CDK5 phosphorylates eNOS at Ser-113 and regulates NO production†
Chien-Hsing Lee and Yin-Win Wei contributed equally to this work.
Abstract
Phosphorylation of endothelial nitric oxide synthase (eNOS) is key mechanism in response to various forms of cellular stimulation. Through protein nitration by peroxynitrite, eNOS is believed to be responsible for the major abnormalities in several important neurodegenerative diseases including Alzheimer's (AD) and Parkinson's diseases (PD). Recent studies provide important in vivo evidence that hyperactivation of Cdk5 by p25 plays an essential role in the cell death of neurons in experimental models of AD and PD. This study focuses on the functional regulation of eNOS by Cdk5/p35 complex in a phosphorylation dependent manner. Our results showed that Cdk5 can phosphorylate eNOS both in vitro and in vivo. In vitro kinase assay together with the bioinformatic analysis and site direct mutagenesis revealed that Ser-113 is the major phosphorylation site for Cdk5. Most interestingly, the nitrite production was significantly reduced in eNOS and Cdk5/p35 co-transfected SH-SY5Y cells when compared with co-transfection of Cdk5/p35 and S113A. Together, our data suggest that Cdk5 can phosphorylate eNOS at the Ser-113 site and down-regulate eNOS-derived NO levels. J. Cell. Biochem. 110: 112–117, 2010. © 2010 Wiley-Liss, Inc.
Nitric oxide (NO) regulates multiple physiological and pathophysiological processes in the nervous system such as learning and memory, neuronal survival and differentiation [De Palma et al., 2008]. NO is generated from NO synthases (NOSs) through the oxidation of a guanidino nitrogen of L-arginine. The NOS isoforms have been identified and divided into two functional classes: constitutive and inducible. The two constitutive isoforms, initially identified in neurons (nNOS) and endothelial cells (eNOS), are expressed in central nervous system neurons [Dinerman et al., 1994; Campese et al., 2007]. In the nervous system, high levels of NO can be either detrimental or beneficial to cell viability and function. The cytotoxic effects of NO may be due to peroxynitrite formation and nitration of tyrosine residues in proteins, or the generation of superoxides [de la Monte et al., 2000]. Moreover, the presence of nitrotyrosination has been described in several neurodegenerative diseases linked to oxidative stress, such as Alzheimer's disease (AD), Parkinson's disease (PD) and amyotrophic lateral sclerosis (ALS) [Guix et al., 2005].
Another important kinase linked to neurodegeneration diseases is cyclin-dependent kinase 5 (Cdk5). Cdk5 is an atypical member of the cyclin-dependent kinase family that has unique functions in the central nervous system. A marked interest among cell biologists and neuroscientists has been generated by the findings that Cdk5 and the regulatory protein p35, also named neuronal Cdk5 activator, which are expressed almost exclusively in postmitotic neurons [Ohshima et al., 2002]. Recent studies provide important in vivo evidence that hyperactivation of Cdk5 by p25 plays an essential role in the phosphorylation of tau and the cell death of neurons in experimental models of AD and PD [Ko et al., 2001; Camins et al., 2006]. Meanwhile, in enhanced NO production in AD and other neurodegenerative diseases implicates also a potential role for eNOS [De Palma et al., 2008]. However, the relationship between eNOS activity and Cdk5 in neuron cells remains unknown.
The eNOS activity is controlled by fatty acid modification, phosphorylation, and direct protein-protein interactions [Zhao et al., 2009]. Here, we investigated whether the protein kinase Cdk5 phosphorylates eNOS and influences eNOS function. The eNOS was analyzed by NetPhos website (www.cbs.dtu.dk/services/NetPhos) server to search for specific phosphorylation site candidates. The results showed that eNOS may be phosphorylated by Cdk5. Seven putative Cdk5 phosphorylation sites in eNOS (Ser53, Ser57, Thr95, Ser113, Ser867, Ser860, and Ser1203) were predicted by NetPhos website server. The phosphorylation of eNOS at Ser-113 has been shown to stimulate by angiopoietin-related growth factor to enhance NO production [Urano et al., 2008], indicating that Ser-113 may be an important regulatory site for the phosphorylation of eNOS. Our results suggested that Cdk5 interacts with eNOS at the Ser-113 site and regulates NO production.
