Expression and localization of estrogen receptor α in the C2C12 murine skeletal muscle cell line
Abstract
The classical model of 17β-estradiol action has been traditionally described to be mediated by the estrogen receptor (ER) localized exclusively in the nucleus. However, there is increasing functional evidence for extra nuclear localization of ER. We present biochemical, immunological and molecular data supporting mitochondrial-microsomal localization of ERα in the C2C12 skeletal muscle cell line. We first established [3H]17βestradiol binding characteristics in whole cells in culture. Specific and saturable [3H]17βestradiol binding sites of high affinity were then detected in mitochondrial fractions (Kd = 0.43 nM; Bmax = 572 fmol/mg protein). Immunocytological studies revealed that estrogen receptors mainly localize at the mitochondrial and perinuclear level. These results were also confirmed using fluorescent 17βestradiol-BSA conjugates. The immunoreactivity did not translocate into the nucleus by 17β-estradiol treatment. Western and Ligand blot approaches corroborated the non-classical localization. Expression and subcellular distribution of ERα proteins were confirmed in C2C12 cells transfected with ERα siRNA and by RT-PCR employing specific primers. The non-classical distribution of native pools of ERα in skeletal muscle cells suggests an alternative mode of ER localization/function. J. Cell. Biochem. 104: 1254–1273, 2008. © 2008 Wiley-Liss, Inc.
The analysis of steroid hormone receptors are important for understanding molecular details of transcriptional control, as well as providing insights as how they contribute to cell identity and function. A variety of methods have been described for determining the presence and distribution of estrogen receptors (ERs) at the cellular level.
The first clues in the identification of estrogen receptors were provided in the late 1950s [Jensen and Jacobson, 1960] and led to the two-step model of steroid hormone action that localized the unoccupied receptor in the cytoplasm [Jensen and Jacobson, 1962; Jensen and DeSombre, 1972]. The utilization of radiolabeled and unlabeled receptor ligands to detect and measure estrogen receptors has been complicated by the presence of other intracellular estrogen binding proteins [Mercer et al., 1981] and by the low concentration of receptors in responsive tissues.
When monoclonal antibodies against the classical estrogen receptor isoform (ERα) were used in immunocytochemical studies in estrogen target tissues and cell cultures, specific staining was confined exclusively to the nucleus [King and Greene, 1984]. Immunochemical and binding studies in cytoplast and nucleoplast fractions from receptor-containing GH3 cells also showed that free ER was associated with the nuclear fraction [Welshons et al., 1984].
In parallel to these observations, several investigators have pointed to the possibility that the ER could be non-classically associated to intracellular membranes [Parikh et al., 1980; Watson and Muldoon, 1985; Muldoon et al., 1988; Craig and Muldoon, 1991; Monje and Boland, 1999; Watson et al., 1999; Monje et al., 2001]. Data supporting this concept from cell-free preparations and characterization by the use of radioligands [Zheng and Ramirez, 1997; Monje and Boland, 1999], as well as cytochemical evidence [Pappas et al., 1995; Nadal et al., 1998; Razandi et al., 1999; Monje et al., 2001], have been accumulated. It has been often reported that 17β-estradiol may trigger a variety of non-genomic events which suggest the ability of the hormone to activate extra nuclear receptors. There is evidence of ER localization in the plasma membrane, although the integral nature of the association has not been proven [Luconi et al., 1999; Norfleet et al., 2000; Monje and Boland, 2001; Monje et al., 2001; Ropero et al., 2002; Li et al., 2003; Levin, 2005]. The presence of estrogen receptors in mitochondria has been shown for several types of ER expressing cell lines by means of immunofluorescence confocal microscopy and in different tissues by subcellular fractionation coupled to immunoblotting and binding analysis of isolated fractions [Zheng and Ramirez, 1999; Horvat et al., 2001; Monje and Boland, 2001, 2002; Cammarata et al., 2004; Chen et al., 2004; Yang et al., 2004; Levin, 2005; Solakidi et al., 2005; Stirone et al., 2005; Pedram et al., 2006]. These findings clearly show that a parallel reservoir of ER or ER-related proteins can also exist at the extra nuclear level.
In this report, we present biochemical and immunological evidence that the ERα or immunoreactive-related proteins are localized at the mitochondrial and microsomal level in the C2C12 murine skeletal muscle cell line. This information may be of functional significance as recent studies from our and other laboratories have demonstrated the participation of mitochondrial ERs in 17β-estradiol inhibition of apoptosis in C2C12 muscle cells [Vasconsuelo et al., 2007] as well as other cell types [Pedram et al., 2006].
MATERIALS AND METHODS
Materials
Estrogen receptor α mouse monoclonal antibodies clones TE111.5D11 (anti-ER ligand binding domain), AER314 (anti-ER transactivation domain) and AER308 (anti-ER hinge region) were purchased from NeoMarkers (Fremont, CA). The anti-lamin B goat polyclonal antibody (C-20) and the anti-ERα rabbit polyclonal antibody (MC-20) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Purified recombinant ERα protein was from PanVera Corporation (Madison, WI). Blocking peptides for N-terminal (aminoacids 10–28; Y-19) and C-terminal (aminoacids 439–458; L-20) antibodies against ERβ were from Santa Cruz Biotechnology, Inc. The chemiluminescence blot detection kit was obtained from Amersham, Buckinghamshire, England. Molecular weight colored markers for electrophoresis were supplied by BioRad (Hercules, CA). Propidium iodide and MitoTracker (MitoTracker® Red CMXRos) dyes were from Molecular Probes (Eugene, OR). [2,4,6,7-3H(N)] 17β-estradiol with a specific activity of 80-115 Ci/mmol was obtained from New England Nuclear (Chicago, IL). Ultrafree®-MC Microcentrifuge Filters (NMWL 30,000 Da, PLTK cellulosic membrane), anti-actin polyclonal antibody (A-506), cytochrome c Oxidase assay kit, secondary fluorescent fluorescein isothiocyanate-conjugated antibody (anti-mouse-FITC), 17α-estradiol, 17β-estradiol, 17β-estradiol derivative (non-conjugated 17β-estradiol-6-(O-carboxymethyl)oxime: bovine serum albumin), 17β-estradiol-BSA-FITC, 17β-estradiol-Peroxidase, tamoxifen and diethylstilbestrol (DES) were purchased from Sigma–Aldrich (St. Louis, MO). ICI182, 780 was obtained from TOCRIS (Ellisville, MO). Estrogen receptor α (ERα) ShortCut®siRNA Mix, fluorescein-siRNA transfection control and TransPass™ R2 Transfection Reagent were purchased from New England BioLabs, Inc., Beverly, MA. TRIzol® Reagent, PCR Reagent System, primers and S.N.A.P DNA purification kit were purchased from Invitrogen Corporation (Carlsbad, CA). Reverse Transcription System was from Promega Corporation (Madison, WI). Other chemicals used were of analytical grade.
Cell Line Culture
C2C12 murine skeletal muscle cells, kindly donated by Dr. Enrique Jaimovich (Universidad de Chile, Santiago, Chile), were routinely cultured in serum-supplemented media composed of Dulbecco's Modified Eagle's Medium (DMEM) without Phenol Red, 10% heat-inactivated (30 min, 56°C) fetal bovine serum (FBS), 1% nistatine and 2% streptomycin. Cultures were maintained at 37°C in a humid atmosphere of 5% CO2 in air and passaged every 2 days into fresh medium.
The MCF-7 (human breast cancer epithelial cell line) was obtained from the American Type Culture Collection (Rockville, MD). MCF-7 cells were routinely cultured in serum-supplemented media composed of DMEM without phenol red, 10% heat-inactivated FBS and 50 µg/ml gentamycin, at 37°C, in a humid atmosphere of 5% CO2 in air. The medium was replaced every 2 days and cells were passaged every 3–5 days.
Unless otherwise noted, cells were cultured in chamber-slides for microscopy (Nunc, Inc., IL).
