Attenuation of estradiol on the reduction of striatal dopamine by amphetamine in ovariectomized rats
Abstract
Amphetamine (AMPH) is a highly addictive drug of abuse which exhibits toxicity to dopaminergic neurons in long-term abusers. Estrogen seems to show neuroprotection in dopamine (DA) deficit caused by AMPH. The present study was to investigate the effects of estradiol on the levels of striatal DA in ovariectomized (Ovx) rats treated with or without AMPH. Female rats were Ovx for 2 weeks before administration of AMPH (5 mg/kg/day, i.p.) with or without 17β-estradiol benzoate (EB) (25 µg/kg/day, s.c.) for 7 days. The striatal tissues were collected, homogenized with DA mobile phase, and centrifuged. The concentrations of DA in the supernatants were detected by HPLC. The protein expressions of dopamine transporter (DAT), vesicular monoamine transporter 2 (VMAT-2), and tyrosine hydroxylase (TH) were analyzed by Western blotting. The results indicated that AMPH could attenuate DA level significantly in striatum (P < 0.01). Comparing to control groups, administration of either EB or EB plus AMPH increased DA level (P < 0.01). The protein expression of striatal DAT was significant greater (P < 0.01) in rats treated with AMPH plus EB than AMPH treated animals. These results suggest that the DA levels in striatum can be enhanced by EB via an increase of DAT expression following administration of AMPH. J. Cell. Biochem. 108: 1318–1324, 2009. © 2009 Wiley-Liss, Inc.
Amphetamine (AMPH) is one of the most common elicit drug abuses in Taiwan. Among them, methamphetamine (METH) is the most common psychostimulants by abusers [National Bureau of Controlled Drugs, Department of Health, Taiwan, 2008]. AMPH was believed to increase dopamine (DA) release from striatum by different mechanisms. First, AMPH increases cytosolic DA by redistribution of neurotransmitters from synaptic vesicles (via vesicular monoamine transporter 2—VMAT-2) to the neuronal cytoplasm and the reverse transport of DA from the cytoplasm into the synapse via the dopamine transporter (DAT) [Sulzer et al., 2005]. Second, AMPH competitively binds with DAT by facilitating exchange of cytosolic DA, or burst channel like transporter to DA efflux [Kahlig et al., 2005]. Third, AMPH inhibits the monoamine oxidase (MAO) to decrease the metabolism of post-synaptic DA [Flatmark, 2000]. However, the depletion of DA in striatum was significant in patients with long-term addiction of METH. Central dopaminergic neurotoxicity has been shown to be induced by METH [Lin et al., 2007], 3,4-methylene-dioxymethamphetamine (MDMA) [Ke et al., 2008] and cannabinoid agonist [Wu et al., 2008]. Reduced DAT density, reduced DA D2 receptors, and VMAT-2 were noted in the striatum of METH abusers by positron emission tomography (PET) [Chang et al., 2007]. In rodents, long-term low-dose administration of AMPH induced release of DA from striatum; while, the high dose of AMPH decreased the release of DA from striatum [Ricaurte et al., 1984]. Estrogen showed neuroprotection in ischemic brain of Ovx rats [Xu et al., 2006]. Estrogen also exhibited neuroprotective effect on the nigrostriatal pathway. Chronic exposure to estrogen increased the density of DA uptake site in the nigrostriatal dopaminergic system, whereas the nucleus accumbens and substantia nigra par reticular were unaffected in Ovx rats [Morissette and Di Paolo, 1993]. Women received oophrectomy increased the risk of parkinsonism. Parkinsonism occurred twice as frequently in women with bilateral versus unilateral oophrectomy [Rocca et al., 2008]. Meanwhile, women tend to increase their rate of consumption of alcohol, marijuana, opioids, and cocaine more rapidly than do men [Lynch et al., 2002]. Once addicted to a drug, women can find it more difficult to quit than men do. In women, several of the positive subjective effects of d-AMPH such as euphoria, desire, increased energy, and intellectual efficiency are potentiated during the follicular phase (when estradiol levels are low firstly and rise slowly; progesterone levels are low) relative to the luteal phase (when estradiol levels are moderate and progesterone levels are high) [Justice and De Wit, 2000]. Ovx rats pre-treated with estrogen resulted in a significant enhancement of the effect of acute estrogen on AMPH-induced striatal DA release and stereotyped behaviors [Becker and Rudick, 1999].
