Target identification of microRNAs expressed highly in human embryonic stem cells†‡§
Authors' Contributions: SSLL designed the experiments, analyzed results and wrote the manuscript. S-LY supervised the determination of both miRNAs and mRNAs. L-PK prepared RNA samples and did part of data analyses. ZYT cultured T3ES and T3DF cells. SS did bioinformatic analyses. BZC prepared T3EB cells. B-CH did the luciferase assay, YHL collaborated to derive hES cell lines. PCY directed the Microarray Core Facility for Genomic Medicine of National Taiwan University College of Medicine where the analyses of miRNAs and mRNAs were done.
The authors declare no competing financial interests.
Database And Accession Number: The original data obtained from Affymetrix human genome U133 plus 2.0 GeneChip have been deposited to NCBI database, and the GEO series number is GSE9440.
Abstract
MicroRNAs (miRNAs) are noncoding RNAs of approximately 22 nucleotides in length that negatively regulate the post-transcriptional expression by translational repression and/or destabilization of protein-coding mRNAs. The impact of miRNAs on protein output was recently shown that although some targets were repressed without detectable changes in mRNA levels, those translationally repressed by more than a third also displayed detectable mRNA destabilization, and, for the more highly repressed targets, mRNA destabilization usually comprised the major component of repression. Thus, comparative profilings of miRNAs and mRNAs from the same samples of different cell types may identify the putative targets of miRNAs. In this investigation, both miRNA and mRNA profiles from the undifferentiated human embryonic stem cell line hES-T3 (T3ES), hES-T3 derived embryoid bodies (T3EB), and hES-T3 differentiated fibroblast-like cells (T3DF) were compared, and 58 genes were found to be targets of four hES cell-specific miRNAs miR-302d, miR-372, miR-200c and/or miR-367 by inverse expression levels (highly negative correlation) of miRNAs to their target mRNAs. Approximately half of these 58 targets are involved in gene transcription. Three common target genes TRPS1, KLF13 and MBNL2 of three highly expressed miRNAs miR-302d, miR-372, and miR-200c were identified, and the target sites of both miR-302d and miR-372 in the 3′UTR of TRPS1, KLF13, and MBNL2 genes were confirmed by the luciferase assay. The highly expressed mRNAs and miRNA target mRNAs involved in KEGG pathways among T3ES, T3EB, and T3DF cells were also compared, and the expression levels of target mRNAs predicted by abundantly expressed miRNAs were found to be three- to sixfold lower than those of non-target mRNAs involved in the same signaling pathways. J. Cell. Biochem. 106: 1020–1030, 2009. © 2009 Wiley-Liss, Inc.
Abbreviations used:
hES, human embryonic stem cells; IVF, in vitro fertilization; MEF, mouse embryonic fibroblasts; miRNAs, microRNAs; T3DF, hES-T3 differentiated fibroblast-like cells; T3EB, hES-T3 differentiated embryoid bodies; T3ES, hES-T3 undifferentiated cells.
Since 1998, when the first human embryonic stem (hES) cell lines were reported [Thomson et al., 1998], a large number of hES cell lines have been derived from the inner cell mass of preimplantation embryos donated at in vitro fertilization (IVF) clinics [Guhr et al., 2006]. These hES cell lines possess remarkable ability of both unlimited self-renewal and pluripotency to generate any cell type differentiated from three germ-layers ectoderm, mesoderm, and endoderm. Thus, these hES cell lines have great potentials for cell therapies in regenerative medicine and experimental models for drug discovery in addition to basic studies on stem cell biology and molecular embryogenesis [Wobus and Boheler, 2005].
The genome-wide gene expression analyses using high-throughput microarray have been used to identify key “stemness” genes responsible for the unlimited self-renewal and pluripotency of hES cells [Ivanova et al., 2002; Ramalho-Santos et al., 2002; Sperger et al., 2003]. A meta-analysis of 20 previously reported transcriptomes had identified 48 genes, as well as several genes involved in signaling pathways such as Wnt, overexpressed in hES cells compared to differentiated cell types [Assou et al., 2007], and these 48 genes, including transcription factors such as OCT4 (also known as POU5F1), SOX2 and NANOG, may be responsible for the unlimited self-renewal and pluripotency of hES cells. However, molecular mechanisms involved in unlimited self-renewal and pluripotency of hES cells remain to be fully understood.
Recently, microRNAs (miRNAs) have been shown to play important roles in mammalian embryo development and cell differentiation. Mammalian genomes encode many hundreds of miRNAs, which are predicted to regulate expression of as many as 30% of protein-coding genes [Bartel, 2004; John et al., 2004; Griffiths-Jones et al., 2006; Landgraf et al., 2007]. Although the biological functions of most miRNAs are unknown, some miRNAs appear to participate in determination of cell fate, in pattern formation in embryonic development, and in control of cell proliferation, cell differentiation and cell apoptosis in animals [Alvarez-Garcia and Miska, 2005; Kloosterman and Plasterk, 2006]. The miRNAs negatively regulate the post-transcriptional expression by translational repression and/or destabilization of protein-coding mRNAs. The impact of miRNAs on protein output was recently shown that although some targets were repressed without detectable changes in mRNA levels, those translationally repressed by more than a third also displayed detectable mRNA destabilization, and, for the more highly repressed targets, mRNA destabilization usually comprised the major component of repression [Baek et al., 2008]. In human and mouse, several ES cell-specific miRNAs were identified by cDNA cloning and sequencing [Houbaviy et al., 2003; Suh et al., 2004]. These miRNAs were shown to be rapidly downregulated during differentiation, while miRNAs reported from other cell types were poorly expressed in ES cell lines. It would be important to identify the target mRNAs of hES cell-specific miRNAs that will lead us to understand the complex and interesting networks of regulation in hES cells. Furthermore, since the hES cell-specific miRNAs can reduce the steady-state levels of their target mRNAs [Bagga et al., 2005; Farh et al., 2005; Lim et al., 2005; Stark et al., 2005; Sood et al., 2006; Baek et al., 2008] and several genes involved in signaling pathways were found to be over-expressed in hES cells compared to differentiated cell types [Assou et al., 2007], it would be of interest to examine if the expression level of the mRNAs targeted by hES-specific miRNAs is lower than that of the non-target mRNAs in the same signaling pathway.
The continuous culture of undifferentiated hES cells either on mouse embryonic fibroblasts (MEF) as feeder layer or in the MEF-conditioned medium bears the risk of transmitting animal pathogens, and limits future medical applications of hES cells. A few human cell systems, including hES-derived fibroblast-like cells as feeder [Stojkovic et al., 2005], with capacity to support the growth of undifferentiated hES cells have been developed to replace the use of MEF. The in vitro differentiation of hES cells into embryoid bodies is an essential step to generate many specific cell types in addition to providing a test of pluripotency of hES cells. Thus, it is of importance to produce uniformed embryoid bodies.