Subcellular Fractionation
C2C12 cell confluent monolayers were scrapped and homogenized in ice-cold TES buffer (50 mM Tris/HCl pH 7.4, 1 mM EDTA, 250 mM sucrose, 1 mM dithiothreitol (DTT), 0.5 mM phenylmethylsulfonyl fluoride (PMSF), 20 µg/ml leupeptin, 20 µg/ml aprotinin, 20 µg/ml trypsin inhibitor) using a Teflon-glass hand homogenizer. A nuclear pellet was obtained by low speed centrifugation (800g, 20 min) of the lysed cell preparation. The supernatant was further centrifuged at 10,000g for 15 min to pellet mitochondria. The remaining supernatant was centrifuged at 120,000g for 90 min, to yield a soluble supernatant (cytosol) and a plasma membrane-containing particulate pellet (microsomes).
Aliquots of the different fractions were frozen in liquid nitrogen and stored in TES buffer at −70°C until measurement of estrogen-binding activity. For ERα immunodetection in isolated mitochondria, the enriched pellet was resuspended in TES buffer and assayed according to the protocol described below for immunocytochemistry assays in whole cells.
To isolate subcellular fractions from mature skeletal muscle, female mice were killed by cervical dislocation, after random selection without regard for the stage of estrous cycle. Skeletal muscle tissue was isolated from legs and placed in ice-cold saline. Connective and adipose tissue were removed. The muscle tissue was homogenized in TES buffer with an Ultraturrax homogenizer using 5 ml buffer/g tissue under ice. The homogenate was filtered through two layers of nylon mesh and subsequently centrifuged as described above for the isolation of subcellular fractions from C2C12 cells.
Protein concentration from each fraction was estimated by the method of Bradford 1976, using bovine serum albumin as standard.
Contamination of nuclear, microsomal and cytosolic fractions with mitochondrial components was assessed by measuring the activity of the mitochondrial marker enzyme cytochrome c oxydase employing the cytochrome c Oxidase assay kit (Sigma–Aldrich) according to manufacturer's instructions. Anti-lamin B antibody was employed for the immunodetection of the nuclear protein marker lamin B in the different fractions.
[3H]17β-estradiol Binding Assays
17β-estradiol binding site concentration was determined by means of [3H]17β-estradiol binding assays using whole-cell [Sadovsky and Riemer, 1992] and cell-free preparations [Monje and Boland, 1999].
For whole-cell binding assays, C2C12 cells were cultured until confluence in six-well plates in serum-supplemented media. Cells were washed with serum-free phenol red-free DMEM and preincubated in the same media for 1 h at 37°C. The media was replaced with 2 ml of DMEM containing 5 nM [3H]17β-estradiol. After 90 min of incubation at 37°C, the cells were exhaustively rinsed with serum-free phenol red-free DMEM to remove the unbound isotope. Trapped radioactivity was extracted with lysis buffer (50 mM Tris/HCl pH 7.4, 1 mM EDTA, 1% Triton X-100). Aliquots from each well were taken in triplicate for both scintillation counting and protein quantitation by the method of Bradford 1976. For determination of non-displaceable binding, a 200-fold molar excess of unlabeled 17β-estradiol was included in the incubation mixture. For displacement studies, an equivalent excess of cold competitors were also included together with the tritiated hormone.
For cell-free binding assays, the total specific estrogen binding capacity (empty plus occupied ER site content) of the subcellular fractions was determined by incubating 0.3 mg protein samples in 0.2 ml of TES buffer. The reactions were begun by the addition of 5 nM [3H]17β-estradiol. A 200-fold molar excess of radioinert 17β-estradiol was used for determination of non-displaceable binding. Specific binding sites were then quantified by subtracting non-specific binding sites from sites bound in presence of [3H]17β-estradiol alone (total binding). After 4 h of incubation in an ice bath with vortex stirring at 30 min intervals, free [3H]17β-estradiol was separated by resin adsorption of the ligand-receptor complex using the hydroxylapatite (HAP) technique [Wecksler and Norman, 1979]. Briefly, 200 µl of HAP slurry were added to each tube and the suspension was incubated for 15 min at 4°C. Then, the mixture was centrifuged 3 min at 800g, and the pellets were washed three times with TE buffer (50 mM Tris/HCl pH 7.4, 1 mM EDTA, 2 mM DTT, 0.3 mM PMSF) containing 0.5% Triton X-100, adjusted to reduce non-specific binding to basal constant levels. HAP pellets were resuspended with 1 ml absolute ethanol and decanted into scintillation vials. Trapped radioactivity was quantified in toluene-based fluid by liquid scintillation spectrometry.
For saturation analysis, samples of the mitochondrial fraction were exposed to a series of [3H]17β-estradiol concentrations ranging from 0.02 to 10 nM. The steroid affinity constant (Kd) and the maximum number of binding sites (Bmax) were estimated according to the Scatchard equation using the nonlinear curve fitting LIGAND program [Munson and Rodbard, 1980].
In competition experiments, where 17β-estradiol-6-(O-carboxymethyl)oxime:bovine serum albumin (E2-BSA) was employed, a stock solution of the estradiol derivative was made by dissolving the powder in buffer A (50 mM Tris–HCl, pH 8.5) at 0.2–1 mg/ml (load). An aliquot (300 µl) of the solution was applied to an ultrafree®-MC microcentrifuge filter (Sigma) and subjected to centrifugation at 13,000g for 30 min to separate 17β-estradiol (E2)-BSA from free 17β-estradiol. The filtrate was recovered and the retentate was washed three times with 300 µl of buffer A, recovered in the same volume as the load sample and analyzed with the load sample and the filtrate [Stevis et al., 1999].
Blocking experiments using different monoclonal antibodies against ERα involved incubation of the cells (1 h, 37°C) or mitochondrial and microsomal fractions (overnight at 4°C) in presence of a 1:100 dilution of the antibody in DMEM or TES buffer, respectively, prior addition of the radioactive ligand. In order to allow blocking of intracellular estrogen binding sites in whole cell experiments, membrane permeabilization with saponin (50 µg/ml), 1 min at 37°C, was performed prior incubation with the antibodies.
In all experiments final isopropanol concentration did not exceed 0.01%, appropriate vehicle controls were used and each condition was assayed in triplicate.
Western Blots
Each subcellular fraction was analyzed for its immunoreactive ERα content. Protein aliquots were combined with sample buffer (400 mM Tris/HCl (pH 6.8), 10% SDS, 50% glycerol, 500 mM DTT and 2 µg/ml bromophenol blue), boiled for 5 min and resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE). Fractionated proteins were then electrophoretically transferred to polyvinylidene fluoride (PVDF) membranes (Immobilon-P, Millipore), using a semi-dry system. Non-specific sites were blocked with 5% non-fat dry milk in PBS containing 0.1% Tween-20 (PBS-T). Blots were incubated for 1 h with the appropriate dilution of each primary antibody. The membranes were repeatedly washed with PBS-T prior to incubation with horseradish peroxidase-conjugated secondary antibodies. The enhanced chemiluminescence blot detection kit was used as described by the manufacturer to visualize reactive products. Relative migration of unknown proteins was determined by comparison with high range molecular weight colored markers.
To verify antibody specificity by antigen (peptide/protein) blocking, an appropriate dilution of primary antibody (1:400) was incubated with or without (control) 50-fold of blocking peptide or recombinant protein at 37°C for 2 h. After incubation, centrifugation at 14,000g for 15 min was performed to pellet any immune complexes. After centrifugation, the supernatant was carefully removed leaving 10 µl at the bottom (immune complex). The complexes were resuspended in PBS-T 1× for Western blot assays.
Ligand Blots
Subcellular fractions were subjected to 10% SDS–PAGE and blotted to PVDF as described above. The membranes were exhaustively washed (at least 24 h at room temperature) with PBS-T followed by PBS, to remove SDS and allow transferred proteins to renaturalize in the membranes. After 1 h blocking with 5% BSA in PBS, the membranes were incubated for 1 h at room temperature with 10−6 M E2-BSA-FITC or E2-Peroxidase conjugates. Visualization of reactive protein bands was performed under a UV light transilluminator (Photodyne). When E2-Peroxidase conjugate was employed reactive bands were detected by enhanced chemiluminescence.