The purpose of this study was to investigate the effect and mechanism of estradiol benzoate (EB) on brain DA levels in Ovx rats treated with AMPH. The protective effect of EB on striatal DA concentration reduced by AMPH was examined and the relationship between the levels of DA and the expression of DAT, VMAT-2, and tyrosine hydroxylase (TH) was established.
MATERIALS AND METHODS
Adult female Sprague–Dawley rats (300–350 g) provided by the Laboratory Animal Center, National Yang-Ming University (Taipei, Taiwan, ROC) were housed in a temperature-controlled (22 ± 2°C) room under 14 h artificial light (lights on from 0600 to 2000). Food and water were provided ad libitum. Female rats were Ovx for 2 weeks before administration of AMPH and being treated with or without EB (0, 6.25, 12.5, or 25 µg/kg) subcutaneously once daily in this experiment. The EB (Sigma, St. Louis, MO) was dissolved in sesame oil (Sigma). The AMPH was dissolved in saline and prepared freshly just before use in the experiments. To determine dose-dependent effects of AMPH, the Ovx rats were received daily intraperitoneal injection of various doses of AMPH (0.2, 1.0, 5.0 mg/kg) for 7 days.
Measurement of Striatal DA Level
Rat brains were quickly removed after decapitation and placed on an ice-cold glass plate for dissection. The striatal tissues were isolated, weighed, frozen on dry ice and stored at −80°C until assay. Tissue samples were homogenized with DA mobile phase (75 mM sodium dihydrogen phosphate, 1.5 mM sodium dodecyl sulfate, 200 µM EDTA, 0.72 mM triethlamine, 13.5% acetonitrile, 12% methanol, pH 4.0) and then centrifuged at 4°C, 8,000g for 10 min. The supernatants were filtered through a 0.22 µm pore membrane (Nanosep® MF) and analyzed for DA by high-performance liquid chromatography (HPLC) with an electrochemical detector (Bioanalytical Systems, model LC-40, W. Lafayette, IN). A 100 mm × 2.1 mm ODS Hypersil C-18 5 µm column (Hewlett-Packard, Corvallis, OR, 79916OD-552) was used. The mobile phase was delivered at 0.3 ml/min by an ISCO 260D pump (Lincoln, NE). DA was detected with a glassy carbon electrode maintained at 0.75 V related to an Ag/AgCl reference electrode. The HPLC chromatogram was recorded by a linear recorder (Model 1202). The retention time of DA was recorded as 13.5 min.
