Increased integrin α5β1 heterodimer formation and reduced c-Jun expression are involved in integrin β1 overexpression-mediated cell growth arrest†
Zhengyu Fang and Wantong Yao contributed equally to this work.
Abstract
Integrins, heterodimers of α and β subunits, are a family of cell surface molecules mediating cell–cell and cell–extracellular matrix interaction. The largest subgroup is formed by the β1 subunit containing integrins which consist of 12 members with different ligand-binding properties. We previously reported that overexpressed integrin β1 subunit in the hepatocellular carcinoma cell line SMMC-7721 imposed a growth inhibitory effect through the upregulation of p21cip1 and p27kip1. In this study, we confirmed the growth inhibitory effect of β1 subunit overexpression in different cancer cell lines. The upregulated CDK inhibitors induced by β1 integrin overexpression were essential for this integrin-mediated growth arrest. Reduced c-Jun level after integrin β1 overexpression plays an important role in the transcriptional activation of p21 through the Sp1 sites. Solely overexpressed β1 subunit could induce the expression of diverse α subunit in different cell lines, among which α5 subunit was found to be correlated with integrin β1-mediated growth arrest. Relative lack of ECM–integrin interaction might be a reason for integrin β1 overexpression-mediated growth arrest. These results helped us understand more about the mechanisms that integrins regulate cell growth. J. Cell. Biochem. 109: 383–395, 2010. © 2009 Wiley-Liss, Inc.
Integrins are cell surface transmembrane glycoproteins that function as adhesion receptors in cell–extracellular matrix (ECM) interaction and link matrix proteins to the cytoskeleton. They play important roles in cytoskeleton organization and transduction of intracellular signals, regulating various processes such as proliferation, differentiation, apoptosis, and cell migration [Clark and Brugge, 1995].
Integrins consist of α and β subunits. Based on extensive search within human and mouse genomic sequences, at least 18α and 8β subunits are now known to assemble into at least 24 integrins [Arnaout et al., 2002]. Among them, β1 is the most ubiquitous and promiscuous integrin, partnering with at least 12 different α integrins. αβ1 integrins have been implicated in diverse roles including cardiac muscle differentiation [Fassler et al., 1996], Schwann cell anchorage, ensheathing axons [Feltri et al., 2002] and wound closure [Grose et al., 2002]. All 12 members of β1 integrins bind to ECM molecules [Brakebusch and Fassler, 2005] and each of them appears to have a specific, non-redundant function.
Until now, no clear correlation between tumor formation, invasion, and β1 integrin expression could be demonstrated. According to the majority of observations, aberrant expression with normal functioning rather than dominant genetic variations of genes coding for integrins has generally been observed. Many studies reported increased expression of β1 integrins in a large number of invasive cancers [Berry et al., 2003; Oshita et al., 2004; Ke et al., 2006]. However, in some cases, reduced levels of β1 integrins were found in certain cancers [Gui et al., 1996]. We previously demonstrated that protein and mRNA level of integrin α5β1 are much lower in human hepatocellular carcinoma cells than that in normal hepatocytes. Additionally, hepatocellular carcinoma cells with poor differentiation or high metastasis failed to express integrin α5β1 [Yao et al., 1997]. Therein we employed the human hepatocellular cancer cell line SMMC-7721, which has been widely used for proliferation [Tang et al., 2002], tumorigenesis [Bu et al., 2008], HBV infection [Lin et al., 2005], growth factor signaling [Li et al., 2001], and gene therapy research [Ye et al., 2006]. After a stable transfection of integrin β1 subunit in the human hepatocellular cancer cell line SMMC-7721, a delay in S-phase was observed, accompanied by the increased expression of cyclin-dependent kinase (CDK) inhibitors including p21Cip1 and p27Kip1 [Liang et al., 2003].
The previous study in our laboratory have demonstrated that overexpression of integrin β1 can upregulate p27 protein level through preventing Skp2-dependent degradation of p27 via the PI3K pathway [Fu et al., 2007], while the biological function and mechanism of the integrin-mediated p21Cip1 regulation remained unknown. Here, we demonstrated that integrin β1 overexpression resulted in impaired c-Jun protein level and activation of p21Cip1 transcription through Sp1 sites. Moreover, we were able to reveal that β1 subunit overexpression resulted in induction of integrin α5 expression, which in turn appeared to be involved in this integrin-mediated cell-cycle control.
MATERIALS AND METHODS
Reagents
We used the following reagents: siRNA against integrin α5 and β1 as well as nonsense RNA (Cell Signaling Technology). JNK inhibitor II and LY294002 (Calbiochem), cycloheximide (Chx) (Calbiochem), anisomycin (Biovision), lipofectamine 2000 reagent (Invitrogen, CA), M-MLV reverse transcriptase (Invitrogen), anti-c-Jun, anti-p473 PKB and anti-p9-Gsk3β from Cell Signaling Technology, anti-integrinα5, anti-integrinβ1, and anti-PARP from BD Pharmingen, anti-Erk, anti-integrin α1, and anti-integrin α6 from Santa Cruz, Ltd, anti-GAPDH and anti-β-actin from Kangchen, anti-p21 from Calbiochem. Fibronectin, laminin, and poly-HEMA were all obtained from Sigma Aldrich.