Immunocytochemistry and Microscopy
Semi-confluent (60–70%) monolayers were washed with serum-free phenol red-free DMEM; incubated 1 h in the same media and then fixed 15 min at 37°C with freshly prepared 3.7% paraformaldehyde in serum-free phenol red-free DMEM. Fixed cells were subsequently incubated in PBS containing 0.2% Triton X-100 at room temperature for 5 min to permeabilize cells and allow intracellular antigen labeling. After fixation, cells were rinsed three times with PBS. Non-specific sites were blocked for 30 min in PBS that contained 5% bovine serum albumin. Cells were then incubated for 60 min in the presence or absence (negative control) of primary antibody (1:50). The specificity of the antibodies was previously checked by Western blot analysis of uterine cytosolic ERα-enriched preparations [Monje and Boland, 1999, 2001], immunocytochemistry of MCF-7 and SHM cells and immunoblots of subcellular fractions derived therefrom [Monje et al., 2001; Monje and Boland, 2002], specific antigen blocking (see Western Blot—Materials and Methods Section), and by the fact that the antibodies did not react with COS-1 cells lacking ER [Monje and Boland, 2002]. After a PBS wash, cells were incubated for another 60 min with a fluorescein isothiocyanate (FITC) conjugated anti-mouse secondary antibody. After another 3 × PBS washes, final 10 min incubation with 1 µg/ml propidium iodide (PI) was performed to label nuclei as a cellular reference. The antibodies were prepared in PBS containing 2% BSA and all steps were done at room temperature.
In parallel experiments cells were incubated in the presence of 1 or 10 nM of 17β-estradiol during 15 min, 30 min or 1 h at 37°C prior fixation and immunostaining.
MitoTracker® Red CMXRos was employed for mitochondria-selective stain into active mitochondria, according to manufacturer's instructions.
Slides were mounted and images were acquired on a NIKON conventional microscope (NIKON Eclipse E 600) equipped with standard filter sets to capture fluorescent signals.
Images were collected using a digital camera (NIKON COOLPIX) and exported to Adobe PhotoShop for digital processing.
Confocal Microscopy
Images were acquired on a Leica TCS SP2 AOBS confocal laser-scanning microscope in an epifluorescence mode. The 488 nm line of an argon ion laser and the 543 nm line of a helium-neon laser were used to excite the samples. A DD 488/543 filter was used to separate red/green fluorescence signals. A 50 µm pinhole was generally used. Cells were imaged through a 63X, 1.3 numerical aperture water immersion objective. Images were collected and saved using the software Meridian, and exported to Adobe PhotoShop for digital processing.
Labeling With E2-BSA:FITC
C2C12 cells were cultured as for immunocytochemistry. Semi-confluent monolayers were washed with serum-free phenol red-free DMEM and incubated for 1 h at 37°C with serum free-DMEM devoid of phenol red. Cells were then incubated at 37°C for 30 min in the presence of 10−6 M FITC-conjugated E2-BSA (E2-BSA:FITC). Permeabilization of the cells with saponin (50 µg/ml) 1 min at 37°C was performed, prior incubation with the FITC conjugates, in order to allow staining of intracellular E2 binding sites. Blocking experiments using TE111.5D11 monoclonal antibody, against the ligand binding domain of the ERα involved the incubation of the cells in presence of a 1:100 dilution of the antibody in DMEM media (1 h, 37°C) prior incubation with E2-BSA-FITC conjugate. The conjugate BSA-FITC was used as a control for non-specific binding.
E2-BSA:FITC was previously used for membrane ER staining in various cell types [Berthois et al., 1986; Benten et al., 1998; Razandi et al., 1999; Monje et al., 2001]. The conjugate was solubilized in 50 mM Tris–HCl pH 8.3 and diluted to final concentration in serum-free culture media. In all the experiments, the concentration of derivative referred to the concentration of the steroid in the final volume. E2-BSA concentration was calculated from the number of E2 molecules attached to each BSA molecule (steroid/BSA molar ratio ∼32:1).
After labeling, the monolayers were extensively washed with serum-free culture media and then fixed 15 min at 37°C with freshly prepared 3.7% paraformaldehyde. MitoTracker® Red CMXRos was employed for mitochondria-selective stain. The slides were mounted and samples were viewed on a NIKON conventional microscope (NIKON Eclipse E 600) using a standard FITC filter set.
Images were collected using a digital camera (NIKON COOLPIX) and exported to Adobe PhotoShop for digital processing.
Transient Transfections With Estrogen Receptor α siRNA
Cells were plated at an appropriate density to reach 40–60% confluence the day of transfection. For transfection experiments, TransPass™ R2 Transfection Reagent was mixed with ERα siRNA. The mix was incubated for 20 min at room temperature and diluted with complete culture medium. The culture medium of the cells was aspirated and replaced with the diluted transfection complex mixture.
To estimate the transfection efficiency of siRNA, 20 pmol of fluorescein-siRNA was used according to manufacturer's instructions. Cells were then visualized, 24 h post-transfection, in a conventional microscope employing an adequate filter for green fluorescence.
To evaluate the effective silencing of ERα, total proteins from transfected and non-transfected cells (controls) were extracted 24 and 48 h post-transfection and ERα expression was tested by Western blot using the AER 314 specific monoclonal antibody. For actin loading control, membranes were stripped with stripping buffer (62.5 mM Tris–HCl pH 6.7; 2% SDS; 50 mM β-mercaptoethanol) and then blocked for 1 h with 5% non-fat dry milk in PBS containing 0.1% Tween-20 (PBS-T). The blots were then incubated 1 h with a 1:10,000 dilution of anti-actin policlonal antibody (A-5060) as primary antibody. After several washings with PBS-T, membranes were incubated with anti-rabbit conjugated to horseradish peroxidase as secondary antibody (1:10,000). Immunoreactive proteins were developed by means of enhanced chemiluminescence (ECL).
For immunocytochemistry and competitive binding assays in transfected C2C12 cells, they were incubated 24 h with 20 pmol of ERα siRNA. In competitive binding assays, 5 nM [3H]17β-estradiol was incorporated into the medium to determine the total binding. For determination of non-displaceable binding, a 200-fold molar excess of unlabeled 17β-estradiol, 17α-estradiol, DES, tamoxifen or ICI182, 720 was included in the incubation mixture. For immunocytochemistry, AER 314 specific monoclonal antibody was employed.
The MCF-7 cell line was used as a positive control for ERα silencing with the specific siRNA mix, employing the same conditions as described above for immunocytochemistry assays in transiently transfected C2C12 cells.
Reverse Transcription-Polymerase Chain Reaction
Semi-confluent cells in 75 cm2 culture flasks were rinsed once with 1 × PBS (pH 7 × 4) and then harvested by scrapping using TRIzol Reagent, for total RNA isolation, according to manufacturer's instructions. The RNA pellet was air-dried for 5 min and subsequently dissolved in 50 µl of deionized water. The concentration and purity of the RNA preparation were determined by measuring the absorbance of RNA at wavelengths of 260 and 280 nm. The RNA integrity was analyzed on a 1% native agarose gel in 1× TBE buffer. RNA was stored at −70°C for subsequent experiments.
cDNA was prepared with AMV reverse transcriptase using random primer hexamers (Promega). The reaction was performed in a total volume of 20 µl containing 5 µg of total RNA, according to manufacturer's instructions. The reaction mixture was incubated at 42°C for 1 h.
For the PCR reaction, the 20 µl sample from the reverse transcription reaction was amplified in a final volume of 100 µl containing 0.5 µM of each primer (forward and reverse). Amplification was performed on an Eppendorf Mastercycler®personal 5332 model for 35 cycles with denaturation at 94°C (30 s), annealing at 58°C (30 s) and extension at 72°C (30 s).