Western Immunoblotting for Protein Expressions of DAT, TH, and VMAT-2
After decapitation, rat brain tissues were dissected from the striatum and homogenized with 100 µl lysis buffer. The lysis buffer consisted of 1.5% Na-lauroylsacrosine, 2.5 mM Tris-base, 1 mM EDTA, 0.68% phenylmethylsulfonyl fluoride (PMSF), and 2% proteinase inhibitors, at pH 7.8. Tissue mixtures were centrifuged for 10 min at 8,000g. The protein concentration in the supernatant was determined by the Bradford method [Bradford, 1976]. Extracted proteins were denatured by boiling for 10 min in SDS buffer (0.125 M Tris-base, 2% sodium dodecyl sulphate (SDS), 0.001% bromophenol blue, 12% sucrose, and 0.15 M dithiothreitol. The proteins in the samples were separated using 8% and 10% SDS–polyacrylamide gel electrophoresis (SDS–PAGE) at 75 V for 15 min and then at 150 V for 40 min using a running buffer. The proteins were electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes (Perkin-Elmer Life Science Products, Inc., Boston, MA) using a Trans-Blot SD semi-dry-transfer cell (170-3940, Bio-Rad, Hercules, CA) for 60 min in a transfer solution. The membranes were washed in buffer (TBS-T buffer, containing 0.8% NaCl, 0.02 M Tris-base, and 0.3% Tween-20, pH 7.6) for 5 min and then blocked by a 120 min incubation in blocking buffer (TBS-T buffer containing 5% nonfat dry milk). These membranes were incubated with a rabbit anti-DAT polyclonal antibody (1:500, Chemicon International, Inc., Temecula, CA), rabbit anti-TH polyclonal antibody (1:1,000, Chemicon International, Inc.) and rabbit anti-VMAT-2 polyclonal antibody (1:1,000, Chemicon International, Inc.), overnight at 4°C. After four washes with TBS-T buffer that were 5 min each, the membranes were incubated for 1 h with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG, 1:6,000 dilution) in 5% nonfat dry milk of TBS-T buffer. The membranes were washed four times with TBS-T buffer, and then the band for steroidogenic acute regulatory protein was visualized by chemiluminescence (ECL Western blotting detection reagents, Amersham Pharmacia Biotech. Buckinghamshire, UK).
Immunohistochemistry
The Ovx rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and the brains were perfusion-fixed with a saline flush (400 ml/rat) following 4% paraformaldehy in 0.1 M phosphate buffer (pH 7.4, 400 ml/rat). The brains were removed after perfusion fixation and were immersed in the same fixative solution at 4°C. Five millimeter coronal slices were embedded in paraffin. Immunohistochemistry was performed on 5 mm thin section. The paraffin sections were dewaxing and dehydration and then were washed for 5 min in 0.01 M phosphate-buffered saline (PBS, pH 7.4). The paraffin sections were treated with 3% hydrogen peroxide for 30 min, following washed for 5 min in 0.01 M PBS and pre-incubation with 2% bovine serum albumin (BSA, UBS®) blocking reagent for 60 min. The brain sections were incubated with primary rabbit anti-DAT polyclonal antibody (1:100, Chemicon International, Inc.) overnight at 4°C. After a 5 min wash in 0.01 M PBS, the sections were incubated with biotinylated secondary IgG for 40 min and then with avidin–biotin peroxidase complex for 30 min in room temperature (ABC kit, Vector Labs, Burlingame, CA). The sections were counter-stained with hematoxylin.
RIA of EB Levels and Measurement of Uterine Weight
The concentration of plasma estradiol was determined by RIA. With anti-estradiol serum No. W1, the sensitivity of estradiol RIA was 1 pg/assay tube. After the Ovx rats were decapitated, the uterus was removed, drained of fluid, and blotted on filter paper. After defatting and cleaning, the uteri were then weighed by an electronic balance (Mettler H33ar, Switzerland).
Statistical Analysis
The treatment means were tested for homogeneity using analysis of variance, and the difference between specific means was tested for significance using Duncan's multiple-range test. A difference between two means was considered statically significant when P was less than 0.01.
RESULTS
Dose-Dependent Effects of AMPH on DA Levels in Striatum of Ovx Rats
The DA levels in striatum of Ovx rats with different doses of AMPH had been examined. Administration of AMPH (0.2, 1.0, and 5.0 mg/kg/day) resulted in a dose-dependent decrease (P < 0.01) in the striatal DA levels of Ovx rats (Fig. 1).