In this investigation, a new method of producing more uniformed sphere of embryoid bodies was developed, and an autogeneic feeder cells with capacity to support the growth of undifferentiated hES cells was established. Both miRNA and mRNA profiles from the undifferentiated human embryonic stem cell line hES-T3 (T3ES), hES-T3 derived embryoid bodies (T3EB), and hES-T3 differentiated fibroblast-like cells (T3DF) were quantitatively determined and compared. Fifty eight targets of four miRNAs expressed highly in human embryonic stem cells were identified by inverse expression levels of miRNAs to their target mRNAs, and approximately half of these 58 targets are involved in gene transcription. The target sites of both miR-302d and miR-372 in the 3′UTR of TRPS1, KLF13 and MBNL2 genes were further confirmed by the luciferase assay. The expression levels of target mRNAs predicted by abundantly expressed miRNAs were also found to be much lower than those of non-target mRNAs involved in the same signaling pathways.
MATERIALS AND METHODS
Human Embryonic Stem Cell Culture
Human embryonic stem cell line hES-T3, which is one of the five hES cell lines derived with institutional review board approval from preimplantation embryos donated at IVF clinics in Taiwan [Li et al., 2006], exhibits normal female karyotype (46, XX), and it has been continuously cultured on mitotically inactivated mouse embryonic fibroblast (MEF) feeder layer in hES medium under 5% CO2 at 37°C and underwent freezing/thawing processes. The hES culture medium consisted of DMEM/F12 (1:1, Gibco) supplemented with 20% KSR, 1% non-essential amino acids, 1 mM L-glutamine, 0.1 mM β-mercaptoethanol, and 4 ng/ml basic fibroblast growth factor (bFGF; Life Technologies). Routine passages of hES-T3 line every 5–7 days were done with collagenase (type IV, 1 mg/ml, Invitrogen) treatment and mechanical scrape. The undifferentiated state of hES-T3 cells (passage 36) grown on MEF feeder layer was indicated by expression of OCT4 and NANOG, and these undifferentiated cells are designated as T3ES.
Formation of Embryoid Bodies
The formation of embryoid bodies (EBs) was induced by mechanically dissecting undifferentiated hES-T3 colonies (passage 36) into pieces that were transferred and grown with very slow shaking (20 rpm) in the EB medium containing 80% knockout DMEM, 20% ES-qualified FBS (Gibco), 1% non-essential amino acids, and 1 mM L-glutamine without MEF feeder layer in bacterial Petri dish plate (α-plus, 10 cm) under 5% CO2 at 37°C for 7 days with change of medium every 2 days. These embryoid bodies are designated as T3EB.
Establishment of Human hES-T3 Differentiated Fibroblast-Like Cells
An autogeneic feeder cells with capacity to support the growth of undifferentiated hES cells was established according to the previously published procedure [Stojkovic et al., 2005]. hES-T3 (passage 19) cells were transferred into feeder-free and noncoated plate (10 cm) in DMEM supplemented with 10% FBS (Gibco) under 5% CO2 at 37°C. After 10 days, cells appeared as fibroblast-like morphology, that is, flat cells with elongated nucleus and branching pseudopodia, and these differentiated fibroblast-like cells are designated as T3DF. These T3DF cells were passaged using trypsin (0.05%, Gibco) every 4 days or cryopreserved. After inactivation using mitomycin C (10 µg/ml), T3DF cells (passage 8) as feeder layer have been shown to support the undifferentiated growth of hES-T3 cells (34 passages on MEF) for more than 14 passages.
Staining of OCT4, NANOG, and Galectin-1
The hES-T3 (T3ES) colonies grown on MEF or T3DF feeder layer were fixed by 4% paraformaldehyde and permeabilized using 0.5% Triton X-100 in the culture dishes. The immunostaining with rabbit polyclonal antibodies against human OCT4, NANOG and galectin-1 (Santa Cruz Biotechnology) were detected with either goat or donkey anti-rabbit IgG.
Extraction of Total RNAs
Total RNAs were extracted using 1 ml TRIZOL reagent (Invitrogen) for approximately 1 × 106 cells of T3ES and T3DF from 10 cm plates, as well as T3EB sample. Total RNAs were quantified spectrophotometrically. The same total RNAs from each sample were used for both mRNA microarray analysis and miRNA quantification.
mRNA Microarray Analysis
The cDNA synthesis was carried out by using SUPERSCRIPT™ one-step RT-PCR kit (Invitrogen). For the reverse transcription step, the whole 5 µl of the resuspended RNAs were incubated for 60 min at 42°C, then 15 min at 72°C in 50 µl of reaction mixture containing 25 µl of 2× Reaction Mix (Invitrogen) and 1 µl of RT/PLANTINUM Taq Mix (Invitrogen). 28.5 µl of the cDNAs present in the 50 µl RT reaction mixture purified by Microarray Target Purification Kit (Roche Applied Science, Indianapolis, ID, http://www.roche-applies-science.com) were used as templates to amplify cDNA by Microarray Target Amplification Kit. The amplified cDNAs were purified by Microarray Target Purification Kit, and complementary RNAs (cRNAs) were synthesized from 200 ng cDNA with Microarray RNA Target Synthesis Kit (Roche Applied Science). The purified 5 µg cRNAs from T3ES, T3EB, and T3DF were analyzed using Affymetrix Human Genome U133 plus 2.0 GeneChip according to the Manufacturer's protocols (Santa Clara, CA, http://www.affymetrix.com) by the Microarray Core Facility for Genomic Medicine of National Science Council in Taiwan. This Affymetrix GeneChip contains 54,675 probe sets to analyze the expression level of 47,400 transcripts and variants, including 38,500 well-characterized human genes. GeneChips from the hybridization experiments were read by the Affymetrix GeneChip scanner 3000, and raw data were processed using GC-RMA algorithm. The raw data were also analyzed by GeneSpring GX software version 7.3.1 (Silicon Genetics, Redwood City, CA, http://www.chem.agilent.com).
Quantification of miRNAs
The expression levels of 250 human miRNAs were determined using the TagMan MicroRNA Assays (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com) as described previously [Chen et al., 2005, 2007]. In brief, TagMan MicroRNA Assays include two steps: stem loop RT followed by real-time PCR (90 ng/Rx, with 24-multiplex primers). Each 10 µl RT reaction that includes 90 ng total RNA, 50 nM stem-loop RT primers, 1× RT buffer, 1.25 mM each of dNTPs, 0.25 U/µl RNase inhibitor, and 10 U/µl MultiScribe Reverse Transcriptase was incubated in the PTC-225 Peltier Thermal Cycler (MJ Research, Watertown, MA) for 30 min each at 16°C and at 42°C, followed by 5 min at 85°C, and then held at 4°C. RT products were diluted 20 times with H2O prior to setting up PCR reaction. Real-time PCR for each miRNA was carried out in triplicates, and each 10 µl reaction mixture included 2 µl of diluted RT product, 5 µl of 2× TagMan Universal PCR Master Mix and 0.2 µM TagMan probe, respectively. The reaction was incubated in an Applied Biosystems 7900HT Sequence Detection System at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The threshold cycle (Ct) is defined as the fraction cycle number at which the fluorescence exceeds the fixed threshold of 0.2. Total RNA input was normalized based on the Ct values of the TagMan U6 snRNA assay as an endogenous control. The fold change was calculated as 2−ΔCT × K, where −ΔCT = −[CTmiRNA−CTU6 snRNA] and K is a constant. The unsupervised hierarchical clustering of 250 miRNAs was performed using standard correlation.