MATERIALS AND METHODS
Plasmid Constructs and Site Directed Mutagenesis
Putative Cdk5 serine/threonine sites in eNOS were predicted by NetPhos (www.cbs.dtu.dk/services/NetPhos). The eNOS-pCMV XL6 plasmid was purchased from OriGene. Full-length eNOS mutant constructs containing alanine codons instead of serine at predicted Cdk5 kinase sites were generated by PCR using eNOS-pCMV XL6 as the template together with the following primers: forward primers 5′-GGC CGG CCC GCC CCC GGC CCC-3′ and reverse primers 5′-GGG GCC GGG GGC GGG CCG GCC-3′. All of the cDNA clones were confirmed by DNA sequencing.
Antibodies and Chemicals
Monoclonal anti-eNOS antibody was purchased from BD Transduction Laboratories. Polyclonal anti-Cdk5 and anti-p35 antibody were obtained from Santa Cruz Biotechnology Inc. Polyclonal anti-Ser (P)113 antibody specific for eNOS phosphorylated on Ser113 was from Cell Signaling Technology. Cdk5/p35 dimeric complex protein was purchased from Cell Signaling Technology. The Cdk5 inhibitor Roscovitine was purchased from Sigma–Aldrich, then prepared as stock solution in DMSO (1 mg/ml) and stored at −20°C.
Cell Culture and Transfection
Human neuroblastoma SH-SY5Y cells were grown in Dulbecco's modified Eagle's/F12 medium supplemented with 5% fetal bovine serum (FBS), penicillin/streptomycin, 2 mM L-glutamine, 1 mM sodium pyruvate and 0.1 mM nonessential amino acids on poly-L-lysine-coated dishes. These Cells were grown in a humidified incubator at 37°C in a 5% CO2 atmosphere.
Human neuroblastoma SH-SY5Y cells were transfected using Lipofectamine 2000 reagent (Invitrogen). After transfection 16 h, the condition medium were harvested and the nitrite concentration were analysis by Griess assay, and following the cells were collected for Western blot analysis, or immunoprecipitation, or flow cytometry.
Immunoprecipitation
Fresh rat brain tissue or transient transfected SH-SY5Y cells were lysed with RIPA buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 0.25% deoxycholic acid, 1% NP-40, 1 mM EDTA, 1 mM PMSF, 1 mM sodium orthovanadate, 1 mM sodium fluoride, 1 µg/ml aprotinin, 1 µg/ml leupeptin, 1 µg/ml pepstatin). For immunoprecipitation of Cdk5 and eNOS, the Catch and Release® v2.0 Reversible Immunoprecipitation System (Upstate) was employed. Briefly, 1× wash buffer, 600 µg of cell lystate, 2 µg of the antibody, and antibody capture affinity ligand were added to a spin column at a final volume of 500 µl. Following 3 h incubation on a rotator at 4°C, the column was centrifuged at 5,000 rpm for 30 s to discard all the non-labeled proteins. The beads were washed three times with 500 µl 1× wash buffer, and bound proteins were used for in vitro phosphorylation studies or Western blot analysis.
In Vitro Phosphorylation Assay
eNOS protein (immunoprecipitated from rat brain lysate) were incubated with the Cdk5/p35NCK dimeric complex kinase (Cell Signaling Technology) for 30 min at 30°C in the presence of 0.1 mM [γ-32P]ATP or cold 0.1 mM ATP in a reaction buffer containing 50 mM Tris–HCl pH 7.5 with 1 mM EGTA, 1 mM DTT, 10 mM MgCl2 and 0.01% Brij 35. Proteins were resolved by 10% polyacrylamide gel, autoradiographed, and the amount of protein was determined by Western blot analysis. Alternatively, on the phosphorylation of eNOS proteins by Cdk5-p35 kinase, the phosphorylation of eNOS could analyzed by Western blot analysis using anti-eNOS or anti-Ser(P)113 antibodies.
Western Blot Analysis
Proteins were resolved by 10% SDS–PAGE, blotted onto a PVDF membrane (Boehringer Mannheim), blocked in 5% skimmed milk, 10% PBS, 0.05% Tween-20 and probed with primary antibodies. Following incubation with horseradish peroxidase-conjugated goat anti-rabbit antibody (Bio-Rad) and horseradish peroxidase-conjugated goat mouse-mouse antibody (Bio-Rad), bound immunoglobulins were detected using enhanced chemiluminescence (Amersham).
