suppressed. Genomic and proteomic analyses suggested that TGF-β1 induces marked changes in a variety of structural genes and proteins associated with EMT. In conclusion, TGF-β1 induces EMT in cultured human pancreatic duct cells, but suppresses its proliferation and transdifferentiation into β cells. Our results are the first report of TGF-β1 effects for EMT and ductal cell transdifferentiation and proliferation at the protein level in human pancreatic duct cells. J. Cell. Biochem. 112: 179–188, 2011. © 2010 Wiley-Liss, Inc.
Pancreatic islet transplantation is potential solution for cure of diabetes mellitus, but serious shortage of human pancreases is remained problem to overcome. As a theoretical alternative, pancreatic duct cells have been proposed to be the precursors of β cells, in both animals and humans [Bogdani et al., 2003], and their underlying molecular mechanisms have been studied extensively [Cras-Meneur et al., 2001; Tatarkiewicz et al., 2003]. Several cytokines, including epidermal growth factor (EGF), insulin-like growth factor (IGF), and transforming growth factor (TGF), are known to be involved in this process and these have been used to manipulate the cells for increment of yield in vitro. In animal studies, TGF-β has been identified as an important factor in pancreatic development, morphogenesis, the control of cell proliferation, and angiogenesis in embryonic and adult animal models [Li et al., 2003]. It is also associated with EMT during carcinogenesis in non-tumorigenic cells [Brown et al., 2004].
Epithelial–mesenchymal transition (EMT) is a complex physiological process which includes the dissolution of adherence junctions, alterations of spindle-like cell morphology, cytoskeletal reorganization, increased cell motility, the loss of epithelial cell markers, and the induction of mesenchymal cell markers. EMT is involved in embryonic period, some diseases and some invasive cancers [Okada et al., 2000; Thiery, 2002; Moustakas and Heldin, 2007]. In recent studies, in vitro human adult β cells have been shown to undergo EMT before redifferentiating into insulin-producing cells [Ouziel-Yahalom et al., 2006]. EMT, therefore, includes several cellular responses that may occur via the activation of different intracellular signaling pathways following TGF-β treatment. However, to the best of our knowledge, the study of the effects of TGF-β on human pancreatic duct cells has not been clearly demonstrated.
In this study, we assessed the effects of TGF-β1 on the proliferation and differentiation of human pancreatic duct cells. We also analyzed the differentially expressed genes and proteins as the result of TGF-β1 treatment.
MATERIALS AND METHODS
Isolation of Human Pancreatic Cells
Three human pancreata were obtained from brain-death organ donors, with permission from their relatives and the institutional ethics committee for organ donation. The procured pancreata were sent to the GMP facility to isolate the islets in accordance with the Edmonton protocol [Shapiro et al., 2000]. After islet isolation using a continuous Euroficoll gradient with a cell separator (Cobe 2991), we received the pellets of pancreatic cells including mainly exocrine pancreatic cells, and then the islets remaining in the pellets were removed completely by handpicking after staining with dithizone.
Dispersion of Human Pancreatic Cell Clusters
Cell clusters were broken up by gentle aspiration with a pipette and incubated with dissociation medium (Sigma Chemical Co.). After three washings with PBS, the dispersed cells were plated at a density of 1 × 107 per 150 mm dish, and cultured in Dulbecco's modified essential medium (DMEM) supplemented with 10% fetal calf serum (FCS). On day 3 and just before TGF-β1 treatment (day 5), the medium in each plate was replaced with 35 ml of fresh medium. After 7 days of culture, the cells were characterized via immunocytochemical staining with cytokeratin, vimentin, and islet hormones. After confirming that more than 80% of cells were comprised duct cells, the following experiments were conducted.
Duct Cells Culture and Immunocytochemical Staining
Cells were treated with human recombinant TGF-β1 (10 ng/ml, R&D Systems Inc., Minneapolis, MN). The cultured cells were fixed with 4% paraformaldehyde and were then permeabilized with 0.05% Triton X-100. Cells were incubated in primary antibodies against pancytokeratin (1:100, DakoCytomation, Glostrup, Denmark), vimentin (1:100, DakoCytomation), or insulin/glucagons (1:100 dilution, Jackson Immuno-Research Lab., West Grove, PA), along with DAPI (1:1,000 dilution; Sigma Chemical Co).
Image Acquisition and Quantification of the Cells
Digital images at two or three fluorescence emission wavelengths were acquired using a confocal microscope (Bio-Rad Laboratories, Hercules, CA). The light source was a multiphoton laser that generates excitation wavelengths in a range of 368–647 nm, with a 512 pixels scan size. The FITC, Texas red, and DAPI images were scanned separately. Fifteen to 20 images were obtained systematically from each 35 mm dish after insulin/glucagon/DAPI or pancytokeratin/vimentin/DAPI staining. Afterward, the numbers of each cell type in all of the sampled images were counted.
Analysis of Cell Proliferation
The cells were then incubated in medium containing human recombinant TGF-β1 for 24 and 48 h with 1 µCi/well of [3H]-thymidine (Amersham Biosciences Corp., Piscataway, NJ). The contents of the wells were transferred to scintillation vials containing 4 ml of scintillation cocktail, and the radioactivity of the samples was measured using a Packard β-counter (Packard Instruments Co., Downers Grove, IL).