Target Prediction of miRNAs
The potential target genes of hES cell-specific miRNAs were first predicted using the TargetCombo open source software [Sethupathy et al., 2006] (http://www.diana.pcbi.upenn.edu/cgi-bin/miRGen/v3/Targets.cgi) which predicts targets by the intersection of miRanda (http://microrna.org), PicTar (4-way, http://pictar.bio.nyu.edu/) and TargetScanS (http://www.targetscan.org/) with a cutoff P value less than 0.05. Since no potential target was predicted using TargetCombo (intersection of miRanda, PicTar and TargetScanS), the methods of both PicTar and TargetScanS were then used to predict potential targets of miRNAs. The expression levels of the predicted target mRNAs were first analyzed by the Volcano plot using parametric test and Benjamini-Hochberg false discovery rate for multiple testing correction. The differentially expressed mRNAs were defined by fold-changes of more than 3 and a P-value cutoff of 0.05. These mRNAs expressed more than threefold of overall mean in T3DF cells were then selected as abundantly differentially expressed genes. These abundantly differentially expressed genes were further analyzed using one way-ANOVA (parametric test, variances assumed equal) with P-value of 0.01 and false discovery rate of 0.01 for their statistically significant differences among T3ES, T3EB, and T3DF cells. The negative correlation coefficients between miRNAs and their target mRNAs among T3ES, T3EB andT3DF cells were calculated to demonstrate their inverse expression levels.
Luciferase Assay
The 3′UTRs of TRPS1, KLF13, and MBNL2 genes were PCR-amplified from genomic DNA of HEK293 cells by using the forward primer TRPS1F (5′-ACTAGTAACATGAAACAAATGAAAGTTCTT-3′) and the reverse primer TRPS1R (5′-ACGCGTGGATCTCAAAGTGCTTTACAAA-3′), KLF13F (5′-ACTAGTCAGAAGGCATGCAGGACCC-3′) and KLF13R (5′-AAGCTTAAATTAAATTAAGTGCTCTCTTTG-3′), and MBNL2F (5′-ACTAGTATTTACAATAAGGAGGAGGCA-3′) and MBNL2R (5′-AAGCTTTTCGGTGAAGTGCTGTTATAT-3′), respectively. All of PCR fragments were cloned into pMIR-reporter luciferase vector (Ambion). All transfections were carried out in triplicate in 96-well plates. Cells (1 × 104) were seeded for 24 h prior to transfection. The luciferase reporter constructs along with the control plasmid (pRL-TK Vector, Promega) were cotransfected into cells at the DNA ratio 5:1 in presence/absence of miRNA precursors (Ambion) as indicated by RNAi fect reagent (Qiagen). After 48 h transfection the Dual-Glo luciferase substrate (Promega) was added to each well and the luminescent signals were measured by Victor3 multilabel counter (PerkinElmer) according to the manufacturer's instructions. The activity of Renilla luciferase was used as an internal control to normalize transfection efficiency [Mayr et al., 2007].
Molecular Functions of hES Cell-Specific miRNA Targets
The GeneOntology, including molecular functions, cellular localization and biological processes, of abundantly differentially expressed targets of hES cell-specific miRNAs were analyzed using GeneSpring GX software version 7.3.1 GeneOntology Browser and MetaCore Analytical Suite (GeneGo, Inc., St Joseph, MI). The current NetAffx annotation of transcripts 26 dated July 8, 2008 was used for analyzing molecular functions of miRNA targets.
Analyses of Gene Expression in Signaling Pathways
The mRNAs expressed more than threefold of overall mean and the target mRNAs predicted by miRNAs that expressed more than 20-fold of U6 snRNA were used to examine whether genes involved in specific pathways showed differential expression among T3ES, T3EB, and T3DF cells. The expression levels of target mRNAs predicted by abundantly expressed microRNAs were also compared with those of non-target mRNAs involved in the same signaling pathways in order to test their differential expression. Pathways were taken from the KEGG database.
RESULTS
Characterization of Undifferentiated hES Cells and Differentiated Derivatives
Human embryonic stem cell line hES-T3 with normal female karyotype (46, XX) was established as previously described [Li et al., 2006]. The undifferentiated state of hES-T3 (designated as T3ES in this investigation) cells grown on mouse embryonic fibroblast (MEF) feeder layer was indicated by positive staining of OCT4 and NANOG (Supplementary online Fig. S1A–H), indicating that the T3ES cell population contained a very high proportion of undifferentiated hES cells. The T3EB embryoid bodies formed with slow shaking appeared to be sphere, while those T3EBs produced without shaking seemed to exhibit irregular shapes (Supplementary online Fig. S2). The T3DF differentiated fibroblast-like cells were derived from hES-T3 cell line according to the previously published procedure [Stojkovic et al., 2005], and they appeared as flat cells with elongated nucleus and branching pseudopodia. The T3DF cells were further shown to be able to support the undifferentiated growth of hES-T3 cells for more than 14 passages (data not presented), and the hES-T3 colonies grown on T3DF feeder layer were stained positively for OCT4 and NANOG (Supplementary online Fig. S1I–P). The expression of transcription factors OCT4, SOX2, and NANOG was also shown to be highly expressed in T3ES cells, and down-regulated in differentiated T3EB and T3DF cells (data not presented).
Expression Profiling of mRNAs
The genome-wide mRNA expression of T3ES, T3EB, and T3DF cells was determined using Affymetrix human genome U133 plus 2.0 GeneChip. The original data have been deposited to NCBI database, and the GEO series number is GSE9440. The gene symbols of the 60 most expressed genes with known functions are summarized in Supplementary online Table S1. The 60 most expressed genes of T3ES cells include OCT4, SOX2, and NANOG. The 60 most expressed genes of T3EB cells contain several germ-layer markers, that is, AFP and H19 of endoderm, HAND1, and COL2A1 of mesoderm, and ISL1 of ectoderm. The 60 most expressed genes of T3DF cells include ten genes TRPS1, KLF13, MBNL2, MTMR3, NF1B, RAB6A, MARCKS, DDEF1, MBNL1, and QKI, which were later identified to be targets of four ES cell-specific miRNAs miR-302d, miR-372, miR-200c, and/or miR-367 (Table II). It is intriguing that the LGALS1 gene encoding soluble galactoside- binding lectin 1 (galectin-1) was extremely abundantly (more than 200-fold of overall mean) expressed in both T3ES and T3DF cells, but very low (less than overall mean) in T3EB cells (Supplementary online Fig. S1Q–T).
