Osteoclast-specific inactivation of the integrin-linked kinase (ILK) inhibits bone resorption
Abstract
Bone resorption requires the adhesion of osteoclasts to extracellular matrix (ECM) components, a process mediated by the αvβ3 integrin. Following engagement with the ECM, integrin receptors signal via multiple downstream effectors, including the integrin-linked kinase (ILK). In order to characterize the physiological role of ILK in bone resorption, we generated mice with an osteoclast-specific Ilk gene ablation by mating mice with a floxed Ilk allele with TRAP-Cre transgenic mice. The TRAP-Cre mice specifically excised floxed alleles in osteoclasts, as revealed by crossing them with the ROSA26R reporter strain. Osteoclast-specific Ilk mutant mice appeared phenotypically normal, but histomorphometric analysis of the proximal tibia revealed an increase in bone volume and trabecular thickness. Osteoclast-specific Ilk ablation was associated with an increase in osteoclastogenesis both in vitro and in vivo. However, the mutant osteoclasts displayed a decrease in resorption activity as assessed by reduced pit formation on dentin slices in vitro and decreased serum concentrations of the C-terminal telopeptide of collagen in vivo. Interestingly, compound heterozygous mice in which one allele of Ilk and one allele of the β3 integrin gene were inactivated (ILK+/−; β ) also had increased trabecular thickness, confirming that β3 integrin and Ilk form part of the same genetic cascade. Our results show that ILK is important for the function, but not the differentiation, of osteoclasts. J. Cell. Biochem. 110: 960–967, 2010. © 2010 Wiley-Liss, Inc.
) also had increased trabecular thickness, confirming that β3 integrin and Ilk form part of the same genetic cascade. Our results show that ILK is important for the function, but not the differentiation, of osteoclasts. J. Cell. Biochem. 110: 960–967, 2010. © 2010 Wiley-Liss, Inc.
Bone continuously undergoes remodeling, a process involving formation and resorption, which are mediated by osteoblasts and osteoclasts, respectively [Boyle et al., 2003]. An imbalance in this process can lead to bone diseases: increased resorption can lead to postmenopausal osteoporosis, while lack or dysfunction of osteoclasts causes the rare congenital disease osteopetrosis [Teitelbaum and Ross, 2003]. The large multinucleated osteoclasts develop from the fusion of multiple precursors of the monocyte-macrophage lineage [Boyle et al., 2003]. The cytokines M-CSF (macrophage colony-stimulating factor) and RANKL (receptor activator of nuclear factor κb ligand) are essential for this process. In vivo, these molecules are provided by bone marrow stromal cells or by osteoblasts, but the availability of the molecules in purified form now allows to efficiently generate osteoclasts in culture by incubating bone marrow derived monocyte/macrophage precursor cells with M-CSF and RANKL [Kobayashi et al., 2009].
Osteoclast bone resorption is a multistep process that begins with matrix recognition and attachment of the osteoclast, followed by polarization, formation of the sealing zone, and secretion of acids and lysosomal enzymes to the resorbing surface [Teitelbaum et al., 1995]. The resorption cycle is initiated by the retraction of the bone-lining cells. This action results in the exposure of the underlying osteoid which is removed by osteoclasts, so that the osteoclast can attach to the mineralized matrix. Interaction of the osteoclast with the mineralized bone matrix induces clustering of the αvβ3 integrin receptor and initiates intracellular signals involving in large part downstream phosphorylation events that enable binding and mediate actin-ring formation, osteoclast migration and bone resorption [Ross, 2008].