PCR primers were specifically designed for mouse ERα employing the Mouse BLASTN program of the Gen Bank Sequence database (Table I). Specificity of primer annealing was analyzed with the same program. No significant match was obtained with the mouse ERβ gene sequence. Total RNA extracted from the MCF-7 cell line was used as a positive control. One sample without RNA was included as a negative control in each experiment. PCR products were purified and sequenced at the University of Chicago, Cancer Research Center, DNA sequencing facility. Results were analyzed with the Mouse BLAST program, Gen Bank Sequence database.
| Transcript (Gene bank accession number) | Primers | Location | PCR product (bp) |
|---|---|---|---|
| NM_007956 | PF1 5′ GGAACGAGCTGGAGCCCCTC 3′ | 241–260 | 380 |
| PR1 5′ CGCGCACGGCGTAGGCGCTGG 3′ | 621–601 | ||
| PF2 5′ CCTTCATGAGGGCTGTAGGG 3′ | 2,701–2,720 | 499 | |
| PR2 5′ CCTGGAGCATCTACAGGAAC 3′ | 3,200–3,181 |
RESULTS
As a first step to identify and characterize 17β-estradiol binding sites in the C2C12 skeletal muscle cell line, we performed competitive binding assays in whole cells in culture. As shown in Figure 1A, reproducible specific binding activity could be measured employing 17β-estradiol as a competitor. The recovered specific binding in C2C12 cells was similar to that previously obtained in MCF-7 cells [Monje et al., 2001]. In order to discriminate intracellular and membrane estrogen binding sites we employed E2-BSA and E2-Peroxidase as competitors, which are membrane impermeable forms of 17β-estradiol (E2). The results presented here show that E2-BSA retained in the column used to separate 17β-estradiol (E2)-BSA from free 17β-estradiol was not able to fully compete with tritiated E2 in whole cell competition assays. E2-BSA loaded produced a partial but not significant competition, which was probably associated to the presence of free E2. Also the macromolecular conjugate E2-Peroxidase was employed as a competitor for surface-located binding sites. Results (Fig. 1A) were similar to those obtained with E2-BSA loaded, and again attributed to the presence of free E2 in the sample. Displacement studies using the stereoisomer 17α-estradiol, the non-steroidal synthetic estrogenic ligand DES, the antiestrogenic ICI and the agonist/antagonist tamoxifen corroborated the expression of ERα-like estrogen binding sites. Also, we could not detect differences in affinity binding between the different compounds employed. Blocking experiments were performed employing specific monoclonal antibodies against different domains of the ERα (Fig. 1B). The different antibodies could partially inhibit [3H]17β-estradiol binding to intracellular sites. The antibodies could not displace tritiated estradiol binding when cells were not subjected to permeabilization prior incubation with the different antibodies. Assuming that the antibodies are not able to permeate the cellular membrane of living cells, this excludes the presence of antigens in the extracellular surface.
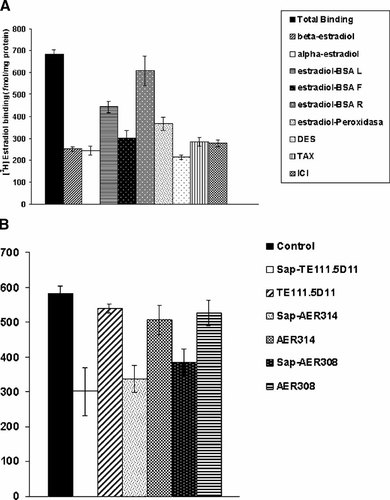
[3H]17β-estradiol binding characteristics of whole C2C12 skeletal muscle cells in culture. A: C2C12 muscle cells were incubated with 5 nM [3H]17β-estradiol and competition assays with 17β-estradiol, 17α-estradiol, unfractionated E2-CMO:BSA (E2-BSA L: load), fractionated E2-CMO:BSA (E2-BSA R: retentate and E2-BSA F: filtrate), estradiol-peroxidase, DES, tamoxifen and ICI182, 720 were performed as described in Materials and Methods Section. B: Blocking experiments with specific monoclonal antibodies against ERα. The different antibodies (AER 314, AER 308, and TE111.5D11) were included in the culture medium of the C2C12 cells prior addition of the radioactive ligand (Materials and Methods Section). Results are expressed in fmol/mg protein and represent the mean of samples analyzed in triplicate ±SD.
To investigate where these intracellular estrogen binding sites were located, we performed competitive binding assays in C2C12 subcellular fractions. As shown in Figure 2A, the ERα-like estrogen binding sites were predominantly detected in mitochondrial and microsomal fractions. Also, low but specific binding sites were present in nucleus and cytosol. The non-classical estrogen binding sites detected in mitochondrial and microsomes were structurally similar to ERα, as an antibody against the ligand binding domain (TE111.5D11) partially blocked the binding of [3H]17β-estradiol (Fig. 2B). This antibody was previously used to block [3H]17β-estradiol binding to the cytosolic ERα receptor [Monje and Boland, 1999]. The magnitude of the displacement by the antibody (∼40% of total binding) was similar to that obtained in other cell lines [Monje et al., 2001]. No blocking effect was observed with a non-specific antibody (data not shown).
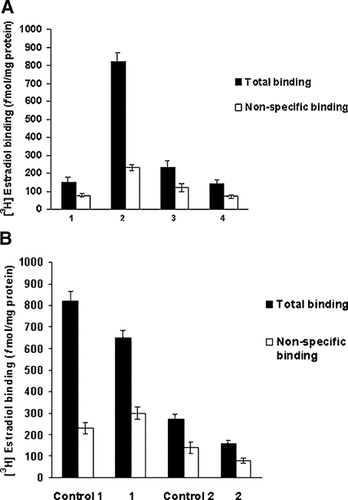
[3H]17β-estradiol binding capacity of subcellular fractions from C2C12 muscle cells. A: Quantification of estrogen binding site concentration by saturation assays. Equal protein samples of nuclear (1), mitochondrial (2), microsomal (3), and cytosolic (4) subfractions were incubated during 4 h in presence of 5 nM [3H]17β-estradiol alone or in combination with 200-fold molar excess of 17β-estradiol. B: Blocking of [3H]17β-estradiol binding in mitochondria and microsomal fractions by a monoclonal antibody (TE111.5D11). Equivalent protein concentrations of mitochondria and microsomal fractions were incubated overnight at 4°C in the absence (control 1 and control 2) and presence of antibody (1 and 2). After treatment, [3H]17β-estradiol binding capacity was measured as is described in Materials and Methods Section. Results are expressed in fmol/mg protein and represent the mean of samples analyzed in triplicate ±SD. 1, mitochondrial fraction; 2, microsomal fraction.
In an attempt to characterize C2C12 cell mitochondria estrogen binding sites, the association of [3H]17β-estradiol to isolated mitochondrial preparations was analyzed in equilibrium binding experiments. As shown in Figure 3-upper panel, the specific binding of the hormone was a saturable process with respect to the ligand concentration. Saturation data analyzed by linear Scatchard transformation revealed a single high affinity binding component for 17β-estradiol (Kd = 0.43 nM) with a maximum binding capacity of ∼572 fmol/mg protein (Fig. 3, lower panel).
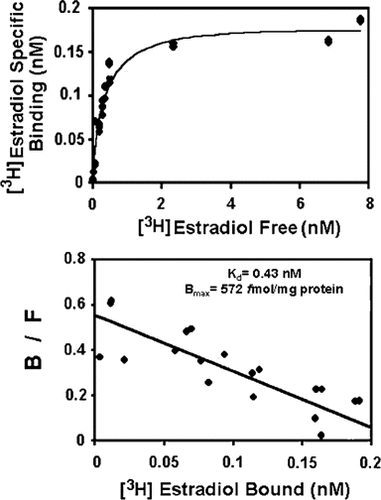
Saturation analysis of [3H]17β-estradiol binding to mitochondrial sites from C2C12 cells. Upper panel: Protein fractions were incubated with increasing concentrations of [3H]17β-estradiol with or without a 100-fold molar excess of unlabeled 17β-estradiol. Incubations were performed for 4 h under ice until separation of unbound steroid by the hydroxylapatite procedure. Specific radioligand binding (B) was plotted against the concentration of free tritiated hormone in the incubation medium (F). Lower panel: Graphical analysis of the same data by the method of Scatchard. Estimated binding parameters at equilibrium are indicated.