Expression Profiling of miRNAs
The expression profiles of 250 human miRNAs in T3ES, T3EB, and T3DF cells were quantitated using TagMan MicroRNA Assays as described previously [Chen et al., 2005, 2007], and the expression level of each miRNA was indicated as folds over U6 snRNA. The average expression levels of triplicate analyses for 250 miRNAs from T3ES, T3EB, and T3DF cells are given in Supplementary online Table S2, and the expression patterns of 250 miRNAs among these three cell types can be roughly classified into five groups with the help of the unsupervised hierarchical clustering using standard correlation. The first pattern (A group) represents a set of 12 miRNAs, including 10 hES cell-specific miRNAs [Suh et al., 2004], in that all 12 miRNAs were expressed at extremely low level (less than 0.07-fold U6 snRNA) in T3DF cells, and six of them were abundantly (more than 20-fold U6 snRNA) expressed in both T3ES and T3EB cells (Table I). The second pattern (B group) consists of 16 miRNAs, including eight members of let-7 family, in that all of them were under-expressed in T3EB cells by comparison with T3ES and T3DF cells. The third pattern (C group) is composed of 47 miRNAs, including pancreas-specific miR-375, in that all of them were over-expressed in T3EB cells compared with T3ES and T3DF cells. The fourth pattern (D group) contains 75 miRNAs, including three heart and skeletal muscle-specific miRNAs, in that most of them were roughly equally expressed in all three cell types. The fifth pattern (E group) contains 100 miRNAs, including 25 placenta-, 6 testis-, and one liver-specific miRNAs, in that all of them were expressed at very low levels (less than onefold U6 snRNA) in all three cell types [Bentwich et al., 2005; Liang et al., 2007].
| miRNAs | T3ES | T3EB | T3DF | T3ES/T3DF | Specificity | Chrom. |
|---|---|---|---|---|---|---|
| hsa-miR-302d | 204.60 | 821.30 | 0.01 | 20,460 | hES | 4q25 |
| hsa-miR-367 | 136.40 | 857.80 | 0.01 | 13,640 | hES | 4q25 |
| hsa-miR-372 | 27.80 | 101.40 | 0.01 | 2,780 | hES | 19q13.42 |
| hsa-miR-200c | 27.73 | 180.60 | 0.03 | 937 | hES | 12p13.31 |
| hsa-miR-20b | 44.68 | 357.90 | 0.06 | 791 | Xq26.2 | |
| hsa-miR-26b | 24.02 | 80.74 | 0.07 | 349 | 2q35 | |
| hsa-miR-302c* | 13.82 | 23.81 | 0.01 | 1,382 | hES | 4q25 |
| hsa-miR-302a* | 1.29 | 5.89 | 0.01 | 129 | hES | 4q25 |
| hsa-miR-302b* | 0.11 | 0.34 | 0.01 | 11 | hES | 4q25 |
| hsa-miR-371 | 3.25 | 8.94 | 0.01 | 325 | hES | 19q13.42 |
| hsa-miR-373* | 0.21 | 0.22 | 0.01 | 21 | hES | 19q13.42 |
| hsa-miR-368 | 0.01 | 0.01 | 0.01 | 1 | hES | 14q32.31 |
Target Identification of Highly Expressed hES Cell-Specific miRNAs
The four hES cell-specific miRNAs miR-302d, miR-367, miR-372, and miR-200c were found to express abundantly (more than 20-fold U6 snRNA) in both T3ES and T3EB cells, but little (0.03-fold U6 snRNA) in T3DF cells (Table I). The targets of these four miRNAs in hES cells are not yet known [John et al., 2004; Griffiths-Jones et al., 2006; Landgraf et al., 2007], and no potential target was predicted using TargetCombo (intersection of miRanda, PicTar, and TargetScanS) [Sethupathy et al., 2006]. The methods of both PicTar and TargetScanS were then used to predict potential targets, and 300, 212, 248, and 395 targets were obtained for miR-302d, miR-367, miR-372, and miR-200c, respectively. The expression levels of these target mRNAs predicted by miR-302d, miR-367, miR-372, and/or miR-200c in T3ES and T3DF cells were analyzed by the Volcano plot, and the differentially expressed genes were defined by more than threefold of changes (T3DF/T3ES) and a P-value cutoff of 0.05. Fifty eight differentially expressed genes were found to express more than threefold of overall mean in T3DF cells (Table II). All of these 58 abundantly differentially expressed genes were further analyzed for their statistically significant differences among T3ES, T3EB, and T3DF cells by using one way-ANOVA with P-value of 0.01 and false discovery rate of 0.01. The inverse expression levels between theses 58 target mRNAs and the miRNAs miR-302d, miR-372, miR-200c, and miR-367 among T3ES, T3EB, and T3DF cells were confirmed by negative correlation coefficients ranging from −0.47 to −0.81 (Supplementary online Table S3). Therefore, these 58 abundantly differentially expressed mRNAs are very likely to be the targets of four abundantly expressed miRNAs miR-302d, miR-372, miR-200c, and/or miR-367 in hES cells.
| miR- | miR- | miR- | miR- | Gene symbol | T3ES | T3EB | T3DF | DF/ES | Description | Probe ID | UniGene |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 302d | 372 | 200c | TRPS1 | 4.21 | 0.46 | 81.92 | 19.5 | Trichorhinophalangeal syndrome I | 234351_x_at | Hs.657018 | |
| 302d | 372 | 200c | KLF13 | 6.13 | 0.75 | 113.70 | 18.5 | Kruppel-like factor 13 | 219878_s_at | Hs.525752 | |
| 302d | 372 | 200c | MBNL2 | 1.47 | 0.28 | 19.69 | 13.4 | Muscleblind-like 2 (Drosophila) | 205017_s_at | Hs.657347 | |
| 302d | 372 | 200c | MTMR3 | 0.99 | 0.78 | 11.28 | 11.4 | Myotubularin related protein 3 | 202198_s_at | Hs.474536 | |
| 302d | 372 | 367 | NFIB | 0.67 | 0.21 | 12.88 | 19.3 | Nuclear factor I/B | 213033_s_at | Hs.644095 | |
| 302d | 372 | RAB6A | 2.37 | 0.41 | 16.23 | 6.9 | RAB6A, member RAS oncogene family | 201048_x_at | Hs.503222 | ||
| 302d | 372 | ZNF238 | 1.76 | 0.69 | 9.84 | 5.6 | Zinc finger protein 238 | 207164_s_at | Hs.69997 | ||
| 302d | 372 | MEF2C | 0.95 | 0.82 | 9.62 | 10.1 | Myocyte enhancer factor 2C | 207968_s_at | Hs.654474 | ||
| 302d | 372 | PURB | 1.03 | 0.78 | 8.49 | 8.3 | Purine-rich element binding protein B | 235711_at | Hs.349150 | ||