Measurement of Insulin and DNA Contents
Total intracellular insulin contents were extracted via the acid/ethanol method. Cells were incubated overnight in 1% hydrochloric acid (ethanol/H2O2/HCl, 14:57:3) at 4°C. The insulin in the supernatant was detected using a radioimmunoassay kit (Dianabot Co., Ltd., Japan). DNA was extracted using a DNA extraction kit (Qiagen Inc., Valencia, CA). The remaining cells were sonicated in acid ethanol and extracted overnight with acid ethanol. The supernatants were used for the insulin assays.
Oligonucleotide Microarray
We used a Mac Array-II 10k chip (Macrogen Inc., Seoul, Korea) with a total spot number of 10,368 and 10,108 human genes. The number of functionally known genes included is 8,032, with 4,270 molecular function genes and 3,672 biological process and cellular component genes. Two hundred sixty of the genes were non-human controls, and the oligonucleotides were 50mers. Total RNA from both TGF-β1 (10 ng/ml) treated and non-treated cells (control) was fluorescently labeled with Cy5-dUTP via reverse transcription. Probes and labeled targets were hybridized. Cy3 and Cy5 fluorescence intensities were determined using a generation III Array scanner. Images from the scanner were imported into the Image (5.5) software. Images were analyzed by calculating the relative fluorescence ratios of the TGF-treated and non-treated cells.
Data Analysis
Fluorescence intensity was processed and measured with Agilent G2566AA Extraction Software Version A.6.1.1. Variance stabilizing normalization, as described by Huber et al. [2002], was applied using the “vsn” package in Bioconductor with the R statistical package. After performing intensity-dependent global LOWESS regression, the spatial and intensity-dependent effects were managed via pin-group LOWESS normalization, according to the approach developed by Yang (http://www.stat.berkeley.edu/users/terry/zarray/Html/normspie.html). Cyber-T is a regularized t-test which utilizes a Bayesian estimate of the variance [Baldi and Long, 2001] between gene measurements within an experiment. This provides a method for the estimation of experiment-wide false-positive and false-negative levels based on the modeling of the P-value distributions. The resources of the GenBank Database were utilized. This database is a DNA sequence database that was built and maintained by the National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov).
Two-Dimensional Gel Electrophoresis and MALDI-TOF Mass Spectrometry Analysis
Cells were homogenized in 400 µl lysis buffer (7 M urea, 2 M thiourea, 2% CHAPS, 2% Pharmalyte pH 3–10, 100 mM dithiothreitol, complete protease inhibitor) and sonicated. Samples (1 mg/ml) were applied to strips that had been rehydrated with sample solution. After rehydration, isoelectric focusing was conducted at 20°C with a current limit of 50 µA/strip, as follows: Immobiline DryStrips (18 cm, pH 3–10, Pharmacia, Peapack, NJ) were gently equilibrated for 20 min. The second-dimensional gels were subsequently overlaid in a solution containing 0.5% agarose, 24.8 mM Tris, pH 8.3, 192 mM glycine, 0.1% SDS, and a trace of bromophenol blue. Using 9–16% vertical SDS gradient slab gels, electrophoresis was conducted at a constant current of 10 mA/gel. Gel images were obtained with a GS-710 image scanner (Bio-Rad) and processed with Melanie 5 software (GeneBio, Geneva, Switzerland). Digitized images were compared via a matching method, and differentially expressed spots (>1.5-fold increase or decrease in intensity) were identified.
RT-PCR Analysis
Total RNA was obtained from rat islets using a Trizol reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized using superscript II RT (Invitrogen) and 1 µg of total RNA, in accordance with the manufacturer's instructions. PCR was conducted with 3 µl of first-strand cDNA as follows: 98°C for 1 min followed by 27 cycles for Pdx-1, NeuroD/BETA2, HNF3 β, Isl-1, and Ngn3 at 94°C for 1 min, 55–58°C for 30 s, 72°C for 1 min, and 72°C for 7 min. The PCR products were then electrophoresed on 1.5% agarose gel. Band intensity was densitometrically calculated using a densitometer VDS analyzer (Pharmacia Biotech AB). Specific primers were designed according to the sequences made available in the databanks or published by others (Supplementary Table I).
MALDI-TOF Mass Spectrometry Analysis
Spots on the gels were excised with end-removed pipette tips to accommodate a variety of spot diameters. The gel slices were destained in microtubes and dehydrated with 50 µl of ammonium bicarbonate:acetonitrile (60:40). The dried gels were digested with GELoader tips (Eppendorf, Wesseling-Berzdorf, Germany) as previously described. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry was conducted using an Applied Biosystems Voyager DE-PRO spectrometer (Applied Biosystems). The spectra were internally calibrated using the trypsin autolysis products (842.51 and 2211.11 Da) and monoisotopic peptide masses were assigned and used for database searches. The proteins were identified by searching the SWISS-PROT and NCBI databases using MS-Fit (Protein Prospector; http//www.prospector.ucsf.edu) and MASCOT (Matrix Science; http://www.matrixscience.com). All searches were analyzed with a mass tolerance of 100 ppm.