Cell Lines and Cultures
Human hepatocellular cancer cell lines SMMC-7721, mock-7721 (mock plasmid stably transfected SMMC-7721) and β1-7721 (integrin β1 subunit stably overexpressed SMMC-7721) cells were introduced previously [Fang et al., 2007]. Mock-7721 and β1-7721 cells were cultured in 1640 (Gibco) containing 400 µg/ml G418 supplemented with 10% fetal bovine serum (PAA) and 1% penicillin/streptomycin (Life Technologies, Inc.). MDA-MB-468 (human breast cancer cell line), Hela (human cervical carcinoma strain), HEK-293T (transformed human renal epithelial cell line), MCF-7 (human breast adenocarcinoma cell line) and GRC-1 (human renal cancer cell line) cells were cultured at 37°C in 5% CO2 in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum (Gibco). SW116 (human colorectal cancer cell line), B16 (mouse melanoma cell line), and DU-145 (human prostate carcinoma, epithelial-like cell line) cells were grown in identical circumstances in RPMI-1640 medium (Gibco) supplemented with 10% newborn calf serum (PAA).
Plasmids Construction
The integrin β1 subunit expression vector, pcDNA3-β1, has been described previously [Liang et al., 2003]. The c-Jun expression vector, pcDNA3-cJun, was generously provided by Dr. D. Thanos (Columbia University, New York). The human wild-type p21Cip1 promoter-luciferase reporter construct plasmid, pGL3-luc, was a kind gift from Dr. Bert Vogelstein (Howard Hughes Medical Institute, Johns Hopkins University). PKB-DD, a constitutively active PKB (protein kinase B) plasmid with serine-473 and threonine-308 mutated to aspartic acid, was a generous gift from Dr. J. Woodgett (Ontario Cancer Institute, Toronto). The full-length human p21Cip1 promoter-luciferase reporter constructs (pGL3-1908) and the 5′ deletion construct of pGL3-1908 containing only the proximal region of p21Cip1 promoter (pGL3-217) were described before [Liang et al., 2004]. The Sp1-site mutants of pGL3-217, including pGL3-217m1-2 (the first two Sp1 sites of p21 promoter were mutated), pGL3-217m3 (the 3rd Sp1 site of pGL3-217 were mutated), pGL3-217m4 (the 4th Sp1 site of pGL3-217 was mutated) and pGL3-217m5-6 (the last two Sp1 sites of pGL3-217 were mutated), were introduced previously [Fang et al., 2007]. Primers to interfere with the expression of p21 were designed using pSilencer 2.0 (Ambion, Austin, TX). The p-Silencer vector produces a hairpin small interfering RNA (siRNA) that induces RNA interference of the target gene. The hairpin siRNA inserts for p21 were as follows: top strand: 5′-GAT CCG TCG ACT TTG TCA CCG AGT TCT CAA GAG AAA CTC GGT GAC AAA GTC GAA GTT TTT TGG AAA-3′, bottom strand: 5′-AGC TTT TCC AAA AAA CTT CGA CTT TGT CAC CGA GTT TCT CTT GAG AAC TCG GTG ACA AAG TCG ACG-3′ (Sangon, Shanghai, China). Each oligonucleotide was annealed and then ligated into the pSilencer-2.0 vector. The constructed plasmid was named pSi-p21. The negative control vector (control) was obtained from Ambion, which is a plasmid with a similar structure but encoding a nonsense minigene with no homology to any known sequences in the human genome.
Western Blotting
Cells were washed with PBS and lysed in a buffer containing 50 mM Tris–HCl (pH 6.8), 2% SDS, 10% glycerol, phosphatase inhibitors (100 mM Na3VO4, 10 mM NaF) and protease inhibitor (1 mM PMSF). Equal amounts of protein were loaded on a SDS–PAGE and transferred to PVDF membrane. After blocking with 5% non-fat milk in TBS-T (containing 0.1% Tween-20), the membranes were incubated with specific primary antibodies, followed by HRP-conjugated secondary antibodies. Proteins were visualized by fluorography using an enhanced chemiluminescence system.
RT-PCR
mRNAs were extracted from 107 cells by using the mRNA Purification Kit (Shanghai Shenergy Biocolor Bioscience & Technology Company, China) according to the manufacturer's guidelines. Then the concentration of mRNA was measured and reverse transcription was performed on 2 µg of mRNA by using reverse transcriptase MMLV (Promega, USA) for first-strand cDNA synthesis with oligo(dT) primer. One-tenth of the cDNA products were used for PCR amplification with specific primers. The primer sequences used in this experiment were listed in Table I. And PCR was carried out as follows: an initial denaturation of 10 min at 94°C was followed by 28–32 cycles of 45 s at 94°C, 45 s at variable temperature (Tm), and 1 min at 72°C, followed by 10 min of final elongation at 72°C. Control PCR amplifications were performed with β-actin-specific primers which were purchased from Watson Biotech (Shanghai, China).