| 302d | 372 | NR4A2 | 1.52 | 0.30 | 7.61 | 5.0 | Nuclear receptor subfamily 4, group A, member 2 | 204622_x_at | Hs.563344 | ||
| 302d | 372 | TAL1 | 1.67 | 0.94 | 7.46 | 4.5 | T-cell acute lymphocytic leukemia 1 | 1561651_s_at | Hs.658150 | ||
| 302d | 372 | PPP3R1 | 1.21 | 0.42 | 5.62 | 4.7 | Protein phosphatase 3 (2B), regulatory subunit B, α isoform | 204506_at | Hs.280604 | ||
| 302d | 372 | FNDC3A | 1.34 | 0.72 | 5.02 | 3.8 | Fibronectin type III domain containing 3A | 241611_s_at | Hs.508010 | ||
| 302d | 372 | ZFP91///Z | 0.65 | 0.25 | 4.79 | 7.3 | Zinc finger protein 91 homolog (mouse) | 224631_at | Hs.524920 | ||
| 302d | 372 | C11orf9 | 0.81 | 0.60 | 4.54 | 5.6 | Chromosome 11 open reading frame 9 | 217462_at | Hs.473109 | ||
| 302d | 372 | IQSEC2 | 0.99 | 0.44 | 4.38 | 4.4 | IQ motif and Sec7 domain 2 | 214819_at | Hs.496138 | ||
| 302d | 372 | RBL1 | 1.24 | 0.68 | 4.31 | 3.5 | Retinoblastoma-like 1 (p107) | 1555003_at | Hs.207745 | ||
| 302d | 372 | ZMYND11 | 0.82 | 0.66 | 3.76 | 4.6 | Zinc finger, MYND domain containing 11 | 1554159_a_at | Hs.292265 | ||
| 302d | 372 | ESR1 | 1.14 | 0.78 | 3.47 | 3.0 | Estrogen receptor 1 | 211627_x_at | Hs.208124 | ||
| 302d | 372 | LMO7 | 0.99 | 0.59 | 3.13 | 3.2 | LIM domain 7 | 1556377_s_at | Hs.207631 | ||
| 302d | 200c | ZFHX4 | 0.27 | 0.15 | 8.46 | 31.8 | Zinc finger homeodomain 4 | 241700_at | Hs.458973 | ||
| 302d | 367 | RUNX1 | 1.16 | 0.77 | 4.02 | 3.5 | Runt-related transcription factor 1 (acute myeloid leukemia 1) | 211180_x_at | Hs.149261 | ||
| 302d | UBE3A | 1.24 | 0.98 | 4.16 | 3.3 | Small nuclear ribonucleoprotein polypeptide N | 234163_at | Hs.654383 | |||
| 200c | 367 | KLF4 | 0.52 | 0.16 | 5.15 | 10.0 | Kruppel-like factor 4 (gut) | 220266_s_at | Hs.376206 | ||
| 200c | 367 | C6orf62 | 0.87 | 0.24 | 3.97 | 4.6 | Chromosome 6 open reading frame 62 | 213875_x_at | Hs.519930 | ||
| 200c | 367 | SYNJ1 | 1.14 | 0.84 | 3.83 | 3.3 | Synaptojanin 1 | 232993_at | Hs.473632 | ||
| 200c | MARCKS | 1.58 | 0.46 | 26.97 | 17.1 | Myristoylated alanine-rich protein kinase C substrate | 201668_x_at | Hs.519909 | |||
| 200c | DDEF1 | 1.51 | 0.38 | 12.34 | 8.2 | Development and differentiation enhancing factor 1 | 236533_at | Hs.655552 | |||
| 200c | MBNL1 | 0.67 | 0.30 | 10.55 | 15.7 | Muscleblind-like (Drosophila) | 1555594_a_at | Hs.478000 | |||
| 200c | ARHGDIA | 0.89 | 0.09 | 10.17 | 11.4 | Rho GDP dissociation inhibitor (GDI) alpha | 201167_x_at | Hs.159161 | |||
| 200c | QKI | 0.94 | 0.81 | 9.55 | 10.2 | Quaking homolog, KH domain RNA binding (mouse) | 214541_s_at | Hs.510324 | |||
| 200c | BAT3 | 1.07 | 0.99 | 7.32 | 6.9 | HLA-B associated transcript 3 | 230513_at | Hs.440900 | |||
| 200c | TSC22D2 | 0.99 | 1.00 | 6.51 | 6.6 | TSC22 domain family, member 2 | 210953_at | Hs.644065 | |||
| 200c | MAP2 | 0.57 | 0.51 | 5.58 | 9.7 | Microtubule-associated protein 2 | 210015_s_at | Hs.368281 | |||
| 200c | CNOT4 | 0.94 | 0.55 | 5.54 | 5.9 | CCR4-NOT transcription complex, subunit 4 | 210204_s_at | Hs.490224 | |||
| 200c | SOX1 | 1.44 | 1.03 | 4.33 | 3.0 | SRY (sex determining region Y)-box 1 | 230982_at | Hs.202526 | |||
| 200c | ETS1 | 0.81 | 0.72 | 4.09 | 5.0 | v-ets erythroblastosis virus E26 oncogene homolog 1 (avian) | 214447_at | Hs.369438 | |||
| 200c | TMEFF2 | 0.58 | 0.68 | 3.80 | 6.5 | Transmembrane protein with EGF and follistatin-like domains 2 | 223557_s_at | Hs.144513 | |||
| 200c | ANKRD15 | 0.92 | 0.73 | 3.55 | 3.9 | MRNA; cDNA DKFZp451J1819 (from clone DKFZp451J1819) | 1565832_at | Hs.306764 | |||
| 200c | RAP2C | 0.84 | 1.31 | 3.39 | 4.1 | RAP2C, member of RAS oncogene family | 218668_s_at | Hs.119889 | |||
| 200c | SNAP25 | 0.89 | 0.78 | 3.36 | 3.8 | Synaptosomal-associated protein, 25 kDa | 202507_s_at | Hs.167317 | |||
| 200c | DMD | 1.05 | 0.86 | 3.35 | 3.2 | Dystrophin (muscular dystrophy, Duchenne and Becker types) | 234752_x_at | Hs.495912 | |||
| 200c | FBXO33 | 0.68 | 0.72 | 3.33 | 4.9 | F-box protein 33 | 227521_at | Hs.324342 | |||
| 200c | VASH1 | 0.60 | 0.89 | 3.32 | 5.6 | Vasohibin 1 | 239810_at | Hs.525479 | |||
| 200c | SEMA6D | 0.61 | 0.52 | 3.27 | 5.3 | Sema transmembrane and cytoplasmic domain (semaphorin) 6D | 220574_at | Hs.511265 | |||
| 200c | GIT2 | 0.95 | 0.97 | 3.15 | 3.3 | CDNA clone IMAGE:5272062 | 1556912_at | Hs.434996 | |||
| 200c | GATA2 | 0.76 | 0.56 | 3.10 | 4.1 | GATA binding protein 2 | 207954_at | Hs.367725 | |||
| 367 | DAB2IP | 1.18 | 0.81 | 8.19 | 6.9 | DAB2 interacting protein | 228942_s_at | Hs.522378 | |||
| 367 | SMAD6 | 1.50 | 1.18 | 6.23 | 4.1 | SMAD family member 6 | 209886_s_at | Hs.153863 | |||
| 367 | CPEB4 | 0.56 | 0.25 | 4.76 | 8.6 | Cytoplasmic polyadenylation element binding protein 4 | 224828_at | Hs.127126 | |||
| 367 | WWP2 | 1.08 | 0.87 | 4.28 | 4.0 | WW domain containing E3 ubiquitin protein ligase 2 | 1554580_a_at | Hs.408458 | |||
| 367 | CACNA1C | 0.97 | 0.56 | 4.20 | 4.3 | Calcium channel, voltage-dependent, L type, alpha 1C subunit | 208020_s_at | Hs.118262 | |||
| 367 | TEF | 0.73 | 0.60 | 4.16 | 5.7 | Thyrotrophic embryonic factor | 210167_s_at | Hs.181159 | |||
| 367 | FMR1 | 0.98 | 1.11 | 4.05 | 4.1 | Fragile X mental retardation 1 | 203689_s_at | Hs.103183 | |||
| 367 | DNAJB12 | 0.87 | 0.29 | 4.02 | 4.6 | DnaJ (Hsp40) homolog, subfamily B, member 12 | 202865_at | Hs.696014 | |||
| 367 | ZNF287 | 1.05 | 0.77 | 3.81 | 3.6 | Zinc finger protein 287 | 216710_x_at | Hs.99724 | |||
| 367 | HAS3 | 1.15 | 0.90 | 3.79 | 3.3 | Hyaluronan synthase 3 isoform b | 228178_s_at | Hs.592069 | |||
| 367 | ATXN3 | 1.12 | 1.35 | 3.48 | 3.1 | Ataxin 3 | 217321_x_at | Hs.532632 |