Western Blotting
Cells were lysed in buffer (150 mM NaCl, 50 mM Tris–HCl, pH 7.4, 2 mM EDTA, 1% NP-40, 10 mM NaF, 1 mM Na3VO4, 10 mM sodium pyrophosphate, 1 mM PMSF, complete protease inhibitor). Usually, 40 µg of protein extracts were subjected to 8% sodium dodecylsulphate (SDS)–polyacrylamide gel electrophoresis. The proteins on the gel were electrophoretically transferred onto Immobilon-P Teflon membranes (Millipore) and Western analysis was conducted with the specific antibodies, anti-Erk 1/2, phospho-Erk 1/2, phospho-Akt, -Akt, phospho-p38, -p38, phospho-JNK, -JNK, phospho-Smad 2 (all from Cell Signaling Technology Inc., Beverly, MA), vimentin, anti-cytokeratin 8/19 (DakoCytomation), Smad 2/3, aldehyde dehydrogenase 2 (ALDH2), β-actin, heat shock protein 70 (Hsp70; all from Santa Cruz Biotechnology Inc., Santa Cruz, CA), villin, Hsp27, heterogeneous nuclear ribonucleoprotein A2/B1 (hnRNPA2/B1), and cytokeratin 18 antibodies (all from Abcam, Cambridge, UK). Proteins were visualized using an enhanced chemiluminescence (ECL) kit in accordance with the manufacturer's recommendations.
Statistical Analysis
All experimental results are described as the means ± SEM. Statistical significance was determined using Student's t-test, and one-way ANOVA, and P < 0.05 was considered significant.
RESULTS
Characterization of Dispersed Pancreatic Cell Clusters
After 5 days of culture, small cell clusters were categorized into two phenotypic groups. One was the epithelial cells, which were stained intensively with pancytokeratin (Fig. 1A a) and the others were round cells stained with pancreatic hormones (Fig. 1A b). Approximately 85% of cells were composed of pancreatic ductal cells, some fibroblasts, and a few endocrine cells. After an additional 2 days of culture with TGF-β1, the proportion of duct, endocrine insulin- or glucagon-producing and mesenchymal cells was changed to 78 ± 15, 8 ± 4, 5 ± 3, and 13 ± 3%. The fraction of mesenchymal cells was increased slightly with no statistical differences, and the number of endocrine cells increased. TGF-β1 treatment induced morphological changes from a rectangular to spindle shape and increased vimentin staining in the borders of the cells (Fig. 1A c). In order to determine whether or not TGF-β1 induced EMT, we measured cytokeratin19 and vimentin levels by Western blotting. Cytokeratin19 levels were reduced and vimentin levels were increased following treatment (Fig. 1B, P < 0.05).
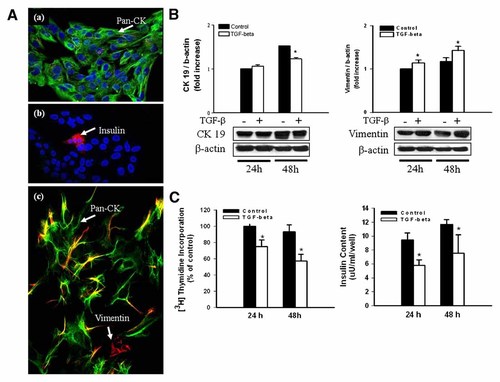
Characterization of the cultured human pancreatic duct cells before and after TGF-β1 treatment. A: Cultured human pancreatic duct cells in the absence of TGF-β1 for 5 days (a,b). Approximately 85% of cells were composed of epithelial cells, some fibroblasts, and a few endocrine cells. After 48 h of TGF-β1 treatment, the cells became elongated and developed actin stress fibers and increased vimentin staining (c). Staining with anti-pancytokeratin (green), anti-vimentin (red), DAPI (blue) staining (a,c): staining with anti-insulin (red) and DAPI (blue) staining (b): original magnification ×400. B: The level of mRNA expression of cytokeratin 19 and vimentin at 24 and 48 h of treatment. Cytokeratin 19 levels were decreased at 48 h and vimentin were increased (*P < 0.05). C: [3H]thymidine incorporation and insulin contents after TGF-β1 treatment. Both were significantly decreased at 24 and 48 h (*P < 0.05).
TGF-β1 Treatment Inhibits Human Pancreas Duct Cell Proliferation and Transdifferentiation Into β Cells
The duct cell proliferation rate represented by [3H]thymidine uptake was reduced after TGF-β1 treatment in a time-dependent fashion. Additionally, the insulin contents, which were used as a marker for the transdifferentiation of duct cells to β cells, were significantly reduced in the treated group at 24 and 48 h (Fig. 1C).
To evaluate the influence of TGF-β1 on key transcription factors for the development of endocrine cells, we conducted RT-PCR for Pdx-1, NeuroD/BETA2, HNF3β, Isl-1, and Ngn3. All mRNA expression levels were reduced (P < 0.05; Fig. 2). These findings indicated that TGF-β1 suppressed the proliferation and transdifferentiation of duct cells.