Western blot analysis employing the nuclear marker Lamin B revealed no nuclear contamination in the other subcellular fractions (data not shown). We also excluded mitochondrial contamination in nuclear, microsomal and cytosolic fractions by measuring cytochrome c oxidase activity. Values obtained for this specific mitochondria marker enzyme in the nuclear fraction were negligible (data not shown). The biochemical methods used to establish the degree of purity of the subcellular fractions have been previously validated by transmission electron microscopy in our laboratory [Drittanti L., Ph.D. thesis, Universidad Nacional del Sur, 1988].
To correlate estradiol binding capacity with ERα-like immunological constituents, subcellular fractions from the C2C12 cell line were resolved by SDS–PAGE followed by Western blot assays employing the TE111.5D11 antibody (Fig. 4A). As expected, high expression levels of the ∼67kDa band for the classical ERα were detected in the total homogenate and cytosolic fraction. However, considerable immunoreactivity was detected in mitochondria and microsomal fractions. Two other different monoclonal antibodies against the ERα (AER 314 and AER 308) rendered the same immunoreactive pattern (data not shown). We propose that some percentage of immunoreactive proteins recovered in nuclear and cytosolic fractions of the total C2C12 cell homogenate could represent protein that is loosely associated to mitochondria and microsomes and causes the receptor to partition into the other subfractions when cells are disrupted [King and Greene, 1984].
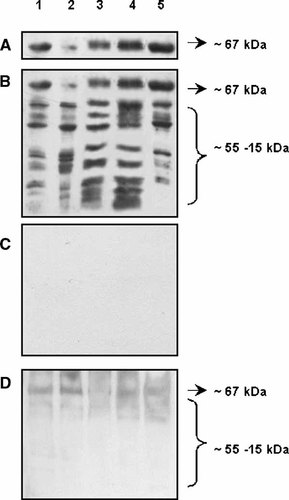
ERα immunochemical reactivity of subcellular fractions from C2C12 cell and mouse skeletal muscle tissue. Samples from C2C12 subcellular fractions containing equivalent protein amounts (20 µg) were fractionated by SDS–PAGE and transferred to PVDF membranes as described in Materials and Methods Section. Probing was done with anti-ERα monoclonal antibody TE111.5D11. A: Image of blot was focused to show the ∼67 kDa protein band expected for this isoform. B: Detection of low molecular weight immunoreactive proteins. A similar pattern of immunodetection was obtained using other anti-ERα monoclonal antibodies (clones AER308 and AER 314). C: Negative control for anti ERα labeling. Subcellular fractions were incubated with anti-mouse peroxidase-conjugated secondary antibody alone. D: Expression and subcellular distribution of ERα in mouse skeletal muscle tissue. Subcellular fractions were isolated as described in Materials and Methods Section. After SDS–PAGE and transference to PVDF membranes, proteins were incubated with anti-ERα policlonal antibody (MC-20). Lane 1, total homogenate; Lane 2, nuclei; Lane 3, mitochondria; Lane 4, microsomes; Lane 5, cytosol.
Figure 4B shows the complete Western blot profile, where additional low molecular weight bands were predominantly detected in mitochondria and microsomes. These results are in agreement with previous studies [Monje and Boland, 1999] and could be consequence of alternative usage of different in-frame initiation codons [Griffin et al., 1999; Osterlund et al., 2000] or splice variants [Ferro et al., 2003; Herynk and Fukua, 2004]. Figure 4C corresponds to a negative control and shows that secondary antibodies did not recognize any proteins ranging within the expected molecular size. The specificity of the immunoreactivity was further evidenced by immuno-neutralization with the recombinant ERα protein and no immuno-neutralization with specific blocking peptides against the ERβ (data not shown).
To investigate whether murine skeletal muscle tissue also expresses ERα immunoreactive proteins with non-classical localization, Western blot assays of subcellular fractions derived therefrom were performed employing MC-20 rabbit polyclonal antibody. ERα was found to be expressed and localized in the microsomal, nuclear and cytosolic fractions (Fig. 4D). ERα was not detected in mitochondria of skeletal muscle tissue differently to C2C12 cells. Although this difference remains unexplained in the present work, it may reflect distinct characteristics of muscle development in vivo and in vitro. This dissimilar subcellular localization of the ER is observed between other cell lines and tissues, for example, MCF-7 breast cancer cells [Chen et al., 2004] and tumorigenic mamary gland.
Figure 5 shows the complete subcellular localization profile for reactive estrogen binding proteins in the C2C12 cell line using E2-Peroxidase (left) and the complete ERα immunoreactive pattern (right). It is clear the labeling of a ∼67 kDa protein band which colocalizes with the immunological detection of ERα. Also, the intensity of the estrogen binding entities correlated with the subcellular expression of the ∼67 kDa band detected with the monoclonal antibody TE111.5D11. Moreover, Ligand blot assays showed a significant proportion of estradiol binding proteins of low molecular weight that could contribute to estrogen binding. Similar results were obtained with E2-BSA-FITC derivative (data not shown). It is likely that the estrogen binding band of ∼45 kDa detected by Ligand blot (Fig. 5, left) may correspond to the ERβ isoform (Milanesi L., Vasconsuelo A., Russo de Boland A. and Boland R., unpublished work).
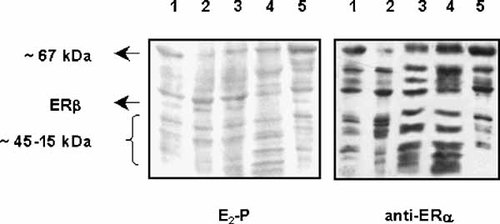
C2C12 muscle subcellular distribution of 17β-estradiol binding proteins by Ligand blot assays. Proteins from C2C12 subcellular fractions were subjected to SDS–PAGE, transferred to PVDF membranes and renatured as described in Materials and Methods Section. Left panel: Estrogen binding proteins labeled with estradiol-peroxidase conjugate (E2-P). Right panel: Western blot with anti-ERα antibody TE111.5D11. Lane 1, total homogenate; Lane 2, nuclei; Lane 3, mitochondria; Lane 4, microsomes; Lane 5, cytosol.
Immunocytochemical detection of ERα isoforms was performed by indirect immunofluorescence. Three different monoclonal antibodies were employed to label paraformaldehyde fixed C2C12 cells. By conventional microscopy we detected two main sources of ERα immunoreactivity: one cytosolic and the other perinuclear.
The ERα cytoplasmic staining showed a punctuate distribution (Fig. 6A). Similar results were obtained with the other two antibodies employed (AER 314 and AER 308; Fig. 6E and F, respectively). To determine whether this cytoplasmic distribution correspond to mitochondrial localization, MitoTracker (MitoTracker Red CMXRos) was employed. As shown in Figure 6B, the perinuclear localization of the green signal, that does not colocalize with the mitochondrial marker MitoTracker, may correspond to ERα association to an intracellular membrane system, possibly the endoplasmic reticulum and Golgi.