Of all the integrins expressed by osteoclasts, the most abundant is the αvβ3 integrin (vitronectin receptor), which is present in high copy number within the cell [Davies et al., 1989; Ross and Teitelbaum, 2005]. Antibodies directed at αvβ3 or inhibitory αvβ3 peptides and peptidomimetics block bone resorption in vitro, confirming that αvβ3 plays a major role in osteoclast function [Davies et al., 1989; Engleman et al., 1997]. Mice deficient for the β3 subunit display late onset osteopetrosis [McHugh et al., 2000]. While increased in number in the bone tissue, osteoclasts from these mice cannot spread, lack actin rings, and have reduced resorption activity [McHugh et al., 2000]. β3-deficient osteoclasts also have abnormal ruffled membranes with wide and blunt folds rather than numerous narrow membrane protrusions. The failure of mutant β3 osteoclasts to normally generate this structure suggests that αvβ3 mediates essential matrix-derived signals [McHugh et al., 2000; Feng et al., 2001]. Rescue of the phenotype is possible by expression of full-length human β3 integrin [Feng et al., 2001]. Interestingly, a truncated β3 lacking a cytoplasmic tail was completely ineffective in restoring the function of the mutant osteoclasts. These studies indicate the importance of the short cytoplasmic domain for osteoclast function [Feng et al., 2001].
Integrin binding to extracellular matrix components induces the assembly of multiprotein complexes that transduce downstream signals [Hynes, 2002; Legate et al., 2009]. A component of these complexes is the multifunctional protein Integrin-Linked Kinase (ILK), which interacts with the cytoplasmic β integrin domain and recruits actin binding regulatory proteins such as α- and β-parvin to organize actin and strengthen adhesion [Hannigan et al., 2005; McDonald et al., 2008; Fielding and Dedhar, 2009]. In addition to this scaffolding role, ILK has been shown to possess serine/threonine kinase activity [Delcommenne et al., 1998; Persad et al., 2001a]. Gene targeting has been used to discriminate between the kinase and adaptor roles of ILK, and the first interpretation of the results favors a major role for ILK as a protein scaffold during development [Lange et al., 2009]. However, not all tissues were examined in the mice models engineered and a physiological role for ILK as a bona fide kinase remains a formal possibility.
From the in vitro work performed to date, it was shown that the kinase activity of ILK is stimulated upon integrin engagement with the surface, as well as by growth factors and chemokines in a PI-3Kinase-dependant manner [Delcommenne et al., 1998], and negatively regulated by the lipid phosphatase tumor suppressor (PTEN) as well as the PP2C protein phosphatase (ILKAP) [Leung-Hagesteijn et al., 2001; Persad et al., 2001b]. ILK phosphorylates protein kinase B/Akt (Akt) at residue serine 473, resulting in Akt activation and promotion of cell survival by inhibition of apoptosis [Delcommenne et al., 1998; Persad et al., 2001a]. ILK-mediated phosphorylation of glycogen synthase kinase 3-β (GSK3 β) at the serine 9 site causes downregulation of its kinase activity, upregulating the expression of cell cycle genes [Delcommenne et al., 1998; Persad et al., 2000]. In bone cells, ILK phosphorylates the transcriptional coactivator αNAC to regulate its subcellular localization within osteoblasts [Quelo et al., 2004]. The physiological relevance of this post-translational modification was recently demonstrated using gene targeting to mutate the ILK phosphoacceptor site within the αNAC sequence in knockin mice [Meury et al., 2010].
The role of ILK in the differentiation or function of osteoclasts and as an effector of αvβ3 integrin-mediated signaling remains unknown, however. We specifically inactivated the Ilk gene in osteoclasts using TRAP-Cre mediated excision of a floxed Ilk allele to uncover a physiological role of ILK in osteoclastic resorptive activity.
MATERIALS AND METHODS
Animal Husbandry and Genotyping
All animal procedures were reviewed and approved by the McGill Institutional Animal Care and Use Committee and followed the guidelines of the Canadian Council on Animal Care. Mice were kept in an environmentally controlled barrier animal facility with a 12-h light, 12-h dark cycle and were fed mouse chow and water ad libitum. The TRAP-Cre mice were engineered by subcloning the Cre recombinase cDNA downstream from the TRAP promoter [Reddy et al., 1995]. The ILKfl/fl mice have been previously described [Terpstra et al., 2003]. The conventional ILK knockout strain was generated by replacing exons 3-12 of the Ilk gene by the PGK-neo selection cassette. The ROSA26R reporter strain [Soriano, 1999] was obtained from Dr. Gérard Karsenty, Columbia University. To generate the osteoclast-specific ILK-ablated mice, mice with one inactivated Ilk allele (ILK+/−) were mated with the TRAP-Cre transgenic mice. Progeny from this cross (TRAP-Cre;ILK+/−) was bred to mice homozygous for the floxed Ilk allele (ILKfl/fl) to yield mutant mice with ILK-deficient osteoclasts (TRAP-Cre;ILK−/fl, hereafter referred to as ILKΔ/−). The mutant animals thus had one Ilk allele inactivated in all tissues, and both alleles disrupted in osteoclasts.