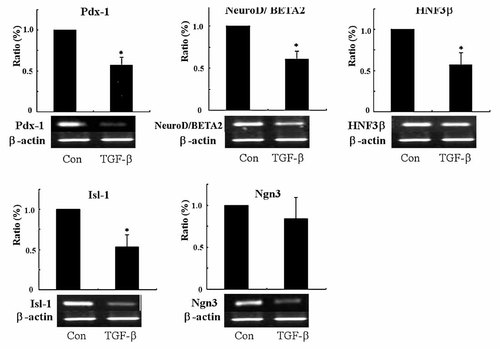
RT-PCR analysis on key transcription factors for the development of endocrine cells before and after TGF-β1 treatment. After 24 h of TGF-β1 treatment, the level of mRNA expression of Pdx-1, NeuroD/BETA2, HNF3β, Isl-1, and Ngn3 were reduced. Data are expressed as the percentage (%) of mRNA levels in control at 0 h of treatment (*P < 0.05).
Intracellular Signaling of TGF-β1 in Human Pancreatic Duct Cells
After TGF-β1 treatment, we analyzed the levels of RAS signaling effectors, including Erk 1/2, p38, JNK, and Akt activity. Erk 1/2 activity increased significantly from 10 min after treatment to 48 h (P < 0.05; Fig. 3). p-38 activity was not altered, but JNK activity was increased up to 30 min after treatment. Akt activity was increased and persisted for more than 24 h. Phosphorylated Smad2 protein levels were also shown to be increased and remained to be elevated (P < 0.05). Thus, Erk1/2, JNK, and AKT are the important Ras downstream effectors that were activated by TGF-β1 in human pancreatic duct cells.
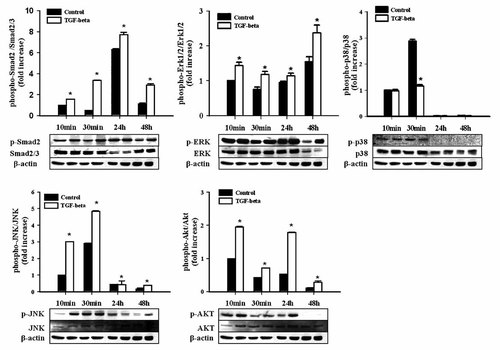
Intracellular signaling of the cultured human pancreatic duct cells before and after TGF-β1 treatment. Human pancreatic duct cells were cultured in the absence or presence of TGF-β1 for up to 48 h. Erk 1/2, JNK, Akt, and Smad2 activity were increased and maintained until 48 h. The signals were measured by scanning densitometry and normalized for β-actin. Data are expressed as the fold value of protein levels in controls on 48 h of treatment (*P < 0.05).
Differentially Expressed Genes Responses to TGF-β1: Oligonucleotide Microarray Analysis
TGF-β1 treatment resulted in the upregulation or downregulation of 33 genes among the 10,368 and 10,108 genes examined. Eight genes were upregulated (Supplementary Table IIA), and 25 were downregulated (Supplementary Table IIB). The responsive genes were subdivided according to their functions in eight categories: (1) cell surface adhesion, matrix and cytoskeletal proteins, (2) cell cycle regulators, (3) enzymes or enzyme regulators, (4) apoptosis regulators, (5) chaperones, (6) defense/immunity, (7) growth factors, their receptors and signaling molecules, (8) other nuclear factors. In order to confirm the microarray results, RT-PCR was conducted for four genes associated with pancreatic development. RACGAP1 expression was increased and MCL1, BST2, and DPT were decreased (Fig. 4). MCL1 is known to evidence antiapoptotic activity during embryonic pancreatic development [Matsushita et al., 2003]. Many other genes have been newly identified as genes that are regulated by TGF-β1.
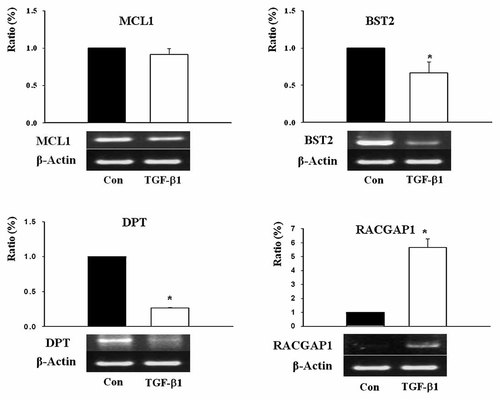
RT-PCR analysis on TGF-β1 responsive genes in the oligonucleotide microarray. Cells were cultured for 24 h in the absence or presence of TGF-β1. The level of mRNA expression of MCL1, BST2, and DPT was reduced and RACGAP1 was increased. Data are expressed as a percentage (%) of mRNA levels in the controls on 0 h of treatment (*P < 0.05).