Flow Cytometry
Cells were transfected with GFP fusion protein for 16 h, collected and resuspended in phosphate buffered saline. A total of 2,000 cells were analyzed by FACScan Flow Cytometer (BD Biosciences).
Nitrite Detection
Nitrite (NO ) generation in SH-SY5Y cells were determined by the Griess assay [Green et al., 1982]. Briefly, SHSY5Y cells, plated in six-well plates, were incubated at 37°C in 1 ml of normal Krebs phenol red-free solution for 5 min. At the end of incubation, 500 µl Griess reagent (1% sulfanilamide, 0.1% naphthylethylenediamine in 2% H3PO4) was added to each sample and left in place for 10 min at room temperature. Absorbance was measured at 550 nm, and NO
) generation in SH-SY5Y cells were determined by the Griess assay [Green et al., 1982]. Briefly, SHSY5Y cells, plated in six-well plates, were incubated at 37°C in 1 ml of normal Krebs phenol red-free solution for 5 min. At the end of incubation, 500 µl Griess reagent (1% sulfanilamide, 0.1% naphthylethylenediamine in 2% H3PO4) was added to each sample and left in place for 10 min at room temperature. Absorbance was measured at 550 nm, and NO concentrations were determined via standard curve by using sodium nitrite. Limits of detection of the method used were 50 nM [Canzoniero et al., 2006].
concentrations were determined via standard curve by using sodium nitrite. Limits of detection of the method used were 50 nM [Canzoniero et al., 2006].
Statistical Analysis
Statistical analysis was performed with one-way ANOVA followed by Turkey's post hoc test. P value of <0.05 was considered to be statistically significant. Data are shown as mean ± SEM.
RESULTS
CDK5 Binds and Phosphorylates eNOS In Vivo and In Vitro
To clarify the interaction between eNOS and Cdk5, we first used co-immunoprecipitation assay to verify this association in vivo. Immunoprecipitation of eNOS by eNOS antibodies co-immunoprecipitate of endogenous Cdk5 in rat brain extracts. Using anti-Cdk5 antibodies, eNOS was co-precipitated with endogenous Cdk5. Conversely, when anti-eNOS antibodies were used, Cdk5 co-precipitated with eNOS (Fig. 1A). Both experiments demonstrated complex formation between Cdk5 and eNOS.
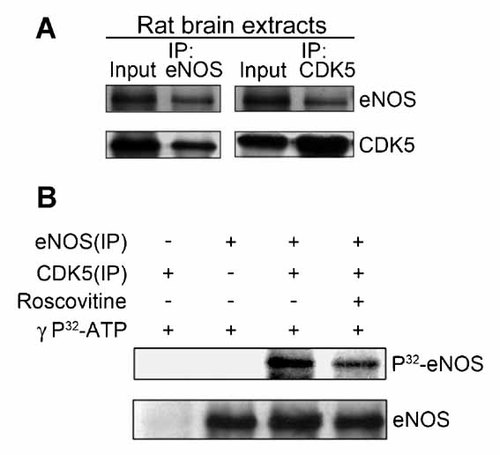
Cdk5 associate with eNOS in vivo and in vitro. A: Rat brain extracts were prepared and immunoprecipitated (IP) with anti-Cdk5 and anti-eNOS antibodies, following immunoblotted with anti-Cdk5 and anti-eNOS antibodies, respectively. B: The eNOS were purified from rat brain by immunoprecipitation, and subjected to an in vitro kinase assay. eNOS were incubated with immunoprecipitated Cdk5 or treated with the Cdk5 inhibitor roscovitine (20 µM) for 2 h at room temperature, and then analyzed by Western blotting assay and autography.
In vitro eNOS phosphorylation was performed by incubating the immunoprecipitated eNOS with Cdk5/p35 dimeric complex protein in the presence of 0.1 mM [γ-32P] ATP. The [γ-32P] ATP was incorporated into eNOS and was detected by radioautography. Roscovitine has been inhibited cyclin-dependent kinase by binding to the catalytic domain of the cyclin-dependent kinase molecule in place of ATP, which prevents transfer of a phosphate group to the substrate [Meijer et al., 1997]. We investigated whether roscovitine abolishes the phosphorylation of eNOS by Cdk5. As shown in Figure 1B, incorporation of [γ-32P] ATP was suppressed in the presence of roscovitine (20 µM). This result demonstrates that eNOS can be directly phosphorylated by Cdk5.