To generate the compound Ilk; integrin β3 heterozygotes, the conventional ILK knockout strain was crossed with the previously described integrin β3-deficient strain [McHugh et al., 2000].
All mice were genotyped by PCR amplification of DNA prepared from tailsnips. Primer sequences are available upon request.
Sample Preparation
Bone samples were fixed in 4% paraformaldehyde (PFA), then either embedded in OCT (OCT Compound Tissue Tek, Electron Microscopy Sciences, Hatfield, PA), methylmethacrylate (MMA), or decalcified and embedded in paraffin prior to sectioning [An and Martin, 2003].
Tartrate Resistant Acid Phosphatase (TRAP) and LacZ Staining
Deplastified, 7 µm sections from decalcified, paraffin-embedded bone samples were immersed in TRAP staining solution (0.3 mM Napthol AS-BI phosphate, 3.7 mM Fast Red Violet LB salt, 50 mM sodium tartrate dihydrate, 2.5 mM MgCl2 in 0.2 M acetate buffer, pH 5.0) for 2.5 min at 37°C, then rinsed under running cold water for 30 min. The samples were allowed to dry at room temperature and then mounted using GVA Mount (Zymed Laboratories Inc., San Francisco, CA). For LacZ staining, 5 µm cryosections were rinsed in PBS and incubated in β-galactosidase staining solution (2 mM MgCl2; 4 mM K3Fe(CN)6; 4 mM K4Fe(CN)6; 0.01% Tween-20; 0.4 mg/mL X-galactosidase in dimethylformamide) within a humidified chamber protected from light for 14 h at 37°C. Sections were then rinsed in PBS, counterstained with 0.1% Safranin O for 30 s, and rinsed again in PBS prior to mounting with GVA.
Reverse Transcription Quantitative PCR (RT-qPCR)
RNA was isolated from primary osteoclastic cultures generated from femur bone marrow using TRIzol Reagent (Invitrogen Canada Incorporated, Burlington, ON), following the manufacturer's protocol. Ten µg of RNA were reverse-transcribed into cDNA using the High Capacity cDNA Archive kit as per the manufacturer's recommendations (Applied Biosystems, Foster City, CA). RT-qPCR amplification was performed on an Applied Biosystems 7500 instrument using specific TaqMan probes and the Taqman Universal PCR Master Mix (Applied Biosystems). Expression level was quantified by the ΔCt method and normalized to Gapdh levels.
Histomorphometry
Images of Goldner stained, undecalcified, MMA-embedded tibial sections were obtained using a Leica DC300F digital camera (Leica Microsystems) connected to a Leica HC DMR Microscope (Leica Microsystems) and quantitatively analyzed using Bioquant NovaPrime software (Bioquant Image Analysis Corporation, Nashville, TN). Parameters were measured within the metaphyseal area of the proximal tibia. Results are presented as mean ± SEM. Statistical analysis was by Student's t-test and P < 0.05 was accepted as significant.
C-Terminal Telopeptide of Collagen Immunoassay
Serum from 6-week-old osteoclast-specific ILK-deficient and wild-type littermates was assayed using the RatLaps ELISA (Nordic Biosciences A/S, Chesapeake, VA) for the quantification of type I collagen fragments released during bone resorption following the manufacturer's instructions.