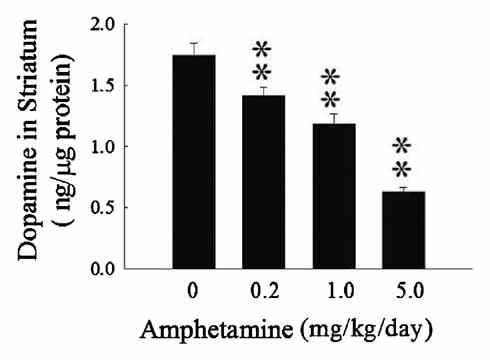
Dose-dependent effects of amphetamine (AMPH: 0, 0.2, 1.0, 5 mg/kg/day) for 7 days in vivo on dopamine levels in striatum of ovariectomized (Ovx) rats by HPLC measurement. Values are mean ± SEM (n = 6 rats). **P < 0.01 as compared to vehicle-treated group.
Effects of AMPH on the Expression of TH, VMAT-2, and DAT in Striatum of Ovx Rat
The effects of AMPH on the protein expressions of TH, VMAT-2, and DAT in striatum of Ovx rats were analyzed by Western blot and used β-actin as an internal control. Administration of AMPH (0.2, 1.0, and 5.0 mg/kg/day) resulted in a dose-dependently decrease for the expressions of VMAT-2, TH, and DAT (Fig. 2).
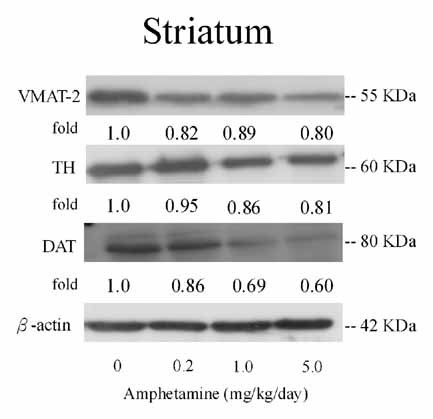
Dose-dependent effects of amphetamine (AMPH: 0, 0.2, 1.0, 5 mg/kg/day) for 7 days on the expression of tyrosine hydroxylase (TH), vesicular monoamine transporter 2 (VMAT-2), and dopamine transporter (DAT) in striatum of ovariectomized (Ovx) rats by Western blotting.
Effects of EB on DA Levels in Striatum of Ovx Rats
The effects of estradiol replacement on the DA levels in striatum of Ovx rats treated with or without AMPH (5 mg/kg/day) were examined. The striatal DA levels were not altered by the treatment of low dose of EB (6.25 µg/kg) in Ovx rats treated with vehicle (Fig. 3, upper panel) or AMPH (Fig. 3, lower panel). Administration of middle (12.5 µg/kg/day) or high (25 µg/kg/day) dose of EB resulted a significant increase (P < 0.01) of striatal DA levels in both vehicle- and AMPH-treated Ovx rats (Fig. 3).
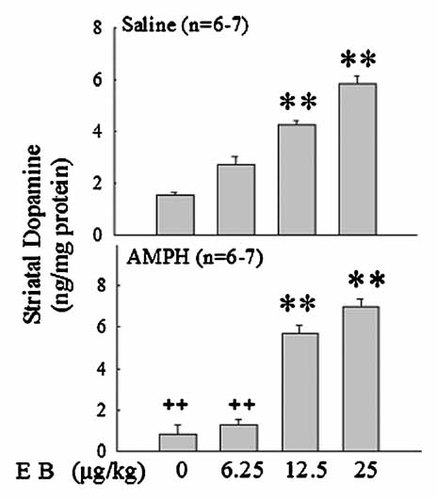
Dose-dependent effects of 17β-estradiol benzoate (EB: 0, 6.25, 12.5, 25 µg/kg/day) for 7 days in vivo on dopamine levels in striatum of ovariectomized (Ovx) rats treatment with AMPH (5 mg/kg, i.p.) by HPLC measurement. Values are mean ± SEM. **P < 0.01 as compared to control group. ++P < 0.01 as compared to control group.