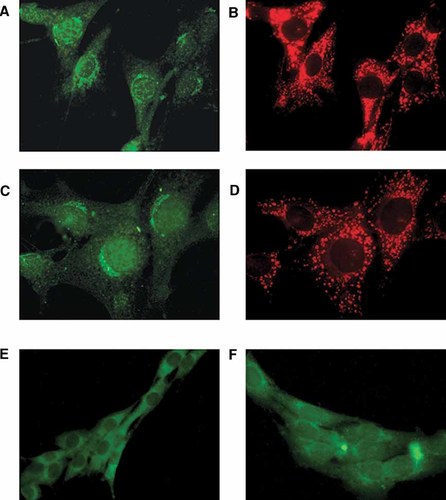
Immunofluorescence conventional microscopy of C2C12 muscle cells stained for ERα antigen. ERα was detected in paraformaldehyde fixed and permeabilized C2C12 cells using clones TE111.5D11, AER 314 and AER 308 as primary antibodies and FITC-conjugated anti-mouse secondary antibody according to Materials and Methods Section. A,C: Mitochondria, perimembrane and nuclear pools of immunoreactive ERα. B,D: Mitochondria localization with the mitochondrial marker MitoTracker. Part (C) is shown to emphasize peripheral staining not detected with MitoTracker (D). Original magnification = 600×. E,F: ERα immunolocalization with AER 314 and AER 308 primary antibodies, respectively. Original magnification: 400×. Negative controls using secondary fluorescent antibodies rendered a negligible cellular background (data not shown). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
By confocal microscopy, immunostaining of the C2C12 cell line with the monoclonal antibodies against the classical ER isoforms exhibited a perinuclear fluorescence and a granular cytoplasmic distribution. The cytoplasmic staining coincided with MitoTracker red (Fig. 7A). When the TE111.5D11 primary antibody was employed, some cells revealed a punctuate nuclear staining (Fig. 7A, merged image at the corner). Figure 7B supports the immunolocalization of the immunoreactive proteins at the mitochondrial level.
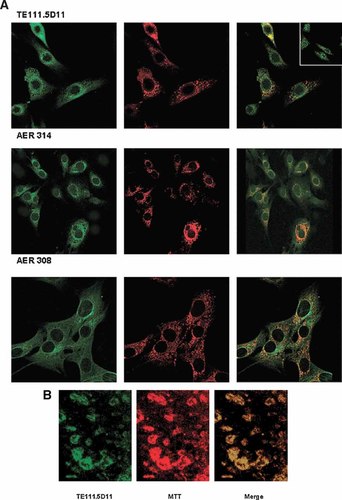
Immunofluorescence confocal microscopy of C2C12 muscle cells and mitochondria after staining for ERα antigen. A: Whole cells. C2C12 cells were labeled using TE111.5D11, AER 314, and AER 308 antibodies as in Figure 6. ERα specific green fluorescence was bright in mitochondria and perinuclear compartment of the cells (left). Mitochondria localization in C2C12 cells with the marker MitoTracker rendered a red signal (center) and the merged image of ERα immunostaining and the mitochondrial marker gave an orange/yellow signal (right). The merged image at the corner show the presence of punctuate nuclear fluorescence in some cells when the TE111.5D11 antibody was employed. A negligible cellular background was observed in negative controls using secondary fluorescent antibodies alone (data not shown). B: Isolated mitochondria. Mitochondrial fractions were labeled with TE111.5D11 antibody as indicated above for whole cells. ERα specific green fluorescence was detected (left). MitoTracker rendered a red signal (center) and the merged image of ERα immunostaining and the mitochondrial marker rendered an orange/yellow signal (right). The same immunoreactive pattern was obtained with AER 314 and AER 308 antibodies (data not shown). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
No ER signal was seen in the cells in which the primary antibody was omitted (data not shown).
No translocation into the nucleus of cytoplasmic and perinuclear green signals were detected when C2C12 cells were subjected to 1 h of 17β-estradiol treatment prior fixation and immunoassaying (Fig. 8B,C). The same results were observed for 15 and 30 min of 17β-estradiol treatment. The green signal, after hormone exposure, showed less fluorescence, respect to the controls, when cells were treated during 1 h with the hormone; probably associated to ligand-dependent switching of the ubiquitin-proteasome pathway for estrogen receptor degradation [Tateishi et al., 2004].
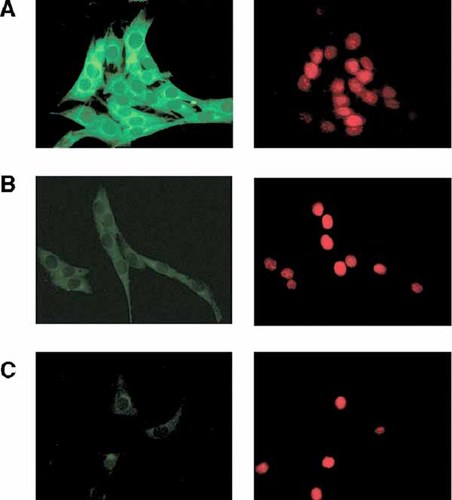
ERα localization after 17β-estradiol treatment in C2C12 muscle cells. Serum was removed after exhaustive washing of the cells and monolayers were treated with 17β-estradiol in DMEM and then fixed for ERα immunodetection as described in Materials and Methods Section. Pictures were taken with the specified scanning parameters. Panels on the right depict nuclear staining with propidium iodide (PI). Left panels represent green ERα fluorescence signal employing TE111.5D11 monoclonal antibody. A: No hormone added, showing basal ERα localization. B,C: 1 h exposure to 1 and 10 nM 17β-estradiol, respectively. Both micrographs show a marked decrease in green fluorescence and no changes or reorganization of ERα immunoreactivity. Original magnification: 400×. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
To evaluate and correlate if the immunostaining detected with the different monoclonal antibodies were also estrogen binding sites, we used the macromolecular complexes E2-BSA-FITC to label living C2C12 cells. Microscopic observations of non-permeabilized cells incubated with the fluorescent conjugate showed a surface labeling (Fig. 9B). Cytosolic and nuclear localization of the derivative was observed after membrane permeabilization with saponin (Fig. 9C). When blocking experiments, employing the monoclonal antibody against the ligand binding domain (TE111.5D11) were performed, cytosolic localization of the fluorescent conjugate was not observed, demonstrating that in cytosol, the E2-BSA-FITC binding was due to ERα like entities structurally similar, at least, in the ligand binding domain. Also, the antibody could not displace the binding of the derivative in the nucleus and surface membrane (Fig. 9D,E), possible due to some non-specific adsorption of the complex to the membrane surface or the presence of estrogen binding entities in nucleus and membrane that do not share structural similarity with ERs ligand binding domains.
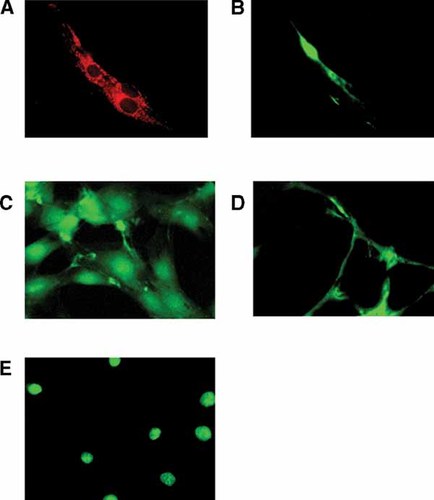
Fluorescence microscopy of muscle cells using 17β-estradiol-BSA-FITC impeded conjugate. For microscopic observation, C2C12 cells were labeled with E2-BSA-FITC as described in Materials and Methods Section. A: Mitochondrial localization with MitoTracker. B,C: Microscopic analysis of fluorescent complex binding to non-permeabilized and permeabilized C2C12 cells, respectively. D,E: Competition with TE111.5D11 antibody in non-permeabilized and permeabilized living cells, respectively. Original magnification = 600×. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Competitive binding assays and immunocytochemistry employing cells transiently transfected with a specific ERα siRNA were performed in order to confirm the functional and structural relationships between the classical ERα and the endogenous ERα detected in C2C12 cells. To estimate the transfection efficiency of siRNA, the fluorescein labeled siRNA was used as a control. Figure 10A depicts a typical pattern of fluorescence, 24 h post-transfection, showing a high transfection efficiency (∼70%). MCF-7 was used as a positive control for selectivity and effectiveness of ERα knock-down with the specific siRNA mix (Fig. 10B). To corroborate the efficacy of ERα silencing with ERα siRNA, two different amounts of siRNA and times of transfection were checked. Effective silencing of ERα was detected 24 h post-transfection with 10 and 20 pmol of siRNA (Fig. 10C, upper panel). Interestingly, some low molecular weight immunoreactive bands were effectively silenced whereas other low molecular immunoreactive bands were not. We suppose that these smaller reactive bands could be generated by different replication origins of the same gene and the mRNAs generated have little or no complementary sequences with the siRNAs. Figure 11A shows competitive binding assays performed in C2C12 whole cells transfected and non-transfected with ERα siRNA. Specific binding, 24 h post-transfection, was reduced compared with controls. Also, transfected cells conserved some specific binding activity that can be attributed to low molecular weight ER-like entities detected by Western blots but not silenced with the siRNAs, ERβ and estrogen binding entities non-structurally related to ERα. The ERβ isoform has been detected in the C2C12 cell line by Western blot analysis, blocking of ligand binding employing specific antibodies, confocal microscopy and PCR with specific primers. This isoform, in C2C12 cells, has a non-clasical mitochondrial localization (Milanesi L., Vasconsuelo A., Russo de Boland A. and Boland R., unpublished work). Finally, ERα immunoreactivity was evaluated by confocal microscopy analysis employing AER 314 monoclonal antibody, in C2C12 cells transiently transfected with ERα siRNA. Figure 11B, lower panel, shows representative micrographs of C2C12 cells 24 h post-transfection and their respective controls (upper panel). Transfected cells revealed a high decrease in ERα immunostaining (lower panel, left), compared with controls (upper panel, left). Similar results were obtained when the other two antibodies (AER 308 and TE111.5D11) were employed.