Pit Formation Assay on Dentin Slices
Marrow from 22-day-old femurs was flushed in αMEM with 10% FBS, 1% penicillin/streptomycin/fungizone, 10−6 M dexamethasone, and 2% glutamine. Cells were plated at a density of 106 cells/ml in 96-well plates containing a sterilized 200 µm-thick slice of dentin. Every 48 h, the wells were washed gently with warm PBS to remove non-adherent cells and fresh media containing 50 ng/ml of macrophage-colony stimulating factor (M-CSF) and 50 ng/ml of receptor-activator of NFκB ligand (RANK-L) was added, for a total culture period of 9 days [Susa et al., 2004]. Cells were then washed away and the dentin slice was stained using toluidine blue. Resorbed area was quantified using the Bioquant NovaPrime software.
Micro-Computed Tomography (µCT)
Trabecular thickness in 2 months old compound heterozygous mice (ILK+/−; β ) and control littermates (ILK+/+; β
) and control littermates (ILK+/+; β ) was measured by µCT. The tibiae were collected and fixed in 4% PFA overnight. The samples were then washed in PBS, placed in 70% ethanol, and analyzed using the SkyScan 1072 instrument from the McGill Center for Bone and Periodontal Research.
) was measured by µCT. The tibiae were collected and fixed in 4% PFA overnight. The samples were then washed in PBS, placed in 70% ethanol, and analyzed using the SkyScan 1072 instrument from the McGill Center for Bone and Periodontal Research.
RESULTS
In order to confirm the ability of the TRAP-Cre transgene to induce osteoclast-specific recombination, TRAP-Cre mice were mated with the ROSA26 reporter (ROSA26R) strain [Soriano, 1999] and examined for tissue-specific recombination. Embryonic femurs from TRAP-Cre X ROSA26R mice were isolated and stained for β-galactosidase expression. LacZ staining was observed near the ends of trabeculae in large, multinucleated cells (Fig. 1B). Sections were also stained for TRAP activity. The corresponding cells stained red (Fig. 1A), confirming that the TRAP-Cre reporter strain allowed for the osteoclast-specific recombination of genes flanked with lox p sites.

Staining of osteoclasts in bone sections from TRAP-Cre; Rosa26R mice. Sagital sections of the proximal femur from a TRAP-Cre; Rosa26R+/− mouse at E15.5. The bone was fixed, embedded in OCT, and cryosectioned. A: TRAP staining. B: LacZ staining. Osteoclasts that express TRAP are stained red; Cre-mediated excision results in the expression of β-galactosidase, causing a blue stain in the LacZ assay.
Mice with an osteoclast-specific inactivation of Ilk (referred to as ILKΔ/−) appeared phenotypically normal and showed no differences in size or weight with their control littermates at birth or throughout their lifespan (data not shown). ILKΔ/− mice had normal tooth eruption. To evaluate the efficiency of TRAP-Cre-mediated excision, primary osteoclast cultures were prepared from ILKΔ/− mice and control littermates. RNA was harvested after 9 days in culture and analyzed for Ilk expression by RT-qPCR as a surrogate marker of Cre-mediated excision. There was no difference in the relative Ilk expression level or any other parameter examined between all control genotypes (ILK+/fl, ILK−/fl, and TRAP-Cre;ILK+/fl, data not shown) and results from these animals were grouped and expressed as “controls.” Ilk expression was significantly reduced by up to 94% in ILKΔ/− osteoclasts, with a mean reduction of 80% (Fig. 2). These results suggest that the floxed Ilk allele was efficiently deleted by the Cre recombinase in osteoclastic cells.
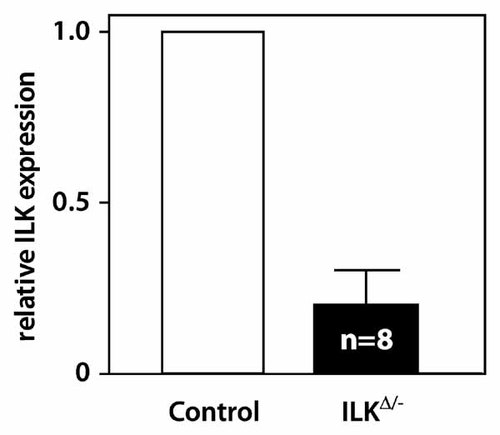
Ilk expression in wild-type and ILKΔ/− osteoclasts. RNA was harvested from primary cultures of osteoclasts from ILKΔ/− mice or wild-type littermates (Control) and analyzed by RT-qPCR. Values are expressed as mean ± SEM of eight cultures of each genotype; expression in cells from control mice was arbitrarily assigned a value of 1. Expression in ILKΔ/− osteoclasts was significantly reduced (P < 0.001).