Biological Effect of EB on Uterine Weight and Serum EB Concentration
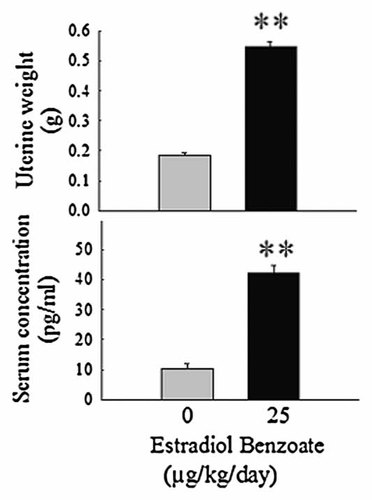
Administration of EB (25 µg/kg/day) increased (P < 0.01) the uterine weight and the concentration of serum EB (Fig. 4).
Effects of the Combination of AMPH and EB on the Levels of DA in Striatum
We utilize HPLC to examine striatal DA level in Ovx rats following the administration of AMPH (5 mg/kg/day) and/or EB (25 µg/kg/day). The result has shown that the DA levels in striatum were 1.91 ± 0.32 (ng/µg protein), 0.43 ± 0.15 (ng/µg protein), 3.16 ± 0.16 (ng/µg protein), 6.88 ± 0.42 (ng/µg protein) in control groups, AMPH groups, EB groups, and EB plus AMPH groups, respectively. Compared with control groups, the DA level of striatum mild increased in EB groups (P < 0.05, Fig. 5) but decreased in AMPH group (P < 0.01, Fig. 5). The DA level of striatum was significantly increased about threefold in EB plus AMPH groups (P < 0.01, Fig. 5).
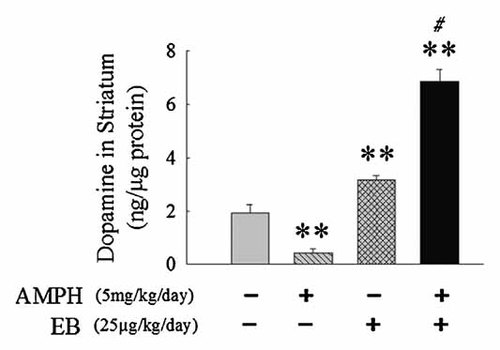
Effects of the administrations of amphetamine (AMPH: 5 mg/kg/day) and/or estradiol benzoate (EB: 25 µg/kg/day) once per day for 7 days on the levels of dopamine in striatum of ovariectomized (Ovx) rats by HPLC measurement. Values are mean ± SEM. **P < 0.01 as compared to vehicle-treated Ovx rats. #P < 0.01 as compared to amphetamine-treated Ovx rats.
Effects of the Administration of AMPH and/or EB on the Expressions of DAT, TH, and VMAT-2 in Striatum of Ovx Rats
To further investigate the effect of EB on the protein expression in striatum following administration of AMPH in Ovx rats, we analyzed the protein expressions of DAT, VMAT-2, and TH by Western blot technique. Administration of AMPH decreased the expression of TH, DAT, and VMAT-2 (P < 0.01, Figs. 6-8). EB did not alter the protein expression of TH, DAT, and VMAT-2 (Figs. 6-8). However, combination of EB and AMPH increased the expression of DAT in striatum of Ovx rats (P < 0.05, Fig. 6).
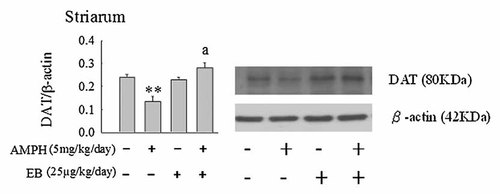
Effects of the administrations of amphetamine (AMPH: 5 mg/kg/day) and/or estradiol benzoate (EB: 25 µg/kg/day) once per day daily for 7 days on the expressions of dopamine transporter (DAT) in striatum of ovariectomized (Ovx) rats by Western blotting. Values are mean ± SEM (n = 4). **Value is significantly (P < 0.01) different from that of vehicle-treated Ovx rats. aValue is significantly (P < 0.01) different from that of amphetamine-treated Ovx rats.