CDK5 Directly Phosphorylates eNOS at Ser-113
To determine whether Ser-113 of eNOS is a major phosphorylation site for the regulation of Cdk5, we co-transfected with Cdk5 and wild-type or S113A eNOS in SH-SY5Y cells for 24 h, the proteins were extracted and analyzed by Western blot assay using Ser(P)113 antibodies and anti-eNOS antibodies (Fig. 2A). Co-transfection of Cdk5/p35 and eNOS induced more phospho-eNOS (Ser-113) than only transfection of eNOS (increased 46%, n = 3, P < 0.05), and the phosphorylation at Ser-113 was down-regulated in the presence of roscovitine (40 µM) (decreased 11%, n = 3, P < 0.05) (Fig. 2B). Moreover, the phosphorylation of eNOS at Ser-113 was inhibited 87% (n = 3, P < 0.05) in Cdk5/p35 and S113A eNOS co-transfected SH-SY5Ycells (Fig. 2A,B). Collectively, these results suggest that Ser-113 of eNOS is a major site for Cdk5 phosphorylation.
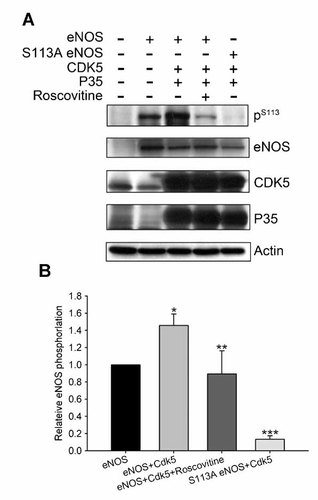
Phosphorylation of eNOS by Cdk5 is in SH-SY5Y cells. A: SH-SY5Y cells were transfected with or without eNOS, or co-transfected with Cdk5/p35 with eNOS or S113A eNOS, and protein extracts were prepared for Western blot analysis using anti-Ser(P)113 and anti-eNOS antibodies. B: The eNOS phosphorylation levels in the culture media. Results represented the mean ± SEM from three experiments. Data were analyzed using one-way ANOVA followed by Turkey's post hoc test. *P < 0.05 versus eNOS groups, **P < 0.05 versus eNOS + Cdk5 groups and ***P < 0.001 versus eNOS group.
To further confirm whether Cdk5 directly phosphorylates eNOS on Ser-113, we transfected with wild-type eNOS or S113A eNOS in SH-SY5Y cells, immunoprecipitated wild-type or S311A eNOS, and determined this interaction using an in vitro phosphorylation assay. To immunoprecipitate using anti-eNOS and anti-Cdk5 antibodies, in vitro phosphorylation assay were performed by incubation of immunoprecipitated eNOS with Cdk5/p35, and analyzed by immunoblot analyses using anti-Ser(P)113, anti-eNOS, and anti-GST antibodies (Fig. 3A). As shown in Figure 3B, the phosphorylation of Ser-113 within eNOS was observed in immunoprecipitated product by anti-eNOS antibodies, and the amounts of phosphorylation were increased on the appearance of recombinant Cdk5 and p35 (from 1 to 4.7 ± 0.1, n = 3). Furthermore, the amounts of phosphorylation were decreased in adding 40 µM roscovitine (4.7 ± 0.1 in eNOS+Cdk5/p35 vs. 3.6 ± 0.3 in eNOS+Cdk5/p35+roscovitine, n = 3). These results suggest that Cdk5 directly phosphorylates eNOS at Ser-113.
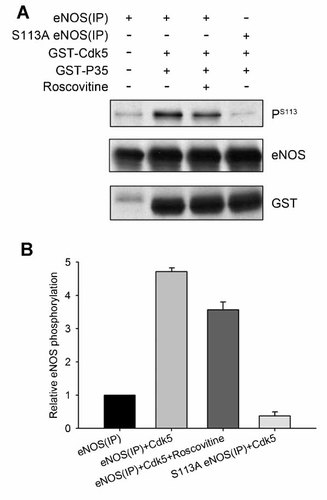
In vitro phosphorylation of eNOS by Cdk5 is inhibited by roscovitine A: Recombinant Cdk5 and p35 were incubated with immunoprecipitated eNOS or S113A eNOS in the presence of Cdk5 inhibitor roscovitine (40 µM) or not. After incubation for 30 min at 30°C, the mixture were analyzed by Western blot analysis using anti-Ser(P)113, anti-NOS and anti-GST antibodies. B: eNOS phosphorylation levels in the culture media. Results represented the mean ± SEM from three experiments.