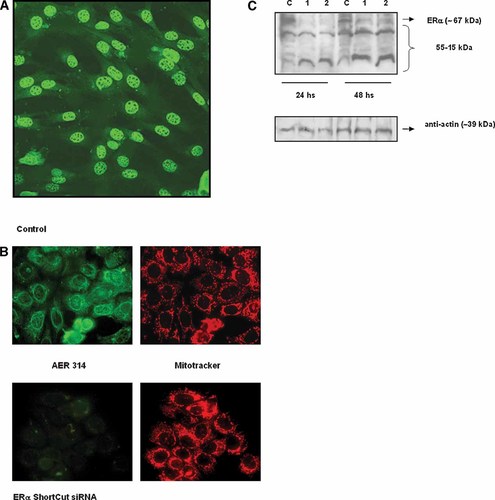
Effects of transfection of ERα siRNA on ERα immunoreactivity. C2C12 cells were transiently transfected with siRNA probes as described in Materials and Methods Section followed by fluorescence microscopy or Western blot analysis. A: Transfection efficiency of siRNA estimated using fluorescein-siRNA transfection control. A typical fluorescence microscopy pattern is shown. Original magnification = 400×. B: Immunofluorescence micrographs of MCF-7 cells transiently transfected with ERα siRNA after staining for ERα antigen. The MCF-7 cell line was employed as a positive control to verify effective silencing of ERα. Cells were transiently transfected with 20 pmol of ERα siRNA, incubated 24 h and then fixed and labeled for immunocytochemistry. Upper panel: Control (non-transfected cells). Left: ERα specific green fluorescence in nucleus, perinuclear region and cytosol employing AER 314 as primary antibody. Right: localization of mitochondria in MCF-7 cells with MitoTracker. Lower panel: cells transfected with ERα siRNA. Left: weak cell ERα staining is observed. Right: Mitochondria staining in MCF-7 cells with MitoTracker. C: Transfection of C2C12 cells with two concentrations of ERα siRNA. Expression of ERα was analyzed 24 h and 48 h post-transfection. Upper panel: Western blots of total cell lysates employing AER 314 monoclonal antibody. C, control; 1, 10 pmol of siRNA; 2, 20 pmol of siRNA. Lower panel: Actin loading control obtained using anti-actin polyclonal antibody. A representative blot from two independent experiments is shown. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
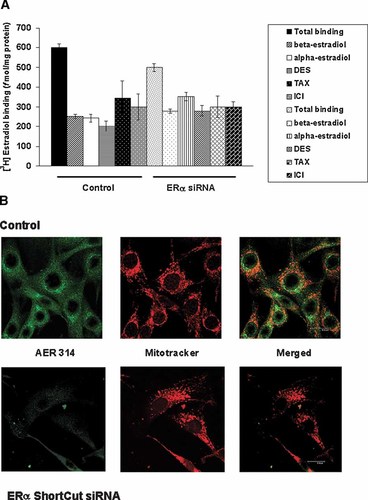
[3H]17β-estradiol binding characteristics and ERα immunodetection in whole C2C12 muscle cells transiently transfected with ERα siRNA. A: Estradiol binding by C2C12 cells. Living cells were transiently transfected with 20 pmol of ERα siRNA as described in Materials and Methods Section, and then incubated with 5 nM [3H]17β-estradiol alone (total binding) or in combination with a 200-fold molar excess of 17β-estradiol, 17α-estradiol, DES, tamoxifen or ICI182, 720 (non-specific binding). Control: non-transfected cells. Results are expressed in fmol/mg protein and represent the mean of samples analyzed in duplicate ±SD. B: Immunofluorescence confocal micrographs of C2C12 cells stained for ERα antigen. C2C12 cells were transfected with 20 pmol of ERα siRNA, incubated 24 h and then fixed and labeled for immunocytochemistry. Upper panel: Control (non-transfected cells). Left: shows ERα specific green fluorescence in mitochondria and perinuclear compartments of the cells employing AER 314 antibody. Center: Localization of mitochondria in C2C12 cells with MitoTracker rendered a red signal. Right: merged image of ERα green fluorescence and mitochondria red fluorescence. Lower panel: cells transiently transfected with ERα siRNA. Left: Weak ERα staining was detected in transfected cells. Center: Mitochondria staining of C2C12 cells with MitoTracker. Right: Merged image. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Coupled RT-PCR was performed with total RNA extracted from the C2C12 cell line. The chosen primer pairs (Table I) amplify 380 and 499 bp fragments from ERα mRNA. An ER positive cell line, the human mammary adenocarcinoma cell line MCF-7, was selected as positive control because it has been shown to be a good source of relatively high levels of ERα mRNA [Barrett-Lee et al., 1987]. As shown in Figure 12, RT-PCR of total RNA extracted from C2C12 and MCF-7 cells yields a single band of the expected fragment size for each primer pair (A, B; F, G). Sequence analysis of PCR products from C2C12 cells showed high sequence homology with the mouse ERα on a Blast search of the sequence database.
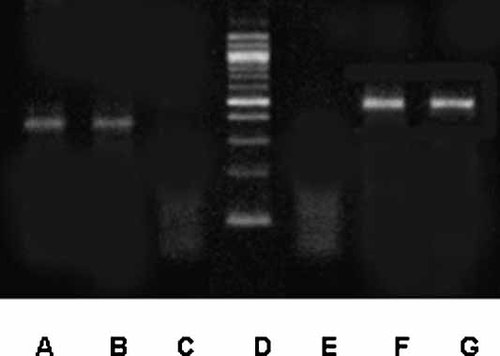
Expression of ERα in C2C12 muscle cells. Total RNA was extracted from cultured MCF-7 cells (positive control) and C2C12 cells. RNA samples were subjected to RT and subsequently to PCR using primer pairs specifically designed to the mouse ERα gene (Table I). Equal amounts of the reaction products were analyzed on agarose gels and stained with ethidium bromide. A, MCF-7 cells; B, C2C12 cells; C, negative control; D, DNA ladder (100 bp); E, negative control; F, MCF-7 cells; G, C2C12 cells. The molecular weight for the ERα PCR products are 380 bp (A,B) and 499 bp (F,G).
These results confirm the structural relation of endogenous C2C12 ERs with the classical and well-known ERα, as well as the subcellular localization of the immunoreactive proteins detected in this cell line.
DISCUSSION
Our work was focused to describe the presence and subcellular localization of native ERα like entities in the C2C12 murine myoblast cell line. We first employed conventional radioligand assays to obtain information about the presence and subcellular localization of estrogen binding sites. The use of different estrogen agonists and antagonists allowed us to characterize the estrogen binding entities as ER-like. Also the immunological approach with specific monoclonal antibodies, in blocking experiments, characterized the ERs as ERα.