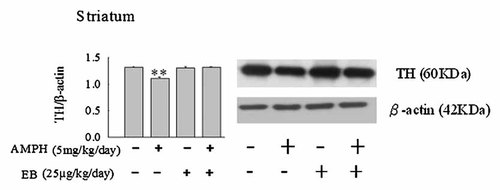
Effects of the administrations of amphetamine (AMPH: 5 mg/kg/day) and/or estradiol benzoate (EB: 25 µg/kg/day) once per day daily for 7 days on the expressions of tyrosine hydroxylase (TH) in striatum of ovariectomized (Ovx) rats by Western blotting. Values are mean ± SEM (n = 4). **Value is significantly (P < 0.01) different from that of vehicle-treated Ovx rats.
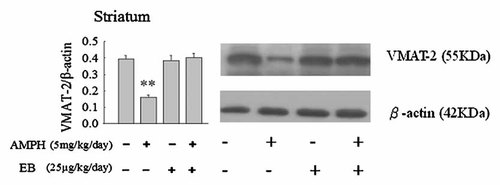
Effects of the administrations of amphetamine (AMPH: 5 mg/kg/day) and/or estradiol benzoate (EB: 25 µg/kg/day) once per day daily for 7 days on the expressions of vesicular monoamine transporter 2 (YMAT-2) in striatum of ovariectomized (Ovx) rats by Western blotting. Values are mean ± SEM (n = 4). **Value is significantly (P < 0.01) different from that of vehicle-treated Ovx rats.
Immunohistochemistry of DAT
To further know neuroprotective effect of estrogen, we have detective expression of DAT by immunohistochemistry. The distribution of DAT was localized in the plasma membranes of axon and terminal of dopaminergic nerve. The control striatum exhibited an immunostaining for DAT while AMPH striatum did not. However, the expression of striatal DAT in EB plus AMPH group was turned to control similarly (Fig. 9).
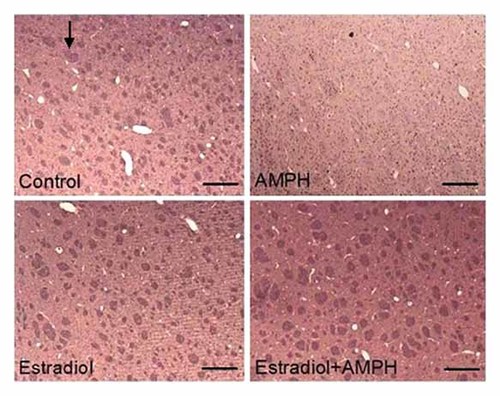
Effects of the administration of amphetamine (AMPH: 5.0 mg/kg/day) and/or estradiol benzoate (EB: 25 µg/kg/day) once daily for 7 days on the expressions of DAT (arrow) in striatum of ovariectomized (Ovx) rats by immunohistochemistry (the scale means 1.0 mm, 10 × 4).
DISCUSSION
Administration of AMPH (0.2, 1.0, and 5.0 mg/kg/day) resulted in a dose-dependent decrease (P < 0.01) in the striatal DA levels of Ovx rats (Fig. 1). Meanwhile, administration of AMPH (0.2, 1.0, and 5.0 mg/kg/day) resulted in a dose-dependently decreased the expressions of VMAT-2, TH, and DAT (Fig. 2). Intraperitoneal administration with varying low doses of methylphenidate (analog of AMPH, 0.25, 0.5, 1.0 mg/kg) produced dose-dependent increasing in extracellular DA levels within ACC [Berridge et al., 2006]. However, repeated high-dose administrations of the AMPH analog, METH, cause persistent dopaminergic deficits (i.e., reductions in striatal DA content, DAT density, and/or activity of the DA synthesizing enzyme TH) in rodents [Hotchkiss et al., 1979; Wagner et al., 1980]. Magnetic resonance imaging (MRI) and PET disclosed reduced DAT and VMAT-2 density in the striatum in human with abuse of METH [Chang et al., 2007]. For both caudate–putamen and nucleus accumbens, the DA response progressively declined with successive injections of AMPH in escalating dose-binge model in rodents [Kuczenski and Segal, 1997; Segal and Kuczenski, 1997].