Differentially Expressed Proteins' Responses to TGF-β1: Proteomic Analysis
We reproducibly detected ∼1,000 protein spots after Coomassie blue staining. We analyzed two gels for each experimental condition, and selected only the spots whose normalized volumes were similar between the gels. We disregarded proteins that evidenced <30% changes between the experimental conditions. Eighty-one protein spots were selected for mass spectrometric analysis. We identified approximately 57 protein spots, encompassing a broad range of molecular weights, pI values, fold changes, and abundance (Supplementary Fig. 1). One of the most striking features was the large amounts of structural proteins detected. One of these spots would be the actin and cytokeratin families. The identified proteins are listed in Tables Ia and Ib. The upregulated proteins identified were as follows: Myh9 protein, ACTB (actin B), villin2, vimentin, cytokeratin18, lamin B2 & A/C, galectin-1, CG-NAP, annexin II & III, Hsp27, Hsp70, ER-60, beta-MPP, hnRNP A2/B1, transgelin2, GAPDH, core I protein, manganese-containing superoxide dismutase (MnSOD), aldehyde dehydrogenase X (ALDH2), thioredoxin peroxidase, oxysterol-binding protein (OSBP)-related protein 2, moesin/anaplastic lymphoma kinase (ALK) fusion protein, anti-elastase, chaperonin subunit 8 and splicing factor (arginine/serine-rich 3). Two additional proteins evidenced no homology with known proteins (Table Ia). The downregulated proteins identified were bullous pemphigoid antigen I-b, dynactin2, caldesmon-1, cytokeratin8&19, hnRNP H1, kappa-B motif-binding phosphoprotein, histone H2B & H3, translin-associated factor X interacting protein I, ras-GTPase activating protein, centrosome associated protein CEP250, core-binding factor (CBFA2T2), nucleobindin 1, tetrahydrofolate dehydrogenase cyclohydrolase (MTHFD2), cytochrome P450 CYP2B21, ATP synthase D chain (My032), F1-aptase. Three additional proteins evidenced no homology with known proteins, and one hypothetical protein, MGC35194, was detected (Table Ib). Cytokeratin 19 (spot no. 1024, 1123, 1126) levels evidenced reductions in response to TGF-β1, whereas vimentin (spot no. 688) was increased. Western blotting was conducted to confirm the expression data derived from proteomics studies. There was good agreement in the expression levels of proteins as determined by 2D gels and by Western blotting (Fig. 5).
| Spot no. | Probability | Accession no. | Protein | Coverage | pI (theoretical unmodified) | MW (kDa) |
|---|---|---|---|---|---|---|
| Structural protein | ||||||
| 719 | 1.00E + 00 | AAH54360.1 | Myosin heavy chain 9 protein [Mus musculus] | 19 | 5.8 | 43.69 |
| 1207 | 1.00E + 00 | AAH12854.1 | ACTB protein [Homo sapiens] | 33 | 5.6 | 40.54 |
| 1169 | 1.00E + 00 | AAH12854.1 | ACTB protein [Homo sapiens] | 41 | 5.6 | 40.54 |
| 331 | 1.00E + 00 | NP_003370.2 | Villin 2; ezrin; cytovillin [Homo sapiens] | 18 | 5.9 | 69.51 |
| 688 | 1.00E + 00 | AAH61847.1 | Vimentin [Rattus norvegicus] | 35 | 5.1 | 53.77 |
| 1022 | 1.00E + 00 | CAA31377.1 | Cytokeratin 18 (424 AA) [Homo sapiens] | 22 | 5.3 | 47.32 |
| Tumor suppress/apoptosis | ||||||
| 531 | 1.00E + 00 | NP_116126 | Lamin B2 [Homo sapiens] | 14 | 5.3 | 67.79 |
| 635 | 1.00E + 00 | NP_005563.1 | Lamin A/C isoform 2; 70 kDa lamin; progeria 1 (Hutchinson–Gilford type); Charcot–Marie–Tooth disease, axonal, type 2B1 [Homo sapiens] | 44 | 6.4 | 65.17 |
| 2029 | 60 | 42542977 | Chain A, X-ray crystal structure of human galectin-1 | 43 | 5.34 | 14.58 |
| Signal transduction | ||||||
| 748 | 1.00E + 00 | BAA78718.1 | Centrosome- & Golgi-localized PKN-associated protein (CG-NAP) [Homo sapiens] | 5 | 5 | 454.1 |
| Signaling intermediates | ||||||
| 1257 | 1.00E + 00 | AAH23990 | ANXA2 protein [Homo sapiens] | 43 | 7.7 | 38.79 |
| 1335 | 1.00E + 00 | 1AXN | Annexin family Mol_id: 1; Molecule: Annexin III; Chain: Null; Other_details: human recombinant | 33 | 5.6 | 36.48 |
| 1574 | 1.00E + 00 | NP_001531 | Heat shock 27 kDa protein 1 [Homo sapiens] | 60 | 6 | 22.82 |