Analysis of Goldner-stained proximal tibial sections revealed an apparent increase in the number and size of the bone trabeculae at the metaphysis in mice with an osteoclast-specific Ilk mutation (Fig. 3A,B). This increase was confirmed upon histomorphometric analysis. We measured an overall 24% increase in bone volume (Fig. 3C) as well as a significant 44% increase in trabecular thickness (Fig. 3D) in ILKΔ/− mutants compared to control littermates. These data suggest that the inactivation of Ilk within osteoclasts may affect resorption, resulting in increased bone volume.
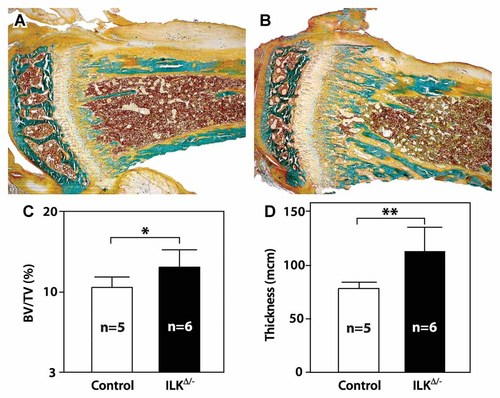
Increased bone volume and trabecular thickness in ILKΔ/− mice. Tibia from 6-week-old wild-type (A) and ILKΔ/− (B) mice were embedded in methylmethacrylate and the undecalcified bones were sectioned, Goldner-stained, and analyzed by histomorphometry at the proximal end. Bone volume/tissue volume (BV/TV; panel C) and trabecular thickness (D) were significantly increased. Results are mean ± SEM of the sample size indicated in the bars of the graphs. *P < 0.05; **P < 0.01.
To determine whether Ilk inactivation affects osteoclast differentiation or activity, we first measured osteoclast numbers in bone sections from ILKΔ/− mice and control littermates. Histomorphometric analysis of TRAP-stained paraffin sections revealed a significant increase in osteoclast number within the primary spongiosa of ILKΔ/− animals (Fig. 4A). We also measured increased osteoclastogenesis in primary cultures of M-CSF and RANKL-treated marrow cells from ILKΔ/− mice as compared to cultures from control animals (Fig. 4B). When these in vitro differentiated primary osteoclasts were plated on dentin slices, we observed a decrease in resorption activity. The pits formed on dentin by ILK-deficient osteoclasts were fewer in number and the resorption tracks were not well demarcated, as compared to those made by control cells (Fig. 4C,D). Quantification of the resorbed surfaces by histomorphometry confirmed a 45% decrease in osteoclastic resorption activity within cells lacking ILK (Fig. 4E). A similar result was obtained when osteoclasts were plated on a synthetic calcium phosphate substrate (data not shown). Interestingly, we also measured a significant 37% decrease in the serum concentration of collagen deoxypiridinoline crosslinks in ILKΔ/− animals (Fig. 4F), supporting the concept that ILK-deficient osteoclasts show impaired resorption activity in vivo as well as in vitro. Taken together, we interpret these data to mean that Ilk inactivation affects the function, but not the differentiation, of osteoclasts.