| Gene | Primer | Sequence | Tm (°C) | Product (bp) |
|---|---|---|---|---|
| p21 | Sense | 5′-GAGGCCGGGATGAGTTGGGAGGAG-3′ | 58 | 159 |
| Antisense | 5′-CAGCCGGCGTTTGGAGTGGTAGAA-3′ | |||
| Integrin β1 | Sense | 5′-AATGAAGGGCGTGTTGGTAG-3′ | 58 | 337 |
| Antisense | 5′-CGTTGCTGGCTTCACAAGTAC-3′ | |||
| Integrin α1 | Sense | 5′-TGCCAGTGAGATTTCAGAGACC-3′ | 60 | 117 |
| Antisense | 5′-GTGATTTCCTGTGTTTTCGTCG-3′ | |||
| Integrin α2 | Sense | 5′-AACTCTTTGGATTTGCGTGTG-3′ | 58 | 82 |
| Antisense | 5′-TGGCAGTCTCAGAATAGGCTTC-3′ | |||
| Integrin α3 | Sense | 5′-ACTGTGAAGGCACGAGTGTG-3′ | 59 | 103 |
| Antisense | 5′-TGCTGGTTCGGAGGAATAG-3′ | |||
| Integrin α5 | Sense | 5′-TGCCTCCCTCACCATCTTC-3′ | 55 | 482 |
| Antisense | 5′-TGCTTCTGCCAGTCCAGC-3′ | |||
| Integrin α6 | Sense | 5′-GTTGTCGTCTCCACATCCCT-3′ | 59 | 125 |
| Antisense | 5′-CACTCTGGAGGCTGAAAAGG-3′ | |||
| β-actin | Sense | 5′-TGATGATATCGCCGCGCTCGTCGT-3′ | 55 | 412 |
| Antisense | 5′-CACAGCCTGGATAGCAACGTACAT-3′ |
MTT Assay
The MTT (3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazoliumbromide) assay was used for measuring cell growth proliferation as described previously [Hansen et al., 1989]. In brief, an equal number of cells were seeded in a 6-well plate, and transfected with pcDNA3-β1 plasmids or integrin α5 SiRNA (Santa Cruz, Ltd). Twenty-four hours after transfection, cells were suspended and seeded into a 96-well plate at the density of 5 × 103 cells/well in DMEM containing 10% FBS. Cells were cultured in fresh medium for 24, 48, and 72 h. Twenty microliters of MTT solution (5 mg/ml in PBS) was added to each well and cells were incubated for 4 h at 37°C. Hereafter, the medium was removed and 200 µl DMSO was added per well. After 5 min of incubation at 37°C, the optical density (OD) values at the wavelength of 490 nm were read with an enzyme-linked immunosorbent assay reader (Bio-Tek, Houston).
Plating Experiment
Target cells were applied to fibronectin (15 µg/ml) or laminin (15 µg/ml) coated tissue culture surfaces and allowed to attach overnight before fixation and adherence, or planted onto poly-HEMA blocked petri dishes; then followed by MTT assay, phase-contrast microscopy or protein extraction.
Flow Cytometry Assay
For cell-cycle assay, cells were digested with 2 mM EDTA in PBS and rinsed twice with ice-cold PBS solution, then fixed by adding them drop wise into 75% ice-cold ethanol while vortexing, followed by incubation in ice for 60 min. The fixed cells were washed with ice-cold PBS and incubated at 37°C for 30 min in 0.5 ml PBS solution containing 20 µg/ml RNase A, 0.2% Triton X-100, 0.2 mM EDTA and 20 µg/ml of propidium iodide. DNA content was determined by FACS analysis (Becton Dickinson). The percentage of cells in G0/G1, S, and G2/M phases was determined using Flowjo software (Tree Star, Inc.) For apoptosis assessment by serum withdrawal, cells were washed twice with phosphate-buffered saline and then starved by exposure to 1 ml of serum-free culture medium for 48 h. At the end of incubation the cells were treated as above for detection by flow cytometry.
Annexin V-FITC Apoptosis Assay
The Annexin V-FITC Apoptosis Detection Kit (ab14085) was obtained from Abcam (UK). Eight hours after serum deprivation culture, 1 × 105 cells were collected by centrifugation and resuspended in 500 µl of 1× Annexin V binding buffer. Add 5 µl of Annexin V-FITC to the cells and incubate at room temperature for 5 min in the dark. Analyze cells by flow cytometry (Ex. = 488 nm; Em. = 530 nm) using FL1.
Dual-Luciferase Reporter Assays
Mock-7721 and β1-7721 cells were cultured in 6-well plates. Cells were co-transfected with luciferase-reporter plasmids (1 µg/ml) and SV40 renilla luciferase plasmids (1 µg/ml) by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Cell lysates were obtained by using 250 ml per well of passive cell lysis buffer (Promega). Luciferase activity was measured by using 20 ml of cell lysate per assay tube in an Autolumat machine (LB 953; EG&G Berthold, Oak Ridge, TN). Luciferase transfection efficiency was normalized by dual-luciferase analysis by using SV40 renilla luciferase control. Reporter constructs included pGL3-basic, pGL3-control, pGL3-217 containing proximal 217 bp region of the p21Cip1 promoter and its Sp1-site mutants [Fang et al., 2007] as described previously.
ChIP Assays
The chromatin immunoprecipitation assays were carried out using the chromatin immunoprecipitation (ChIP) assay kit (UPSTATE, USA) as introduced previously [Fang et al., 2007]. Specific primers spanning proximal promoter of the p21Cip1 gene were used to carry out PCR from DNA isolated from ChIP experiments and input samples. The primer pairs used here was: 5′-ACC AAC GCA GGC GAG GGA CT-3′ (uP1), 5′-CCG GCT CCA CAAGGAACT GA-3′ (dP1) [Fang et al., 2007].
Statistics
Data are presented as means ± SD. The number of experiments represents the independent experiments at least two times. An independent-samples t-test was used to compare different conditions. Differences were considered statistically significant at P < 0.05.