The effects of EB replacement on the DA levels in striatum of Ovx rats treated with or without AMPH (5 mg/kg/day) were also examined in this study. The striatal DA levels were not altered by the treatment of low dose of EB (6.25 µg/kg/day) in Ovx rats treated with vehicle (Fig. 3, upper panel) or AMPH (Fig. 3, lower panel). While, administration of middle (12.5 µg/kg/day) or high (25 µg/kg/day) dose of EB resulted a significant increase (P < 0.01) of striatal DA levels in both vehicle- and AMPH-treated Ovx rats (Fig. 3). The basal extracellular concentrations of DA in the striatum, determined by quantitative microdialysis, are greater on estrus than on diestrus [Xiao and Becker, 1994]. Similarly, there are estrous cycle-dependent differences in AMPH stimulated behaviors and striatal DA release; intact female rats exhibited a greater behavioral response to AMPH on estrus than on other days of the cycle. AMPH-induced striatal DA response was attenuated following Ovx. Prior treatment with estrogen resulted in a significant enhancement of the effect of acute estrogen on AMPH-induced striatal DA release and stereotyped behaviors [Becker and Rudick, 1999]. Compared to the concentration of EB 12.5 and 25 µg/kg/day, the 6.25 µg/kg/day seemed not enough to enhance the effect of acute estrogen on AMPH-induced striatal DA level. Two mechanisms were postulated to contribute to the effect of EB on stimulated DA release in female rats. First, estrogen acts to inhibit intrinsic GABA neurons that have recurrent collaterals onto DA terminals. This results in greater DA release. Second, estrogen acts on DA terminals to enhance DA release by downregulating pre-synaptic D2 DA receptor function [Becker, 1999].
To check the biological effect of EB on uterine weight and serum EB concentration, we administered EB (25 µg/kg/day) subcutaneously. Increased uterine weight and the concentration of serum EB (P < 0.01) were noted (Fig. 4). The amount of reproductive hormones is changing at different stages of estrous cycle in rats. It has been demonstrated that the levels of plasma estradiol ranged from 17 ± 2 to 21 ± 2 pg/ml between midnight of estrus and 1500 h of metestrus and reached the peak level at noon of proestrus (88 ± 2 pg/ml) [Butcher et al., 1974]. In the present study, we found the level of plasma estradiol increased from 10.31 ± 1.69 to 42.19 ± 2.34 (pg/ml) after administration of EB (25 µg/kg/day) for 1 week. It is within the normal range and may be close to the level in the morning of proestrus. Therefore, estradiol administration is effective and physiological to modulate the response of DA release in this study.