| 467 | 1.00E + 00 | NP_694881 | Heat shock 70 kDa protein 8 isoform 2 [Homo sapiens] | 24 | 5.6 | 53.61 |
| 698 | 1.00E + 00 | AAC51518 | ER-60 protein [Homo sapiens] | 39 | 5.9 | 57.16 |
| 931 | 1.00E + 00 | 29840827 | Mitochondrial processing peptidase beta subunit (beta-MPP) (P-52) | 25 | 6.4 | 55.09 |
| 1264 | 1.00E + 00 | NP_002128 | Heterogeneous nuclear ribonucleoprotein A2/B1 isoform A2 | 46 | 8.7 | 36.05 |
| 1857 | 1.00E + 00 | AAH02616 | Transgelin 2 [Homo sapiens] | 53 | 8.6 | 22.56 |
| 1204 | 1.00E + 00 | CAA25833 | Glyceraldehyde-3-phosphate dehydrogenase [Homo sapiens] | 24 | 8.4 | 36.21 |
| 926 | 1.00E + 00 | BAA05495 | Core I protein [Homo sapiens] | 14 | 5.9 | 53.31 |
| 1742 | 1.00E + 00 | AAP34410 | Manganese-containing superoxide dismutase [Homo sapiens] | 42 | 6.9 | 23.77 |
| 1743 | 1.00E + 00 | AAP34410 | Manganese-containing superoxide dismutase [Homo sapiens] | 39 | 6.9 | 23.77 |
| 799 | 1.00E + 00 | P30837 | Aldehyde dehydrogenase X, mitochondrial precursor (ALDH class 2) | 19 | 6.4 | 57.66 |
| 1557 | 1.00E + 00 | NP_006397 | Thioredoxin peroxidase (antioxidant enzyme) [Homo sapiens] | 25 | 5.9 | 30.75 |
| Metabolism | ||||||
| 1148 | 1.00E + 00 | Q8BX94 | Oxysterol binding protein-related protein 2 (ORP-2) | 17 | 5.8 | 55.82 |
| Enzyme or enzyme regulator | ||||||
| 442 | 1.00E + 00 | AAK71522 | Moesin/anaplastic lymphoma kinase fusion protein [Homo sapiens] | 41 | 7.7 | 62.03 |
| 1027 | 1.00E + 00 | NP_109591 | Serine (or cysteine) proteinase inhibitor, anti-elastase [Homo sapiens] | 23 | 5.9 | 42.84 |
| Unknown | ||||||
| 1591 | 1.00E + 00 | AAC27822 | Unknown gene product | 23 | 9.5 | 17.66 |
| 648 | 1.00E + 00 | AAH09007 | Chaperonin subunit 8 (theta) [Mus musculus] | 16 | 5.4 | 60.11 |
| 1730 | 1.00E + 00 | NP_003008 | Splicing factor, arginine/serine-rich 3; 20-kD [Homo sapiens] | 33 | 11.8 | 19.54 |
| 1454 | 1.00E + 00 | AAH09544 | Unknown (protein for IMAGE:3897065) [Homo sapiens] | 26 | 5.2 | 17.99 |
| Spot No. | Probability | Accession No. | Protein | Coverage | pI (theoretical unmodified) | MW (kDa) |
|---|---|---|---|---|---|---|
| Structural protein | ||||||
| 759 | 1.00E + 00 | XP_237042 | Similar to bullous pemphigoid antigen 1-b [Rattus norvegicus] | 3 | 5.4 | 1095.31 |
| 827 | 54 | NP_081427 | Dynactin 2 [Mus musculus] | 17 | 5.14 | 44.09 |
| 408 | 75 | NP_149131 | Caldesmon 1 isoform 5 [Homo sapiens] | 21 | 6.4 | 61.18 |
| 912 | 1.00E + 00 | AAA19668 | Cytokeratin 8 polypeptide | 17 | 5.5 | 52.69 |
| 1024 | 1.00E + 00 | KRHU9 | Keratin 19, type I, cytoskeletal—human | 23 | 5 | 44.07 |
| 1123 | 1.00E + 00 | NP_002267 | Keratin 19; keratin, type I cytoskeletal 19; keratin, type I, 40 kDa; cytokeratin 19; 40-kDa keratin intermediate filament precursor gene [Homo sapiens] | 32 | 5 | 44.09 |
| 1126 | 1.00E + 00 | AAH34561 | Keratin complex 1, acidic, gene 19 [Mus musculus] | 23 | 5.3 | 44.52 |
| Transcription Regulators | ||||||
| 2140 | 1.00E + 00 | NP_005511 | Heterogeneous nuclear ribonucleoprotein H1 [Homo sapiens] | 24 | 5.9 | 49.5 |
| 575 | 1.00E + 00 | A54143 | Kappa-B motif-binding phosphoprotein—mouse | 21 | 5.2 | 51.31 |
| 1952 | 1.00E + 00 | 0503212A | Histone H2B | 55 | 10.3 | 13.76 |
| 1942 | 1.00E + 00 | 0503212A | Histone H2B | 44 | 10.3 | 13.76 |
| 1909 | 5.30E + 01 | CAH73371 | H3 histone, family 3A [Homo sapiens] | 30 | 11.34 | 14.04 |
| 383 | 1.00E + 00 | NP_060900 | Translin-associated factor X interacting protein 1 [Homo sapiens] | 11 | 4.6 | 42.34 |
| Signaling transduction | ||||||
| 1922 | 1.00E + 00 | T08621 | Centrosome-associated protein CEP250—human | 6 | 5 | 281.9 |
| 610 | 1.00E + 00 | AAH40344 | Core-binding factor, runt domain, alpha subunit 2 (CBFA2T2) protein [Homo sapiens] | 12 | 8.5 | 68.75 |
| 655 | 1.00E + 00 | NP_006175 | Nucleobindin 1 [Homo sapiens] | 17 | 5.1 | 53.86 |
| Signal intermediates/metabolism | ||||||
| 654 | 1.00E + 00 | 4558256 | A chain A, human tetrahydrofolate dehydrogenase cyclohydrolase | 36 | 6.1 | 32.88 |