RESULTS
Integrin β1 Overexpression Induces Growth Arrest Through Upregulation of p21 and p27 in Most Cancer Cell Lines
Preliminary experiments were conducted to examine the effect of integrin β1 overexpression on various cancer cell lines derived from different tissues which have low intrinsic integrin β1 expression. We observed overexpression of integrin β1 subunit could induce the expression of the p21 and p27 in most cell lines including cervical, melanoma, breast, kidney, liver, and prostate cancer cells. In the breast cancer cell line MDA-MB-468, however, the protein level of the CDK inhibitors did not alter significantly after integrin β1 overexpression (Fig. 1A).
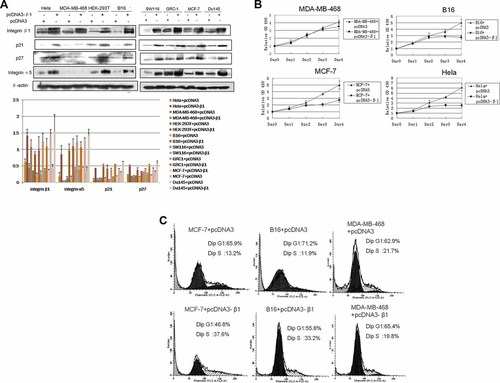
Integrin β1 subunit overexpression induces growth arrest through upregulation of p21 and p27 in most cancer cell lines. A: Cells from the eight indicated cancer cell lines were transfected with pcDNA3-β1 or pcDNA3 plasmid for 48 h, and total cell lysates from eight cell lines were examined by Western blotting. The mature form of integrin β1 subunit (125 kDa) and α5 subunit (155 kDa) were detected and β-actin was used to normalized the amount of loaded protein. B: Hela, B16, MDA-MB-468, and MDA-MB-435 cells were transfected with pcDNA3-β1 or pcDNA3 plasmid, and cell proliferation was determined by MTT assay. Error bar, SD of the samples. C: Cells were transfected with pcDNA3-β1 or pcDNA3 plasmid, and cell-cycle distribution was determined by propidiumiodide flow cytometry. Data bars represent the mean absorbance ± SD from triplicate wells from three separate experiments.
To ascertain whether the cell growth ability correlated with the altered level of CDK inhibitors induced by integrin β1 overexpression (all Western blots showed the mature type of integrin β1 in this article), MTT assay was carried out to estimate cell growth ability. Three cancer cell lines, in which p21 and p27 were significantly upregulated after integrin β1 was overexpressed, together with MDA-MB-468 cells, were used here. We found that increased expression of mature integrin β1 resulted in marked growth inhibition in B16, Hela, and MCF-7 cells, but had little effect on MDA-MB-468 cells (Fig. 1B). Results from flow cytometry also showed that increased expression of integrin β1 could induce S-phase delay in the above-mentioned cell lines except for MDA-MB-468 cells (Fig. 1C). We were unable to clarify why the proliferation of MDA-MB-468 cells was not influenced by integrin β1 overexpression. One possible explanation might be that integrin α5 expression (Fig. 1A) was lost in MDA-MB-468 cells.
Upregulated p21 Levels Induced by Integrin β1 Overexpression Play Opposite Roles in Cell Growth
In our previous works, we have demonstrated that accumulated p27 after integrin β1 overexpression was involved in cell growth control [Fu et al., 2007]. In this study, we first investigated the biological consequence of the integrin-induced p21 expression. The p21 siRNA expression vector (pSi-p21) was constructed as introduced in the Materials and Methods Section and was proved to inhibit the p21 expression in both nucleus and cytoplasm (Fig. 2A). We found that downregulation of p21 by siRNA resulted in enhanced cell growth in β1-7721 cells (Fig. 2B). Furthermore, the S-phase delay of β1-7721 cells could be reversed by knockdown of p21 (Fig. 2C). However, we noticed that cell growth was not fully restored by knockdown of p21 in β1-7721 cells, indicating the involvement of other factors in this integrin-mediated cell-cycle control.
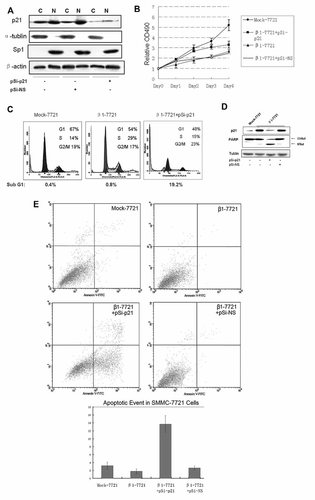
Upregualtion of p21 is essential for integrin β1 overexpression-induced growth arrest. A: β1-7721 cells were transfected with pSi-p21 or pSi-NS plasmids for 48 h, the nuclear and cytoplasmic extracts from cells were examined by Western blotting. Sp1 was used to normalize the amount of nuclear protein and α-tublin for the cytoplasmic protein. B: β1-7721 cells were treated as in (A), and cell proliferation was determined by MTT assay. Error bar, SD of the samples. C: β1-7721 cells were treated as in (A), and cell-cycle distribution was determined by propidiumiodide flow cytometry compared to Mock cells. D: β1-7721 cells were treated as in (A), and both Mock-7721 and β1-7721 cells were cultured in serum-deficient RPMI-1640 medium for 8 h, total cell lysates of were examined by Western blotting. Cleavage of PARP as a apoptotic marker was detected. E: Flow cytometric analysis of apoptotic cells using Annexin V-FITC. β1-7721 cells were treated as in (D), and both the Mock-7721 and β1-7721 cells were incubated with Annexin V-FITC in the annexin-binding buffer and analyzed by flow cytometry. Shown is representative example of multiple experiments.