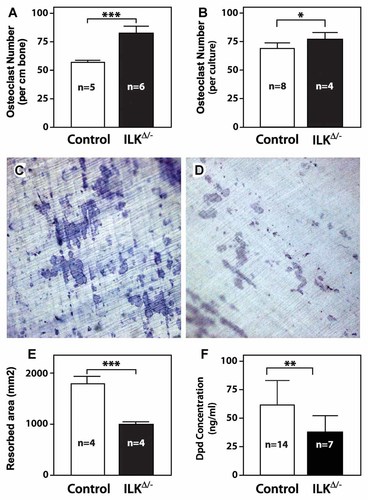
Increased differentiation of osteoclasts with reduced activity in ILKΔ/− mice. A: osteoclast numbers in TRAP-stained sections from control and ILKΔ/− mice were quantified by histomorphometry. B: Marrow cells from control and ILKΔ/− mice were differentiated into osteoclasts by treatment with M-CSF and RANKL and the number of TRAP-positive cells in each culture was quantified. C,D: Pit formation on dentin slices. Primary cultures of osteoclasts from control (C) or ILKΔ/− (D) mice were plated on dentin for 9 days. Cells were then washed away and dentin discs were stained with Toluidine blue. The resorbed area was quantified by histomorphometry and is graphed in panel E. F: Concentration of Dpd crosslinks in blood from 6-week-old control and ILKΔ/− mice were assayed by ELISA. For all bar graphs, results are mean ± SEM of the sample size indicated in the bars. *P < 0.05; **P < 0.01; ***, P < 0.001.
ILK is a downstream effector of integrin signaling [McDonald et al., 2008] and interacts with the cytoplasmic tail of β3 integrin [Hannigan et al., 1996]. Mice deficient for β3 integrin display late-onset osteopetrosis with an augmented number of osteoclasts that have decreased resorption activity [McHugh et al., 2000], a phenotype similar, albeit more pronounced, to what we observed in ILKΔ/− animals. To determine if β3 integrin and Ilk are part of a common genetic pathway, we generated compound Ilk; integrin β3 heterozygotes (ILK+/−; β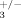 ) and compared their bones to control littermates (ILK+/+; β
) and compared their bones to control littermates (ILK+/+; β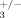 ) using micro-computed tomography. Mice with lower gene dosage for both Ilk and β3 integrin (ILK+/−; β
) using micro-computed tomography. Mice with lower gene dosage for both Ilk and β3 integrin (ILK+/−; β ) displayed a significant increase in the thickness of the trabeculae at the primary spongiosa (Fig. 5), as was observed in ILKΔ/− animals (Fig. 3D). These results confirm that β3 integrin and Ilk form part of the same genetic cascade.
) displayed a significant increase in the thickness of the trabeculae at the primary spongiosa (Fig. 5), as was observed in ILKΔ/− animals (Fig. 3D). These results confirm that β3 integrin and Ilk form part of the same genetic cascade.
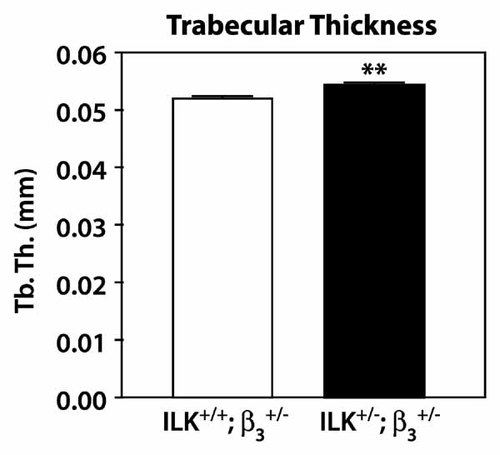
Increased trabecular thickness in compound Ilk; integrin β3 heterozygotes. Trabecular thickness (Tb. Th.) was measured by µCT in 2 months old compound heterozygous mice (ILK+/−; β , n = 5) and control littermates (ILK+/+; β
, n = 5) and control littermates (ILK+/+; β , n = 7). **P < 0.01.
, n = 7). **P < 0.01.
DISCUSSION
We have successfully engineered mice with an osteoclast-specific mutation for ILK. Phenotypic analysis of these mice revealed increased bone volume and an augmented number of osteoclasts, but reduced circulating levels of collagen degradation products. The increased osteoclastogenesis and inhibited resorptive activity were confirmed in vitro. We conclude that the Ilk gene is important for osteoclast function, but not differentiation.