Validation of TRPS1, KLF13, and MBNL2 Targets
The three genes TRPS1, KLF13, and MBNL2 were found to express extremely abundantly (more than 20-fold overall mean) in T3DF cells in which miR-302d, miR-372, and miR-200c had almost no expression (Tables I and II), whereas these three genes TRPS1, KLF13, and MBNL2 exhibited relatively low mRNA expression in both T3ES and T3EB cells in which miR-302d, miR-372, and miR-200c were expressed abundantly (more than 20-fold U6 snRNA). The inverse expression levels between these three miRNAs and their three common target mRNAs were confirmed by the highly negative correlation coefficients ranging from −0.65 to −0.75 (Supplementary online Table S3). Therefore, the TRPS1, KLF13, and MBNL2 genes are very likely to be the common targets of three abundantly expressed miRNAs miR-302d, miR-372, and miR-200c in hES cells. The potential binding structures of the 3′UTR of TRPS1, KLF13 and MBNL2 genes by miRNAs miR-302d and miR-372 were predicted using PicTar program, and those binding sites by miR-200c were predicted using TargetScan method (Supplementary online Fig. S3). The fragment of 3,187 bp amplified from the 3′UTR of TRPS1 gene contains three of four predicted sites for miR-302d and miR-372, as well as one predicted site for miR-200c. The fragment of 1,791 bp amplified from the 3′UTR of KLF13 gene includes one binding site for miR-302d and two predicted sites for miR-372. The fragment of 352 bp amplified from the 3′UTR of MBNL2 gene has only one binding site for miR-302d and miR-372. The base-pairings between miRNAs and their targets, as well as the constructions of luciferase reporter vectors, were shown in Figure 1A,B. To demonstrate directly whether TRPS1, KLF13, and MBNL2 were indeed the targets of miR-302d and miR-372, the luciferase reporter vectors harboring the amplified fragments of target genes were cotransfected with and without miRNA precursor of either miR-302d or miR-372 in HEK293T cells. miR-302d specifically suppressed the luciferase activity to 57%, 62%, and 80% of reporter vector harboring TRPS1, KLF13, and MBNL2, respectively (Fig. 1C). miR-372 inhibited the luciferase activity to 86%, 37%, and 47% for TRPS1, KLF13, and MBNL2, respectively. These results implied that miR-302d and miR-372 were able to regulate negatively the same targets due to their same seed sequence. However, miR-302d showed stronger inhibition to TRPS1 target than did miR-372, whereas miR-372 inhibited KLF13 and MBNL2 targets much more than did miR-302d. Furthermore, in order to confirm the predicted miRNA binding sites, the 4-bp mutations of miR-302d and miR-372 binding sites on KLF13 and MBNL2 genes were generated by site-directed mutagenesis. Because miR-302d and miR-372 could bind to the similar sequences of their targets, a 4-bp mutation was introduced in the distal corresponding site of KLF13 at either (nt 2,582–2,606) for miR-302d or (nt 2,570–2,606) for miR-372 binding site named KLF13 MUT2 and in the proximal corresponding site (nt 922–951) named KLF13 MUT1. Similarly, a 4-bp mutation was introduced in the 3′UTR of MBNL2 at either (nt 2,558–2,591) for miR-302d or (nt 2,566–2,590) for miR-372 (Supplementary online Fig. S4). The results of these reporter assays showed that miR-302d and miR-372 were indeed able to suppress specifically the luciferase activities of the reporter vectors harboring the predicted binding sites of wild-type 3′UTR of KLF13 and MBNL2, but not mutant-types (Fig. 1D).
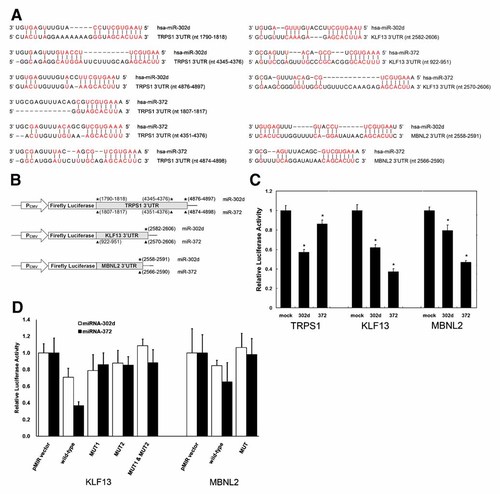
TRPS1, KLF13, and MBNL2 targets of miR-302d and miR-372. A: Predicted binding sites of miR-302d and miR-372 within the 3′UTRs of TRPS1, KLF13, and MBNL2. The base-pairings were indicated by short vertical lines between miRNAs and 3′UTRs of target genes. B: The construction of luciferase reporter vectors. The nucleotide positions of potential miRNA binding sites were denoted by stars and arrow heads for miR-302d and miR-372, respectively. C: The effects of miR-302d and miR-372 on the luciferase activity of TRPS1, KLF13 and MBNL2 reporter vectors. HEK293T cells were cotransfected with the reporter vector and miRNAs (as indicated), and luciferase activities were measured 48 h later. Shown are means with SE values. *P < 0.0001 when compared with mock control (Reporter vector cotransfected with negative control miRNA). D: The effects of miR-302d and miR-372 on the luciferase activity of reporter vectors containing wild-type and mutant 3′UTRs of KLF13 and MBNL2. HEK293T cells were cotransfected with the reporter vector and miRNAs (as indicated), and luciferase activities were measured 48 h later. Shown are means of triplicates with SE values. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Molecular Functions of hES Cell-Specific miRNA Targets
The molecular functions of 58 abundantly differentially expressed targets of miRNAs miR-302d, miR-372, miR-200c and/or miR-367 expressed abundantly in hES cells were analyzed using GeneSpring GeneOntology Browser and MetaCore Analytical Suite. The top four molecular functions of eight categories obtained by GeneSpring with P-value less than of 0.005 were found to be the same to the top four molecular functions of eight categories obtained by MetaCore (Table III). All of the top four molecular functions are involved in gene transcription. Among the other different categories between GeneSpring and MetaCore, zinc ion binding and sequence-specific DNA binding are also involved in gene transcription. In other words, the molecular functions of approximately half of these 58 targets are involved in gene transcription in hES cells.