We utilized HPLC to examine striatal DA level in Ovx rats following the administration of AMPH (5 mg/kg/day) and/or EB (25 µg/kg/day). The result has shown that the DA levels in striatum were 1.91 ± 0.32 (ng/µg protein), 0.43 ± 0.15 (ng/µg protein), 3.16 ± 0.16 (ng/µg protein), 6.88 ± 0.42 (ng/µg protein) in control groups, AMPH groups, EB groups and EB plus AMPH groups, respectively. Compared with control groups, the DA level of striatum mild increased in EB groups (P < 0.05, Fig. 5) but decreased in AMPH group (P < 0.01, Fig. 5). The DA level of striatum was significantly increased about threefold in EB plus AMPH groups (P < 0.01, Fig. 5). Many studies have indicated that female rodents are more sensitive than male rodents to the behavioral effects of stimulants (AMPH and cocaine) and that estradiol enhances the subjective response to stimulants. The expression of sensitization to AMPH is attenuated in ovariectomized (Ovx) rats [Forgie and Stewart, 1993]. Estradiol treatments in Ovx rats enhance sensitization of locomotor activity induced by AMPH or cocaine [Peris et al., 1991]. Meanwhile, AMPH-stimulated behaviors and striatal DA release are also related to estrous cycle in rats. Intact female rats exhibit a greater behavioral response to AMPH on estrus than they do on other days of the cycle [Becker et al. 2001]. In humans, sex differences are present for all of the phases of drug abuse. Women begin regularly self-administering illicit (i.e., AMPH cocaine, heroin, and cannabis) drugs of abuse at lower doses than do men, use escalates more rapidly to addiction, and are at greater risk for relapse following abstinence [Becker and Hu, 2008]. Acute subjective effects of AMPH in women, higher levels of estrogen were associated with greater AMPH-induced increases in “euphoria” and “energy and intellectual efficiency” [Kosten et al., 1996].
Effects of the administration of AMPH and/or EB on the expressions of DAT, TH, and VMAT-2 in striatum of Ovx rats were examined. Administration of AMPH decreased the expression of TH, DAT, and VMAT-2 (P < 0.01, Figs. 6-8). EB did not alter the protein expression of TH, DAT, and VMAT-2 (Figs. 6-8). However, combination of EB and AMPH increased the expression of DAT in striatum of Ovx rats (P < 0.05, Fig. 6).
The VMAT-2 transports intraneuronal DA into vesicles for storage and subsequent release and is thus an important regulator of cytoplasmic DA levels and dopaminergic function. Disruption of VMAT-2 function likely contributes to the dopaminergic deficits caused by high-dose administrations of d- and d/l-METH, as experiments with VMAT-2-knockout mice indicate that disruption of vesicular DA transport potentiates METH-induced persistent dopaminergic deficits [Fumagalli et al., 1999]. Both AMPH and METH directly inhibit the VMAT-2. Both electrically and AMPH-stimulated extracellular DA release are lower in VMAT-2-knockout mice than in wild-type mice suggesting that the knockout mice have smaller vesicular DA stores [Wang et al., 1997; Patel et al., 2003].
Although AMPH and its analogs generally display comparable actions at the various transporters, including DAT, serotonin (5-hydroxytryptamine, 5HT) transporter and norepinephrine transporter, the DAT is the protein most frequently implicated in their reinforcing properties and abuse potential [Fleckenstein et al., 2007]. To further know neuroprotective effect of estrogen, we have detective expression of DAT by immunohistochemistry. The distribution of DAT was localized in the plasma membranes of axon and terminal of dopaminergic nerve. The control striatum exhibited an immunostaining for DAT while AMPH striatum did not. However, the expression of striatal DAT in EB plus AMPH group was turned to control similarly (Fig. 9). Reduced levels of three dopamine nerve terminal markers (DA, TH, and DAT) in post-mortem striatum (nucleus accumbens, caudate, putamen) of chronic METH users. However, levels of DOPA decarboxylase and the vesicular monoamine transporter were normal. This suggests that chronic exposure to METH did not cause permanent degeneration of striatal dopamine nerve terminals. The decreased DAT could provide the basis for dose escalation occurring in METH users [Wilson et al., 1996].
CONCLUSION
The present study has shown that AMPH could attenuate DA level significantly in striatum. Comparing to control groups, administration of EB or EB plus AMPH increased DA level. The protein expression of striatal DAT was significant greater in rats treated with AMPH plus EB than in AMPH-treated animals. These results suggest that the DA levels in striatum can be enhanced by EB via an increase of DAT expression following administration of AMPH.