| 389 | 9.90E-01 | NP_942028 | Cytochrome P450 CYP2B21 [Rattus norvegicus] | 12 | 6.7 | 56.71 |
| Metabolism | ||||||
| 1741 | 1.00E + 00 | AAG43146 | ATP synthase D chain, My032 protein [Homo sapiens] | 67 | 6.6 | 15.81 |
| 796 | 1.00E + 00 | 1MABB | Chain B, Rat Liver F1-Atpase | 49 | 4.9 | 51.33 |
| Unknown | ||||||
| 516 | 65 | BAC37665 | Unnamed protein product [Mus musculus] | 22 | 5.18 | 47.46 |
| 873 | 1.00E + 00 | BAC04534 | Unnamed protein product [Homo sapiens] | 17 | 5 | 47.87 |
| 364 | 1.00E + 00 | BAC33545 | Unnamed protein product [Mus musculus] | 18 | 6.3 | 59.34 |
| 366 | 1.00E + 00 | NP_689503.1 | Hypothetical protein MGC35194 [Homo sapiens] | 37 | 9.7 | 23.07 |
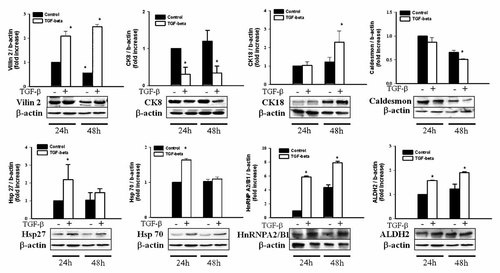
Expression of TGF-β1-regulated protein identified in 2-DE experiments. The level of vilin, CK18, Hsp27, Hsp70, hnRNP, and ALDH2 expression was increased after TGF-β1 treatment, and the level of CK8 and caldesmon was decreased after treatment. Data are expressed as the fold value of protein levels in controls on 48 h of treatment (*P < 0.05).
DISCUSSION
Pancreatic duct cells have the potential for transdifferentiation into all types of pancreatic cells with appropriate external stimulus [Swenne, 1992; Nielsen et al., 1999; Bogdani et al., 2003]. Among the many cytokines related to pancreatic endocrine cell differentiation [Larsson, 1998], TGF-β1 is considered to be related to a variety of biological processes, including growth, differentiation, morphogenesis, development, angiogenesis, and apoptosis [Miralles et al., 1998; Tulachan et al., 2007]. However, the mechanisms underlying the effects on pancreatic duct cell proliferation and transdifferentiation are currently poorly understood, and this is particularly true in human tissues.
In our study, dispersed human pancreatic duct cells proliferated rapidly and formed the clusters comprised mainly epithelial cells. It is well known that a standard islet and ductal cell isolation technique using Cobe 2991 have about 80% average purity. And after second purification process using hand-picking removal of dithizone stained cells, an average purity is over 95% [Ricordi et al., 1988; Noguchi et al., 2010]. On the 5th day of culture, more than 80% of the cells were comprised duct cells with a small number of insulin-producing cells and fibroblasts. This result is consistent with recent study, which described that cell population after 8 days culture of human adult exocrine pancreatic cells consisted of ductal cells (75–82%), acinar cells (12–19%), and a few endocrine cells (3.5%) [Fanjul et al., 2010]. Thus, those cells were considered appropriate for the evaluation of the effects of TGF-β1 and had appropriate purity. During embryonic pancreatic organogenesis, TGF-β appears to play a significant role in the regulation of pancreatic growth and differentiation. It seems to favor the development of endocrine cells in animal models and performs an important role in the regulation of EMTs [Crisera et al., 1999; Rane et al., 2006]. Our experiments also demonstrated that TGF-β1 clearly inhibited the proliferation of human pancreatic duct cells. We observed the typical morphologic and functional changes using immunocytochemical staining method in EMT after TGF-β1 treatment. Additionally, TGF-β1 treatment reduced insulin contents and suppressed the expression of transcription factors associated with endocrine cell differentiation, such as Pdx-1, NeuroD/BETA2, Isl-1, and Ngn3. These findings suggest strongly that TGF-β1 induces EMT but inhibits the proliferation and differentiation into endocrine cells of pancreatic duct cells. Our results are different from those of Seeberger et al. [2009], who demonstrated EMT is an artifact of cell culture within human pancreas. But many other studies confirmed possibilities of EMT in human pancreatic epithelial cells in vitro and in vivo conditions [Dalvi et al., 2009; Fanjul et al., 2010]. So further researches are required to confirm the controversial results.