| Molecular functions | GeneSpring | MetaCore | ||
|---|---|---|---|---|
| Genes | P-value | Genes | P-value | |
| Transcription factor activity | 18 | 1.03E−08 | 28 | 8.87E−16 |
| Transcription regulator activity | 20 | 2.81E−07 | 30 | 1.40E−13 |
| Nucleic acid binding | 29 | 2.78E−06 | 38 | 5.03E−10 |
| DNA binding | 22 | 1.80E−05 | 31 | 2.01E−9 |
| Zinc ion binding | 19 | 0.000595 | ||
| Calmodulin binding | 4 | 0.0039 | ||
| Ciliary neurotrophic factor receptor binding | 1 | 0.00495 | ||
| Calmodulin inhibitor activity | 1 | 0.00495 | ||
| Protein N-terminus binding | 6 | 2.65E−7 | ||
| Estrogen receptor activity | 3 | 2.67E−7 | ||
| Sequence-specific DNA binding | 12 | 1.91E−6 | ||
| Nitric-oxide synthase regulator activity | 3 | 5.53E−6 | ||
Expression Levels of Genes Involved in Signaling Pathways
There are 1,772, 2,925, and 1,622 gene probes expressed more than threefold of overall mean among T3ES, T3EB, and T3DF cells, respectively (Supplementary online Fig. S5). The numbers of gene probes involved in the KEGG pathways with random probability of less 5% are indicated in Supplementary online Table S4, and the expression levels of these gene probes involved in specific KEGG pathways are given in Supplementary online Table S5. The miRNAs (miR-302d, 367, 372, 200c, 20b, and 26b) expressed more than 20-fold U6 snRNA in T3ES and T3EB cells (Table I) were used to predict their target genes. The numbers of predicted target gene probes in the KEGG pathways with random probability of less 5% are indicated in Supplementary online Table S4, and the expression levels of the predicted target gene probes in specific KEGG pathways are given in Supplementary online Table S5. The expression levels of mRNAs and predicted target mRNAs in KEGG pathways from T3ES, T3EB, and T3DF cells are compared in Table IV, indicating that the expression levels of target mRNAs predicted by abundantly expressed miRNAs were found to be three-six folds lower than those of mRNAs involved in the same signaling pathways. T3ES, T3EB, and T3DF cells appeared to express different signaling pathways except that both T3ES and T3EB cells have Wnt signaling pathway and cell adhesion molecules (CAMs) in common. The Wnt signaling pathway and CAMs in both T3ES and T3EB cells were further analyzed to examine if the abundantly expressed miRNAs target different components of the same pathway in different cell types. In the Wnt signaling pathway, 28 gene probes were commonly targeted, whereas 27 and 12 gene probes were differentially targeted by miRNAs in T3ES and T3EB cells, respectively (Supplementary online Table S6). As to CAMs, 15 gene probes were commonly targeted, while 18 and 6 gene probes were differentially targeted by miRNAs in T3ES and T3EB cells, respectively. Furthermore, the expression levels of target mRNAs were found to be five- to ninefold lower than those of non-target mRNAs in Wnt signaling pathway and CAMs in T3ES and T3EB cells.
| Pathways | mRNA | miRNA | ||||
|---|---|---|---|---|---|---|
| mRNAs | No. of common genes | Random P-value | Target mRNAs | No. of common genes | Random P-value | |
| T3ES cells | ||||||
| Tight junction | 7.10 | 29 | 0.000183 | 1.11 | 60 | 9.86E−11 |
| Wnt signaling pathway | 6.99 | 31 | 0.000202 | 1.47 | 76 | 2.16E−16 |
| Adherens junction | 5.24 | 19 | 0.0232 | 1.17 | 61 | 7.55E−15 |
| Cell cycle | 5.10 | 17 | 0.0249 | 1.68 | 39 | 2.51E−06 |
| MAPK signaling pathway | 6.68 | 37 | 0.0279 | 1.57 | 133 | 3.70E−28 |
| Cell adhesion molecules (CAMs) | 9.19 | 20 | 0.0315 | 2.31 | 43 | 2.63E−05 |
| Regulation of actin cytoskeleton | 7.23 | 30 | 0.0467 | 1.37 | 133 | 1.71E−37 |
| Mean of 7 pathways | 6.79 | 1.53 | ||||
| Ratio of mRNAs/miRNA targets | (6.79/1.53) | 4.44 | ||||
| T3EB cells | ||||||
| TGF-beta signaling | 9.17 | 31 | 6.67E−06 | 3.54 | 40 | 4.43E−10 |
| Cell adhesion molecules (CAMs) | 8.48 | 36 | 0.00119 | 2.89 | 46 | 1.05E−06 |
| Axon guidance | 11.39 | 38 | 0.00171 | 4.04 | 99 | 8.02E−35 |
| Wnt signaling | 7.47 | 41 | 0.00181 | 1.92 | 61 | 6.13E−10 |
| ECM-receptor | 27.00 | 26 | 0.00567 | 9.60 | 48 | 3.72E−12 |
| Focal adhesion | 16.62 | 51 | 0.0153 | 5.61 | 93 | 2.50E−15 |
| Mean of 6 pathways | 13.35 | 4.60 | ||||
| Ratio of mRNAs/miRNA targets | (13.35/4.6) | 2.90 | ||||
| T3DF cells | ||||||
| Calcium signaling | 4.18 | 29 | 0.00327 | 1.04 | 44 | 0.0308 |
| Focal adhesion | 8.67 | 30 | 0.0283 | 0.94 | 111 | 6.20E-22 |
| Mean of 2 pathways | 6.42 | 0.99 | ||||
| Ratio of mRNAs/miRNA targets | (6.42/0.99) | 6.49 | ||||
DISCUSSION
Expression Profiles of mRNAs and miRNAs
The hES-T3 cell line [Li et al., 2006] was differentiated into uniformed spheres of T3EB embryoid bodies using a newly developed method of slow shaking (20 rpm) and fibroblast-like T3DF cells according to the published procedure [Stojkovic et al., 2005]. The established T3DF cells were further shown to be able to support the growth of undifferentiated hES-T3 cells for more than 14 passages. The mRNA expression profiles of the undifferentiated T3ES cells, T3EB embryoid bodies, and T3DF differentiated fibroblast-like cells were determined. The 15 genes, including transcription factors OCT4, SOX2, and NANOG, of the 60 most expressed mRNAs of T3ES cells were found to be in common with the 48 over-expressed genes in undifferentiated hES cells reported previously [Assou et al., 2007]. The expression of OCT4, SOX2, and NANOG was shown to be down-regulated in differentiated T3EB and T3DF cells. The presence of three germ-layer markers among the 60 most expressed genes of T3EB cells confirms the pluripotency of hES-T3 cell line as previously demonstrated by formation of teratoma in SCID mouse [Li et al., 2006]. The expression of galectin-1 mRNAs was the second highest abundant in T3ES and T3DF cells, but very low in T3EB cells. The T3ES and T3DF cells were further stained positively for galectin-1 protein. The galectin-1 protein has been reported to have many diverse biological functions [Camby et al., 2006], and its specific roles in T3ES and T3DF cells remains to be illustrated.