We also noticed that the percentage of sub-G1 cells containing amounts of DNA was increased significantly after p21 was downregulated in β1-7721 cells (Fig. 2C), foreshowing the anti-apoptotic activity of p21. To further investigate the role of this integrin-induced p21 upregulation in cell surviving, β1-7721 cells were transiently transfected with pSi-p21 or pSi-NS vectors and cultured in RPMI 1640 medium without serum for 8 h. As shown in Figure 2C, downregulation of p21 in β1-7721cells induced the increased proportion of apoptosis cells as reflected by sub-G1 peak, indicating that knockdown of p21 in β1-7721 cells results in increased cell apoptosis sensitivity to serum deprivation, which further proved the protective role of p21 in cell survival. Apoptosis initiation was further confirmed by proteolytic cleavage of poly-ADP-ribose polymerase (PARP) which serves as a biochemical marker of cells undergoing apoptosis (Fig. 2D). P21 knockdown also strongly promoted the degradation of PARP. Similar results were obtained from the flow cytometric analysis of apoptotic cells using the Annexin V-FITC staining (Fig. 2E). Thus the upregulated p21 induced by integrin β1 overexpression might play opposite roles in inhibiting proliferation and preventing apoptosis.
Integrin β1 Overexpression Activates p21 Transcription Through Downregulation of c-Jun Protein Level
Although we have made a preliminary attempt to understand the linkage between integrin β1 overexpression and increased p21 transcription [Fang et al., 2007], the pathway through which signal is transduced from the cell surface to the nucleus remained unclear. Among the integrin-mediated signaling pathways [Clark and Brugge, 1995], PI3K/PKB (phosphatidylinositol 3′-OH kinase/protein kinase B), MAPK/Erk (mitogen-activated protein kinase/extracellular signal-regulated protein kinase) and JNK (c-Jun NH2-terminal kinase) pathways are known to play important roles in cell growth regulation. We next screened for potential molecular targets within signal transduction pathways involved in this integrin-mediated p21 regulation (Fig. 3A). We found that the level of Erk and its phosphorylated form did not differ from the Mock-7721 to β1-7721 cells, while the phosphorylation of PKB and GSK-3β (glycogen synthase kinase 3β) was reduced significantly. We then investigated whether PI3K/PKB pathway was involved in this progress. After Mock-7721 and β1-7721 cells were treated with Ly294002, the PI3K/PKB inhibitor, or transfected with constitutively active PKB mutant (PKB-DD), the expression level of p21 was detected by Western blot. As shown in Figure 3B,C, the protein level of p21 was not influenced by inhibition or activation of PI3K/PKB pathway, therefore inhibited PI3K/PKB pathway induced by integrin β1 overexpression did not participate in the integrin-mediated regulation of p21.
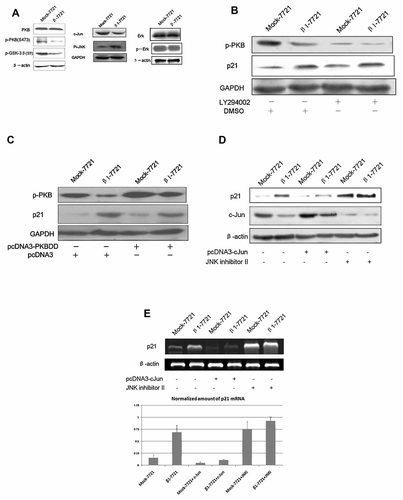
Integrin β1 overexpression activates p21 transcription through the downregulation of c-Jun protein level. A: PI3K/PKB, JNK, and MAPK/Erk pathway-associated molecules in Mock-7721 and β1-7721 cells were examined by western blotting. B: Mock-7721 and β1-7721 cells were treated with 20 µM LY294002 for 24 h, and the protein level of p21 and phosphorylated PKB (p473) was determined by Western blot. C: Mock-7721 and β1-7721 cells were transfected with PKB-DD vector and mock vector for 36 h, and the protein level of p21 and phosphorylated PKB (p473) was determined by Western blot. D: Mock-7721 and β1-7721 cells were treated with 0.1 µM JNK inhibitor II (Calbiochem) for 12 h or transfected with the pcDNA3-cJun and pcDNA3 mock plasmid for 48 h, and the protein level of p21 and c-Jun were then determined by Western blot. E: Both cells were treated as in (D), and p21 mRNA level were analyzed by RT-PCR analysis. Shown is representative example of multiple experiments.
It is well known that p21 gene expression was controlled by various transcription factors together with some co-activators or co-repressors, especially Sp1/Sp3, Jun and their co-activators [Gartel and Radhakrishnan, 2005]. In this study we found that the expression level of c-Jun, one of the downstream molecules of integrin signaling, markedly decreased after integrin β1 overexpression (Fig. 3A). C-Jun is an immediate early gene, whose expression is rapidly and transiently induced by extracellular stimuli. It acts as a nuclear messenger converting a cytoplasmic signal to alterations in gene expression. To investigate whether c-Jun was involved in this integrin β1-mediated p21 regulation, we performed the transfection of c-Jun expression vector into the Mock-7721 and β1-7721 cells. As shown in Figure 3D, overexpression of c-Jun leads to impaired p21 expression in both cells, especially in β1-7721 cells. On the other hand, after c-Jun was downregulated by JNK inhibitors, the protein level of p21 was significantly elevated in Mock-7721 cells compared with β1-7721 cells. Next we investigated whether p21 transcription level was hanged in this process. And the similar results were observed (Fig. 3E), indicating that c-Jun played the important role in this integrin β1-mediated p21 regulation.