Gene expression monitoring by RT-qPCR suggests that the floxed Ilk allele was efficiently excised by the Cre transgene. TRAP is expressed in multiple tissues but promoter fragments with restricted expression patterns have been identified [Reddy et al., 1995; Pan et al., 2005]. We ascertained that in bone, the only cell type expressing the TRAP-Cre transgene was the osteoclast and thus the bone-related phenotype that we report is caused by osteoclast-specific inactivation of the target gene.
ILK was identified as a protein interacting with the cytoplasmic domain of β integrin chains and protein–protein interaction have been demonstrated between ILK and β1 and ILK and β3 integrins [Hannigan et al., 1996]. Ilk inactivation in osteoclasts did not affect expression of either αv or β3 integrins (data not shown). Signaling downstream from integrins involves numerous effectors [Hynes, 2002; Legate et al., 2009] and ablating β3 function most likely impinges on several cascades. It is thus not surprising that the phenotype of integrin β3-deficient bones would be more severe than a bone phenotype caused by the inactivation of a single, specific downstream effector. Nevertheless, the phenotypes of mice deficient for molecules operating within the same pathway should be similar and can be affected by gene dosage. Albeit with reduced severity, there is striking similarity between the phenotypes of Ilk- and β3-deficient bones: increased number of osteoclasts with reduced resorptive functionality. More than these phenocopy aspects, the effect of gene dosage reduction in compound heterozygotes confirm that β3 and Ilk reside within the same pathway and establish ILK as a bona fide downstream effector of the integrin signal in vivo.
β3-deficient osteoclasts show difficulties in spreading, lack actin rings, and form irregular ruffled borders [McHugh et al., 2000]. It will prove interesting to study these aspects of osteoclast function in ILK-deficient cells as ILK has been shown to affect cell adhesion [Hannigan et al., 1996; Attwell et al., 2003]. Preliminary data using transmission electron microscopy suggest ruffled border anomalies in osteoclasts of ILKΔ/− mice (not shown).
ILK is a multifunctional protein that functions as a scaffold in addition to acting as a kinase [Hannigan et al., 2005; McDonald et al., 2008; Fielding and Dedhar, 2009]. In its scaffolding role, it regulates actin dynamics by recruiting actin binding regulatory proteins such as α- and β-parvins [Legate et al., 2006]. Using mice targeted for either the kinase or the protein interaction domains of ILK, it has been proposed that the kinase activity of ILK is not necessary for mammalian development while its scaffolding role would be critical [Lange et al., 2009]. It should be noted that the bone phenotype of the mutant animals used in these studies was not examined [Lange et al., 2009]. Treating primary osteoclast cultures with ILK kinase inhibitors [Persad et al., 2000] or attempting to rescue the phenotype of ILKΔ/− osteoclasts with expression vectors for kinase-dead ILK would allow to determine if the kinase activity of ILK is necessary for osteoclast function. There is genetic evidence that the kinase activity of ILK is important for bone cell activity: mutating the ILK phosphoacceptor site within the αNAC transcriptional regulator affects matrix gene expression and leads to osteopenia [Meury et al., 2010].
Considering the multifunctional nature of ILK, several downstream mechanisms could contribute to the impaired function of ILK-deficient osteoclasts. Attachment and spreading could be affected by abnormal recruitment of PINCH, paxillin, or α- or β-parvin to focal adhesions or reduced activation of the Rho family of GTPases Rac or Cdc42 [Nakrieko et al., 2008]. Survival could be modulated through impaired PKB/Akt activation [Delcommenne et al., 1998]. Detailing the mechanisms affected downstream of the inactivation of ILK in osteoclasts will provide increased understanding of the molecular effectors of skeletal resorption.
Acknowledgements
We thank Dr. Svetlana Komarova of McGill University for help with the dentin slices resorption pit assay. The ROSA26R reporter strain was obtained from Dr. Gérard Karsenty, Columbia University. We used the microcomputed tomography instrument from the Center for Bone and Periodontal Disease, McGill University. Mia Esser, Louise Marineau, and Nathalie Guevremont provided expert help in animal care and use. Guylaine Bédard prepared the figures. Supported by a research grant from the Shriners of North America to R.St-A.