CDK5 Phosphorylates eNOS at Ser113 and Regulates NO Production
To characterize whether the interaction between Cdk5 and eNOS is mediated by Ser 113, HA-Cdk5 and eNOS or S113A eNOS were transfected to SH-SY5Y cells. Transfected cells were lysed and subjected for immunoprecipitation using either anti-eNOS or anti-Cdk5 antibodies. Each complex was then immunoblotted against anti-eNOS or anti-Cdk5 antibodies. As shown in Figure 4A, Cdk5 specifically co-immunoprecipitated with eNOS and also coimmunoprecipitated with S113A eNOS, indicating that Ala substitute for Ser-113 does not significantly interfere with the binding of eNOS to Cdk5. In other words, the binding of eNOS to Cdk5 is in a phosphorylation independent manner.

Cdk5 phosphorylated eNOS on Ser113 and regulated NO production. A: eNOS and Cdk5 co-immunoprecipitate. The eNOS (WT) and eNOS (S113A) were immunoprecipitated (IP) from lysates of SH-SY5Y cells transfected with indicated genes using anti-eNOS and anti-Cdk5 antibodies. Each complex was analyzed by Western blot using the indicated antibodies. B: Human neuroblastoma SH-SY5Y cells were transient expressed eNOS, S113A eNOS, Cdk5 and p35 before treated with or without Cdk5 inhibitor roscovitine (40 µM) for 12 h. The analyses of Nitrite (NO ) generation were performed by Griess assay. Each bar represents the mean ± SEM from three experiments. Data were analyzed using one-way ANOVA followed by Turkey's post hoc test. *P < 0.01 versus transient expression eNOS groups; **P < 0.05 versus transient expression eNOS and Cdk5/p35 groups; ***P < 0.05 versus transient expression eNOS groups.
) generation were performed by Griess assay. Each bar represents the mean ± SEM from three experiments. Data were analyzed using one-way ANOVA followed by Turkey's post hoc test. *P < 0.01 versus transient expression eNOS groups; **P < 0.05 versus transient expression eNOS and Cdk5/p35 groups; ***P < 0.05 versus transient expression eNOS groups.
To demonstrate the regulation of eNOS activity by Cdk5 in SH-SY5Y cells, we co-transfected eNOS and Cdk5/p35 in SH-SY5Y cells for 24 hours. Measurement of eNOS activity (nitrite production) was analyzed by Greiss assay. As shown in Figure 4B, nitrite production was increased in eNOS transfected cells (126.2 ± 31.8 in control vs. 479.1 ± 37.6 in eNOS n = 3, P < 0.05), and nitrite production was reduced in eNOS and Cdk5/p35 co-transfected cells compared with eNOS transfected cells (from 479.1 ± 37.6 to 287.3 ± 42.2, n = 3, P < 0.01). Incubation of 40 µM roscovitine for 18 h rescued the reduction of nitrite production in eNOS and Cdk5/p35 co-transfected cells (381.2 ± 8.3 in S113A eNOS+Cdk5/P35 vs. 287.3 ± 42.2 in eNOS + Cdk5/P35, n = 3, P < 0.05). Moreover, co-transfection of Cdk5/p35 and S113A eNOS also recovered the reduction of nitrite production in eNOS and Cdk5/p35 co-transfected cell (620.8 ± 59.2 in S113A eNOS + Cdk5/P35 vs. 287.3 ± 42.2 in eNOS + Cdk5/P35, n = 3, P < 0.05). Our results showed that Cdk5 phosphorylates and regulates eNOS activity.
DISCUSSION
The present study is to describe that a novel mechanism of Cdk5 phosphorylates eNOS and modulates its activity. Our results demonstrated that Cdk5 can phosphorylate eNOS both in vitro and in vivo (Fig. 1). We revealed that the Ser-113 of eNOS is a major site for Cdk5 phosphorylation by using in vitro phosphorylation assay, the bioinformatic analysis and site direct mutagenesis (Figs. 2 and 3). Furthermore, the nitrite production was significantly reduced in eNOS and Cdk5/p35 co-transfected SH-SY5Y cells, whereas increased in eNOS at S113A and Cdk5/p35 (Fig. 4). Together, our data suggest that Cdk5 can phosphorylate eNOS at Ser-113 and down-regulate eNOS-derived NO levels.