c-Jun Represses p21 Transcription Through Sp1 Sites
Previously we have demonstrated that integrin β1 overexpression mainly activated p21 transcription through the proximal region on the p21 promoter. And analyzing of a series of deletion and point mutants of p21 promoter, we found that Sp1–3-binding site (−77 and −83 relative to the transcription start site) played an important role in integrin β1-mediated p21 regulation [Fang et al., 2007]. In this study, to investigate whether altered expression of c-Jun was the necessary for this signal transduction pathway, a series of mutation of p21 proximal promoter reporter constructs as introduced before [Fang et al., 2007] were used and assayed for luciferase activity in the Mock-7721 or β1-7721. As shown in Figure 4A, after c-Jun expression vector was transfected into β1-7721, the activity of p21 promoter decreased markedly. When the Sp1–3-binding site (−82 to −77) was mutated, neither integrin β1 nor c-Jun overexpression could influence the activity of p21 proximal promoter. These data indicated that the Sp1–3-binding site (−82 to −77) might be the c-jun cis-element in p21 promoter.
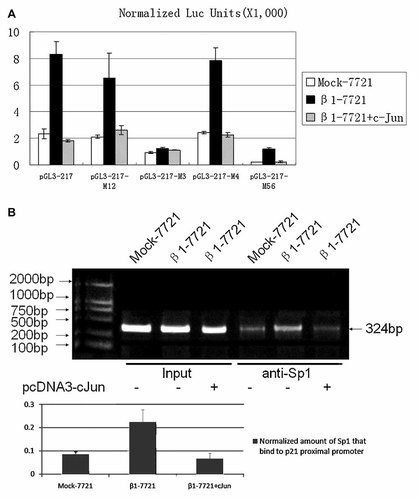
c-Jun protein represses p21 transcription through Sp1 sites. A: pGL3-217 and its Sp1-site mutants were transiently transfected into the Mock-7721 and β1-7721 cells. The luciferase activities were analyzed and the data were normalized to the activity of SV-40 renilla construct and are shown as means ± SD NLU. B: Soluble chromatin was immunoprecipitated with anti-Sp1 antibodies from the Mock-7721, β1-7721, or β1-7721 cells transfected with c-Jun expression vector. PCR primers for the regions of the p21(WAF1) gene as indicated above were used to amplify the DNA isolated from the immunoprecipitated chromatin.
We next investigated whether altered c-Jun protein level could influence the interaction between transcription factor Sp1 and p21 promoter. Chromatin immunoprecipitation assays (ChIP) were carried out in Mock-7721, and β1-7721 cells, using anti-Sp1 antibody and polymerase chain reaction (PCR) primers spanning the proximal 324 bp region (−324 to 0) of p21 gene which contains all of the six Sp1-binding sites. It was found that after the reduced c-Jun level in β1-7721 cells was restored, the interaction between Sp1 and p21 proximal promoter was impaired evidently (Fig. 4B), which further proved the important role of c-Jun in this integrin-induced p21 transcription through Sp1 sites.
Integrin α5 Induced by β1 Subunit Overexpression Was Involved in the Integrin β1-Mediated Cell Growth Regulation
Formation of the integrin αβ heterodimer is essential for cell surface expression and function; heterodimers of the β1 integrin subfamily must bind various components of the ECM including fibronectin (α3, α4, α5, α8, αv), laminins (α1, α2, α3, α6, α7, α9), collagens (α1, α2), vitronectin (αv) as well as cell counter-receptors such as vascular cell adhesion molecule 1 (VCAM-1)(α4) and fertilin (α6), which is mediated and determined by all kinds of α subunits [Brakebusch et al., 1997; Irie, 2005]. Accordingly, α group is the prerequisite molecules for β group to play major roles in a variety of biological processes ranging from cell migration to tissue organization, growth, differentiation, and apoptosis. Elevated expression of integrin β1 chain precursors must bind to certain α subunits before they locate at the membrane and exert growth inhibitory effect. Therein we further investigated whether extrinsic mature integrin β1 could trigger the change of α subunits. As anticipated, interestingly, we found that integrin β1 overexpression could induce the expression of diverse α subunits in different cell lines. The type of α subunits induced by the β1 subunit overexpression seemed to be the same as α subunits originally expressed on the cell surface (a part of data is shown in Fig. 5A,B). Among the α subunits induced by β1 mature subunit overexpression, α5 subunit was considered to be the one that correlated with the integrin β1-mediated growth control basing on the previous findings.