EMT is a complex, extreme manifestation of epithelial plasticity [Thiery, 2002]. TGF-β1 was initially described as an EMT inducer in normal mammary epithelial cells, which functioned by signaling through receptor serine/threonine kinase complexes [Miettinen et al., 1994]. During pancreatic development, EMT is considered one of the essential processes for the differentiation of pancreatic β cells from the epithelial layer [Cole et al., 2009]. The significance of the EMT of pancreatic duct cells has yet to be clearly explained, but it also needs to be determined whether the gain of plasticity in epithelial cells is one of the stages in differentiating into other cells or not.
In order to determine the Ras downstream effectors of TGF-β1 in humans, we analyzed the activities of Akt, PI3 kinase, and MAPK—including Erk 1/2, p-38, and JNK—after treatment. The phosphorylation of Smad2, Erk 1/2, JNK, and Akt are increased significantly and persisted after treatment. These data demonstrated that Akt, Erk 1/2, JNK, and Smad 2 phosphorylation are the principal signaling pathways of TGF-β1 in human pancreatic duct cells for the induction and maintenance of EMT. Other reports also demonstrated that TGF-β1 stimulates ERK activity in EMT culture models [Ellenrieder et al., 2001; Zavadil et al., 2001; Xie et al., 2004].
Based on these results, we attempted to identify the TGF-β1 regulated target genes. Via microarray analysis, the RACGAP1 gene was one of the genes upregulated via TGF-β1 treatment. The genetic alteration of the RACGAP1 gene results in carcinogenesis via the dysregulation of Rho/Rac/Cdc42-like GTPase [Katoh, 2004]. G protein-related gene expression (GPR38 & 55, RGS20) was reduced in human pancreatic duct cells with TGF-β treatment (Table Ib). Therefore, the results of the present study provide further evidence to suggest that changes in the level and activity of G protein-coupled receptor involve alterations in the expression and function of cytoskeletal proteins. Budding uninhibited by Bub1 also plays a critical role in maintaining the organization of the cytoskeletal network that affects cell morphology. The overexpression of Bub1 induced a change in the cell shape and a reduction in cell motility, which can be modulated by structural alterations in both intrafilament and actin networks [Ando et al., 2008]. Therefore, our data show that two genes are functionally linked to cytoskeletal systems.
Among 25 downregulated genes, MCL1, BST2, and DPT were considered to involve pancreatic development. MCL1 trangenic mice evidenced hyperplasia of pancreatic β cells and inhibited the apoptosis of β cells [Matsushita et al., 2003]. NF-κB is involved in the upregulation of antiapoptotic genes, including Bcl2, Bcl-xL, Mcl-1, and inhibitor of apoptosis protein [Dasari et al., 2006]. Bone marrow stromal antigen 1 (BST-1) is expressed in pancreatic α and β cells [Kajimoto et al., 1996], however, the precise role of BST-2 in pancreatic development will have to be clarified further in the future. DPT is a tyrosine-rich acidic extracellular matrix protein with possible functions in cell–matrix interactions [Kuroda et al., 1999]. The reduction in DPT by TGF-β1 may be associated with increased fibrosis in islets.
In addition to EMT, the process known as “cadherin switching” which includes the downregulation of E-cadherin and the upregulation of mesenchymal cadherins was also noted [Hazan et al., 2004]. In our protein work, we were noted marked changes in the expression of a variety of structural proteins following TGF-β1 treatment. Those proteins belonged to the actin family (myosin heavy chain, ACTB, villin2, Bub1, dynactin) and the cytokeratin family [Tulachan et al., 2007] (Tables Ia and Ib). Gamma actin, tubulin, and mutant beta-actin were identified by mass spectrometry, although the ratio was below two times that of the controls. TGF-β1 induced an increase in transgelin2 as a smooth muscle actin alpha 22 homolog, as well as the downregulation of cytokeratin19. Thus, we considered this result to be the effect of TGF-β1-induced EMT. Our data did not show any change in cadherin as an indicator of TGF-β1-induced EMT either on microarray or proteomics.
Furthermore, TGF-β1 has been shown to induce a host of cytoskeleton rearrangement-related proteins. Hsp27 is involved in the pathogenesis of kidney tubulointerstitial fibrosis [Vargha et al., 2008]. Additionally, Hsp70 can interact with cytoskeletal elements, including microtubules, microfilaments, actin, and tubulin, and the proteins are required during extensive cytoskeletal rearrangement and disassembly, as was also shown to occur in the TGF-β2/FGF-2 treated rat lens epithelial explants [Takenaka and Hightower, 1992]. Therefore, TGF-β-induced actin disorganization as well as cytoskeleton disassembly occurs concurrently with the upregulation of Hsp70 and Hsp27 protein expression.
Collectively, these observations suggest that TGF-β1 induces EMT in cultured human pancreatic duct cells and suppresses the proliferation and transdifferentiation via the regulation of various genes' expression and the activation of Erk 1/2, JNK, and Akt pathways. Our results are the first report of TGF-β1 effects for EMT and ductal cell transdifferentiation and proliferation at the protein level in human pancreatic duct cells.