NO mediates the majority of endothelium-dependent responses in the brain and the source of this NO is the eNOS [Faraci and Heistad, 1998; Faraci, 2006]. The up-regulation of hippocampal eNOS levels has been induced by the kainic acid to cause cell death and blood-brain barrier leakage [Shin et al., 2007]. Furthermore, eNOS has been reported to participates in the pathogenesis of brain edema through the reduction of brain eNOS mRNA [Jiang et al., 2009]. The treatment of combination increased eNOS activity to protect mice against focal cerebral ischemia-induced neuronal damage [Nonaka et al., 2009]. Therefore, the activity of eNOS may involve in neurodegenerative diseases.
The regulation of eNOS activity is through protein phosphorylation. eNOS is primarily phosphorylated on serine residues and to a lesser extent on tyrosine and threonine residues [Bucci et al., 2005]. Co-transfection of wild-type Akt and eNOS in COS cells increases eNOS phosphorylation and NO production, whereas the mutation of serine 1177/1179 to alanine prevent Akt-dependent NO release [Fulton et al., 1999]. Moreover, the bradykinin-stimulated NO release is associated with an increase in phosphorylation at Ser-635, and Ser-617 [Michell et al., 2002]. AMP-activated kinase and PKC phosphorylate eNOS at Thr-495 and reduce eNOS catalytic activity [Chen et al., 1999]. These results suggest that protein phosphorylation plays an important role in the regulation of eNOS activity.
In addition, a recent study has suggested that angiopoietin-related growth factor stimulates phosphorylation of eNOS at ser-113 and enhances NO production in endothelial cells [Urano et al., 2008]. Meanwhile, Ser-113 has been located in an N-terminal oxygenase domain of eNOS, which belongs to the class of heme-thiolate proteins with the zinc tetrathiolate center [Alderton et al., 2001]. Zinc tetrathiolate center are essential for eNOS dimer stability, and eNOS dimmer formation contribute to fully enzymatic activity [Chen et al., 1995]. Our results reveal that the Ser-113 of eNOS is the main Cdk5 phosphorylation site. The phosphorylation of eNOS at Ser-113 may induce conformational change, decrease dimer stability, and reduce nitrite (NO ) production. Similar findings were also been reported in the phosphorylation of eNOS at S1179 induces a conformational change that shifts the entire FMN domain to allow enhanced electron transfer through the reductase domain, activating the eNOS activity [Atochin et al., 2007]. The phosphorylation of eNOS by Cdk5 related to induce conformational change should be an interesting subject for further investigations.
) production. Similar findings were also been reported in the phosphorylation of eNOS at S1179 induces a conformational change that shifts the entire FMN domain to allow enhanced electron transfer through the reductase domain, activating the eNOS activity [Atochin et al., 2007]. The phosphorylation of eNOS by Cdk5 related to induce conformational change should be an interesting subject for further investigations.
The activity of Cdk5 has been increased in several neurodegenerative diseases, including amyotrophic lateral sclerosis [Nguyen et al., 2003], Huntington's disease [Luo et al., 2005], PD [Smith et al., 2003], and AD [Maccioni et al., 2001]. In addition, three isoforms of NOS have participated in the progression of neurodegenerative diseases [Guix et al., 2005]. eNOS was responsible for the abnormalities in Huntington's disease [Aguilera et al., 2007] and AD [Sohn et al., 1999]. Our results presented that Cdk5 phosphorylates eNOS and down regulates its activity, indicating the phosphorylation of eNOS by Cdk5 may be implicated in pathogenesis of neurodegenerative disease.
In conclusion, Cdk5 interacts with eNOS and that it is also responsible for phosphorylation of eNOS at the Ser-113 site. Increased phosphorylation of eNOS by Cdk5 correlated with decreased nitric oxide production. The novel mechanism may be of relevance in AD and will be valuable in the possible therapeutic approaches on AD.
Acknowledgements
We thank the grant support from National Cheng Kung University Hospital Grant (NCKUH-9701004 to P.-J. Lu), National Science Council (NSC 97-2311-B-006-003-MY3 to P.-J. Lu and NSC-98-3112-B-006-012 to Y.-L. Chen).