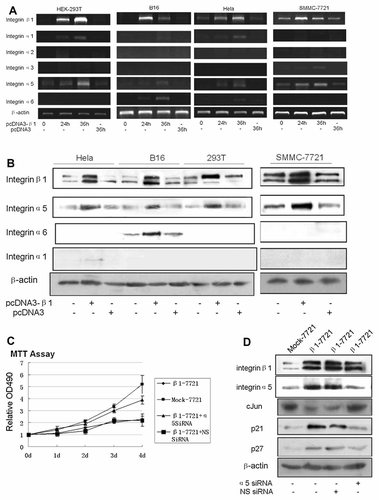
Integrin α5 induced by β1 subunit overexpression was involved in the integrin β1-mediated cell growth regulation. A: The HEK-293T, Hela, B16, and SMMC-7721 cells were transfected with pcDNA3-β1 or pcDNA3 plasmid for 24–36 h, mRNA level of integrin α1, α2, α3, α5, α6, and β1 were next analyzed by RT-PCR analysis as described under the Materials and Methods Section, β-actin was used to normalized the amount of loaded RNA. B: The cells were treated as in (A) for 48 h, and protein level of integrin α1, α5, α6, and β1 were determined by Western blotting. C: β1-7721 cells were transfected with integrin α5 siRNA or nonsense siRNA for 24 h, and proliferation of Mock-7721 and β1-7721 cells was determined by MTT assay. Error bar, SD of the samples. D: β1-7721 cells were treated as in (C) for 48 h, and total cell lysates of Mock-7721 and β1-7721 cells were examined by western blotting. Here shown are representative data from three independent experiments.
Integrin α5β1, a widely expressed fibronectin (FN) receptor, is one of the best-characterized integrins that recognize the tripeptide sequence, Arg-Gly-Asp. The association between fibronectin (FN) and α5β1 integrin is involved in regulating not only cell adhesion and migration, but also cell differentiation and apoptosis [Ruoslahti, 1996; Frisch and Ruoslahti, 1997]. To investigate the role of the α5 subunit in β1 subunit overexpression-mediated cell growth, α5 siRNA was used to specially inhibit integrin α5 expression. As shown in Figure 5C,D, knockdown of integrin α5 in β1-7721 cells resulted in significantly faster proliferation than that in control cell lines accompanied by impaired expression of p21 and p27, representing the occurrence of alleviated S-phase delay. These data indicated that increased integrin α5β1 heterodimers formation plays an important role in integrin β1 overexpression-mediated growth arrest.
Inadequate Integrin–ECM Interactions Might Be a Reason for the Integrin β1 Overexpression-Mediated Growth Arrest
Integrins could bind to either ECM macromolecules or counter-receptors on adjacent cell surface, which is important process for integrins-mediated signal transduction. All 12 members of β1 integrins bind to ECM molecules. We hypothesized that the altered integrin–ECM interaction might be the reason why the PI3K/PKB and JNK pathways and growth arrest after integrin β1 was overexpressed. Here, we performed plating experiments to elucidate its mechanism.
Mock-7721 and β1-7721 cells were cultured on fibronectin (the ligands of α5β1 integrins)-coated culture dishes. As shown in Figure 6A, the S-phase delay in β1-7721 cells was reversed after cells attached to fibronectin, the ligand of integrin α5β1, while no evident change occurred in Mock-7721 cells. Results from MTT assay also showed that the proliferation of β1-7721 cells was increased accompanied by the decreased expression of p21 and p27 when cell attached to fibronectin (Fig. 6B,C). These data further confirmed the important role of integrin α5β1 heterodimer formation in β1 subunit-mediated growth arrest.
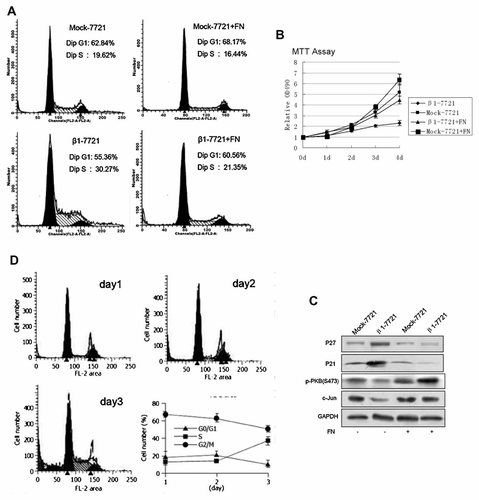
Inadequate integrin–ECM interactions might be a reason for the integrin β1 overexpression-mediated growth arrest. A: Mock-7721 and β1-7721 cells were cultured on FN-coated dishes or FN-free dishes for 72 h, and cell-cycle distribution was determined by propidiumiodide flow cytometry. B: Mock-7721 and β1-7721 cells were treated as in (A), and proliferation of Mock-7721 and β1-7721 cells was determined by MTT assay. Error bar, SD of the samples. C: Mock-7721 and β1-7721 cells were treated as in (A), and total cell lysates were examined by Western blotting. D: The parental SMMC-7721 cells were plated on poly-HEME coated petri dishes, and cultured in normal medium for 24 (A), 48 (B), or 72 h (C), respectively, then collected and analyzed by flow cytometry. Shown is representative example of multiple experiments.
Remarkably, when the adhesion of the parental cells to ECM was blocked by plating them onto poly-HEMA-coated petri dishes, the percentage of both cells in S-phase was increased (Fig. 6D). That is, when the parental SMMC-7721 cells were prevented from interaction with ECM through the coated poly-HEMA the same effects of S-phase accumulation took place as that in β1-7721. These results suggested that S-phase delay induced by overexpressing β1 in SMMC-7721 cells might be the result of the relative lack of integrin–ECM interaction.
DISCUSSION
In the previous studies of my laboratory, we examined the relationship between the cell surface integrin β1 level and p21 expression in human hepatocellular cancer cells [Liang et al., 2004; Fu et al., 2007]. In this study, to confirm the role of integrin β1 in the regulation of p21 expression, we selected several cancer cell lines with low intrinsic integrin β1 expression. And the results were in accordance with our previous findings that integrin β1 overexpression could negatively regulate cell growth through the CDKs. And we also found that the proliferation of cells with high intrinsic β1 expression such as human glioblastoma (U87) cells could not be influenced by integrin β1 overexpression (data not shown here).