While this manuscript is in preparation, comprehensive profiles of miRNAs from undifferentiated and differentiated hES cells, as well as somatic stem cells, were reported [Laurent et al., 2008], and hES cells were found to possess a unique miRNA signature, with the upregulated miRNAs dominated by a single seed sequence, including members of the miR-302 cluster. Although it is difficult to compare exactly the miRNA profiles of undifferentiated and differentiated hES cells determined in this investigation using the ABI TagMan MicroRNA Assay with the recent report using Illumina miRNA microarray, the hES cell-specific miRNAs of miR-302d, miR-367, miR-372, and miR-200c were found to be abundantly expressed in undifferentiated cells and downregulated in differentiated cells by both investigations.
Target Identification of hES Cell-Specific miRNAs
With stringent criteria of both more than threefold expression of overall mean and more than threefold of changes, 58 targets were identified by the inverse expression levels (highly negative correlation) of miRNAs miR-302d, miR-372, miR-200c, and/or miR-367 to their predicted target mRNAs in T3ES, T3EB, and T3DF cells. These results are in agreement with the previous reports that highly expressed tissue (cell type)-specific miRNAs can reduce the endogenous steady-state levels of their target mRNAs by promoting destabilization through deadenylation in addition to translational repression in animals [Bagga et al., 2005; Farh et al., 2005; Lim et al., 2005; Stark et al., 2005; Sood et al., 2006; Baek et al., 2008]. The three abundantly (more than 20-fold of overall mean in T3DF cells) differentially (more than 10-fold of changes between T3DF and T3ES cells) TRPS1, KLF13, and MBNL2 genes were found to be common targets of three highly expressed hES cell-specific miRNAs miR-302d, miR-372, and miR-200c. The luciferase assay confirmed that both miR-302d and miR-372 directly interact with the target sites in the 3′UTR of TRPS1, KLF13, and MBNL2 genes, resulting in the mRNA destabilization and/or translational inhibition.
It may also be noted that the TRPS1, KLF13, and MBNL2 genes were found to contain no predicted sites for the abundantly expressed miR-367, as well as the low expressed miRNAs miR-302c*, miR-302a*, miR-302b*, miR-371, mir-373*, and miR-368 (Supplementary Table S7). However, the TRPS1 gene, but not KLF13 and MBNL2 genes, was found to contain potential binding sites for both miR-20b and miR-26b which were not reported to be hES cell-specific but exhibited similar pattern of expression with hES cell-specific miR-302d, miR-372, and miR-200c (Table II). Thus, the TRPS1 gene may also be a putative target of miR-20b and miR-26b. Recently, 253 target mRNAs of miR-290 cluster (corresponding to human miR-302 cluster) were found by the transcriptome analysis of mouse ES cells lacking Dicer of miRNA processing enzyme [Sinkkonen et al., 2008]. A comparison of these 253 targets with the 58 targets of human miR-302d, miR-367, miR-372, and/or miR-200c revealed four common targets TRPS1, MBNL2, MBNL1, and NF1B in which TRPS1 and MBNL2 were experimentally confirmed and MBNL1 and NF1B were among top 10 predicted targets in this investigation. Further, the Rbl1 gene, one of five members of cyclin E-CDK2 cell cycle regulatory pathway, directly targeted by mouse ES cell-specific miR-290 family [Wang et al., 2008] was also found to be targeted by the corresponding human ES cell-specific miR302d and miR-372 in this investigation.
The GeneOntology of the 58 targets by hES cell-specific miRNAs was analyzed. Forty eight (P-value of 6.09E−10) of these 58 targets are localized in nucleus, and 39 (P-value of 4.38E-10) of them are involved in regulation of gene expression processes. As to their molecular functions, approximately half of them were involved in gene transcription (Table III). In particular, among the three common target genes of miR-302d, miR-372, and miR-200c, TRPS1 gene encodes zinc finger transcription factor, and patients with tricho-rhino-phalangeal syndrome type 1 were reported to contain deletion and nonsense mutations in TRPS1 gene [Momeni et al., 2000]. The TRPS1 malformation syndrome is characterized by distinctive craniofacial and skeletal abnormalities. In mice, heterozygous deletion of TRPS1 gene displayed facial anomalies that overlapped with the phenotypes of human TRPS1 syndrome, while homozygous mutation showed a complete absence of vibrissae [Malik et al., 2002]. These findings implicated that the TRPS1 zinc finger transcription factor regulates bone development, and suggested that skeletal abnormalities in TRPS1 syndrome are only the extreme manifestation of a generalized bone dysplasia. Kruppel-like factor 13 (KLF13) gene encodes zinc finger transcription factor which binds to GC-rich sequences, and KLF13 protein is the dominant transactivator of RANTES gene induced at T-cell differentiation stage [Song et al., 1999]. Human MBNL2 gene encodes a protein related to Drosophila muscleblind protein 2, and this protein may play a role in the expansion of short nucleotide repeats resulting in myotonic dystrophy [Miller et al., 2000]. In short, all of these three genes appear to play important roles in cell differentiation and embryo development.
Differential Expression of Target and Non-Target mRNAs in the Same Signaling Pathway
The T3ES, T3EB, and T3DF cells appeared to have different major signaling pathways, although there are some common ones such as Wnt signaling pathway in both T3ES and T3EB cells. The highly expressed mRNAs and the target mRNAs predicted by abundantly expressed miRNAs appeared to be involved in different component steps of the same signaling pathways, and the expression levels of target mRNAs were found to be much lower than those of non-target mRNAs involve in the same signaling pathways. The lower expression levels of miRNA target mRNAs in major signaling pathways are in agreement with the lower expression levels of mRNAs targeted by highly expressed tissue (cell type)-specific miRNAs [Bagga et al., 2005; Farh et al., 2005; Lim et al., 2005; Stark et al., 2005; Sood et al., 2006; Baek et al., 2008]. The dual attenuation mechanisms (mRNA destabilization and translational inhibition) of highly expressed miRNAs can certainly increase their efficiencies and flexibilities to regulate the expression of many target genes, especially different components of major signaling pathways, in order to facilitate a more rapid transition to a new gene expression program during differentiation processes. The relative contribution to overall gene expression through mRNA destabilization and translational inhibition by highly expressed hES cell-specific miRNAs remains to be determined.
Acknowledgements
We thank Dr. Chien-Chih Chiu and Dr. Bai Hsun Chen as co-mentors to Z.Y. Tsai and B.Z. Chen, respectively. We also thank the technical assistance by the research assistants at Microarray Core Facility of National Research Program for Genomic Medicine of National Science Council in Taiwan.