Integrin-mediated cell adhesion impacts on two key aspects of growth regulation. First, integrin-mediated adhesion can influence the activity of the basal cell-cycle machinery, which consists of various CDK complexes [Walker and Assoian, 2005; Walker et al., 2005]. In this study, we found that p21 and p27 was upregulated after β1 subunit was stably overexpressed in human hepatocellular carcinoma cells, which was responsible for the induced growth arrest. This make us understand more about the mechanism of integrin-mediated cell-cycle control. Second, integrin-mediated anchorage is also a key regulator of apoptosis [Cordes, 2006]. As shown in Figure 2D,E, p21 knockdown in β1-7721 cells resulted in increased cell apoptosis, indicating the protective role of p21 in integrin-mediated cell death (IMD). Thus, the p21 induced by integrin β1 overexpression has dual roles in regulating cell growth.
The regulation of p21 protein expression and stability is rather complex, since many factors are involved. Several kinases such as JNK, AKT/PKB, ERK, and p38 MAPK have been demonstrated to play important roles in regulating p21 protein expression and stability [Kobayashi and Tsukamoto, 2001; Li et al., 2002; Kim et al., 2005]. In this study, we found the reduced c-Jun protein level plays an important role in this integrin-mediated p21 transcription. The transcription factor c-Jun is a prominent member of the AP-1 transcription family. Its transcriptional activities are regulated by changes in the level of c-jun expression level as well as post-translational modifications of the c-Jun protein. It was found that c-Jun, in addition to its repressive ability on p53, can downregulate p21 through the Sp1-binding sites [Wang et al., 2000; Kolomeichuk et al., 2008]. However, the effect of c-Jun on p21 is not straightforward as it has also been seen that c-Jun can interact with Sp1 and induce p21 in some cases [Kardassis et al., 1999]. The exact relationship between c-Jun and p21 transcription remained not clarified. In this study, we found that overexpression of integrin β1 can repress the transcription of p21 through Sp1 sites, which is consistent with Wang et al.'s findings. And we provided direct evidence of the altered interaction between Sp1 and p21 promoter after the protein level of c-Jun was differed. Taken together, our finding provided one possible mechanism to understand β1 integrin-mediated cell-cycle regulation.
It was interesting that solely overexpression of integrin β1 could induce diverse α subunits in different cancer cell lines, though the mechanism remained unknown. To find out why integrin β1 overexpression could not induce expression in MDA-MB-468 cells, we have examined the level of methylated histone proteins on the promoter of integrin α5 gene. We found the methylation of histone proteins on integrin α5 promoter was much higher in MDA-MB-468 cells than that in Hela and B16 cells (data not shown here), which might partially explain why expression of α5 subunit could not be induced by integrin β1 overexpression in this MDA-MB-468 cells.
Our data suggested that increased integrin heterodimers could negatively regulate cell growth, which was consistent with Varner et al.'s findings [Varner et al., 1995]. However, the precise mechanisms are not yet fully understood. The mechanism underlying this integrin overexpression-mediated growth arrest may be due to two kinds of possibilities. One hypothesis is “integrin-mediated death” (IMD) described by Stupack and coworkers [Stupack et al., 2001; Cheresh and Stupack, 2002]. It is well known that α5β1 integrin, in general, acts as the effector protein of cell proliferation, such as endothelial cells in the vascular system [Yang et al., 1993]. In this study, however, we showed that overexpression of β1 subunit induced S-phase delay, which could be reversed by attachment to fibronectin. Moreover, for the parental cells, they underwent S-phase delay and apoptosis when they were deprived of attachment by plating them on poly-HEME-coated petri dishes. Therefore, we postulated that the relative lack of ECM might be involved in S-phase delay triggered by overexpression of α5β1 integrin gene in SMMC-7721 cells. The other possibility is that the trans-dominant integrin inhibition, which is defined as the occupancy of one integrin by its ligand, can inhibit the functions of other integrins [Blystone et al., 1994; Diaz-Gonzalez et al., 1996; Blystone et al., 1999; Schwartz and Ginsberg, 2002]. For example, integrin α5β1 is essential for angiogenesis [Yang et al., 1993], but may suppress the functions of integrin α5β1 if integrinβ3 prevail in the endothelial cells [Simon et al., 1997]. It was reported that the basal integrin repertoire in hepatocellular carcinoma cells was characterized by the expression of several potential laminin receptors of the integrin family, such as α1β1, α2β1, α6β1 [Volpes et al., 1993; Torimura et al., 1997; Ozaki et al., 1998; Masumoto et al., 1999; Nejjari et al., 1999]. And the overexpression of β1 may preferentially dimerize with α1, α2, and α6, the subunits of the receptors of laminin or collagen (they were lost in this in vitro model). Therefore, if these subunits are occupied, the functions of integrin α5β1 may be suppressed, including its capacity to block cell-cycle arrest. The roles of other α subunits involved in integrin β1 overexpression-mediated cell functional changes remain to be elucidated.
Acknowledgements
We are grateful to Dr. Bert Vogelstein from Howard Hughes Medical Institute, Johns Hopkins University for his gift of plasmids. This work was supported by Shanghai Leading Academic Discipline Project (B110) and National Key Sci-Tech Special Project of China grant (2008ZX10002-018 and 2008ZX10002-019).