The ERα-like specific binding sites, detected in whole cells in culture, were present not only in cytosol and nucleus but also in association with particulate subfractions. This is in agreement with other observations, where an important content of ERs was detected in mitochondrial and microsomal preparations in different cell types [Luconi et al., 1999; Zheng and Ramirez, 1999; Norfleet et al., 2000; Horvat et al., 2001; Monje and Boland, 2001, 2002; Monje et al., 2001; Ropero et al., 2002; Li et al., 2003; Cammarata et al., 2004; Yang et al., 2004; Solakidi et al., 2005; Stirone et al., 2005; Chen et al., 2005 and references therein; Levin, 2005 and references therein; Pedram et al., 2006].
The specific binding in mitochondrial fractions isolated from C2C12 cells was a saturable process with respect to the ligand concentration. Scatchard linearization of the saturation binding data was consistent with a single set of high affinity binding sites.
Immunoblots with anti-lamin B antibody excluded the possibility of nuclear contamination of the particulate preparations. Also cytochrome c oxidase measurements demonstrated no cross-contamination with mitochondria components in the other subfractions.
Western Blots of subcellular fractions showed high expression levels of ERα immunoreactivity not only in cytosol but also in mitochondria and microsomes. Low molecular weight bands were mostly detected in these subfractions and, as shown by Ligand Blot assays, they may contribute to the total estradiol binding capacity.
In agreement with these findings, immunocytochemical assays with conventional and confocal microscopy confirmed the mitochondrial and microsomal localization of ERα-like entities. Both, the perinuclear and mitochondrial pools of ERα may be considered as non-classical reservoirs. Immunostaining of mitochondria enriched fractions with the specific monoclonal antibody TE111.5D11 against the ligand binding domain also established the non-classical mitochondrial location of ERα in C2C12 muscle cells.
Western blots of subcellular fractions derived from mouse skeletal muscle tissue demonstrated expression and non-classical localization (microsomal) of the ERα isoform.
ERs have been demonstrated to be predominantly nuclear receptors, however, increasing evidence points to the presence of extranuclear ER-like entities, structurally and functionally similar to the well-known ERs. Our findings also suggest specificity of the immunoreactivity entities detected by the monoclonal antibodies, as 17β-estradiol treatments were shown to alter the intensity of ERα immunodetection and this can be attributed to a ligand-dependent switching of the ubiquitin-proteasome pathway for ER. In addition, the subcellular location of the immunoreactive entities was not modified as shown for other cell lines, like the SHM rabbit uterine myocyte cell line, which showed a rapid reorganization of perinuclear ERα into the nucleus, induced by 17β-estradiol [Monje et al., 2001]. The fact that ERα perinuclear immunoreactivity did not translocate into the nucleus of C2C12 cells, during 17β-estradiol treatments, might be another evidence re-enforcing the hypothesis that one of the two putative ERα localizations detected by immunocytochemical approaches could be an internal membrane system such as the endoplasmic reticulum and Golgi. The other, mitochondrial, was confirmed by a colocalization with a specific mitochonrdrial fluorescent marker (Mitotracker) in confocal microscopy. Finally, we employed a BSA-conjugated 17β-estradiol ligand to correlate immunochemical and functional data. This macromolecular complex has been previously used to study the existence of binding entities at the outer surfaces of the cells [Berthois et al., 1986; Valverde et al., 1999; Monje et al., 2001]. Our results suggest the presence of real estrogen binders localized in cytosol, perinuclear and nuclear compartments when cells where permeabilized with saponin prior treatment with the specific macromolecular complex. Non-permeabilized living cells showed a plasma membrane labeling of 17β-estradiol-BSA-FITC complex. Interestingly, preincubation with the specific antibody against the ligand binding domain of the ERα blocked the binding of the estradiol derivative to the cytosolic and perinuclear compartments but did not affect membrane and nuclear binding of the complex. We propose that in cytosol and perinuclear compartments may reside estrogen binding entities structurally and functionally similar to the ERα, at least with respect to its ligand binding domain. In agreement with these observations, in competitive binding assays, where the E2-BSA derivative devoid of free estradiol was employed (E2-BSA retentate), a minor specific binding localized in the cell membrane. Although by confocal immunocytochemistry some cells showed a punctuate nuclear staining with the TE111.5D11 antibody, fluorescence labeling with E2-BSA-FITC in the nucleus was more intense and homogeneous. Nuclear and membrane labeling of the estradiol derivative may indicate the presence of other estrogen binding entities not related to the estrogen receptors, as previously described by Valverde et al. 1999. Also we do not exclude non-specific binding of the estradiol derivative to the cell membrane and nucleus. Berthois et al. 1986 reported that trypsin treatment of cells markedly increased the binding of the fluorescent complex and this was not displaceable by E2-BSA. Lipophilic components located at the plasma membrane level and in membrane subcellular organelles, shielded by proteins, can be made accessible by limited proteolytic treatment unmasking hydrophobic constituents for E2-BSA-FITC binding.
Competitive binding assays and immunocytochemistry approaches employing transiently transfected cells with a specific ERα siRNA mix confirmed functional and structural relationships between the classical ERα and the endogenous ERα detected in C2C12 muscle cells. In addition, we could verify the non-classical localization of these ERα proteins. Interestingly, competitive binding assays, in transfected cells, showed a conserved specific binding with the different competitors. ERβ, detected in C2C12 cells (Milanesi L., Vasconsuelo A., Russo de Boland A. and Boland R., unpublished work) and other estrogen binding proteins, like ERα-like binding proteins of low molecular weight that were not silenced with the ERα siRNAs (as shown by Western blot analysis) could contribute to the specific binding. Also, these ER binding proteins of low molecular weight represent a minor population of ER-like immunoreactive entities, as shown by a marked decrease in immunostaining in immunocytochemistry assays, when cells were transiently transfected with specific ERα siRNAs.
Furthermore, PCR fragments of the expected size obtained from RNA of C2C12 cells showed a high sequence homology with the mouse ERα cDNA.
Of interest, we could not obtain the predicted PCR products when using mitochondrial mRNA from the C2C12 cells (data not shown), thereby suggesting that the ER is translocated into mitochondria. There are no data published demonstrating a mitochondria targeting domain in ERα. Only experiments that employ the ligand binding domain of ERα targeted to the nucleus and mitochondria of ER negative and breast cancer cell lines using targeting vectors containing mitochondria or nucleus localization sequences have been performed to demonstrate an antiapoptotic role of the mitochondrial ER [Pedram et al., 2006]. It is possible that a posttranslational modification could occur in some cell types that express mitochondrial ER. This may imply the existence of proteins and/or a lipid microenvironment in mitochondria which serve as anchorage of the receptor.
In view of the results of this study, we propose the presence of extranuclear ERα in the C2C12 murine myoblast cell line. The structurally similarity between the reactive entities detected in mitochondria and perinuclear compartments with the ERα isoform strengthen the general idea on the existence of a subpopulation of ER with a non-classical location.
It has been reported that 17β-estradiol treatment inhibits apoptosis in MCF-7 cells and other cell types via ER isoforms localized in mitochondria. The antiapoptotic role of estradiol through mitochondrial ERs in MCF-7 cells involved inhibition of cytochrome c release, ROS formation, JNK activation, PKCα phosphorylation and BAX dimerization and translocation into mitochondria [Chen et al., 2004, 2005; Pedram et al., 2006].
Separate studies of our laboratory have shown that 17β-estradiol, at physiological concentrations, abrogates cytochrome c release, PARP cleavage and DNA damage, induced by H2O2 or etoposide in C2C12 muscle cells. Antibodies against the estrogen receptors or specific ER siRNAs inhibited this protective action [Vasconsuelo et al., 2007], demonstrating a functional role of estrogen receptors detected, to a greater extent, in mitochondria of skeletal muscle cells.
Finally, there is evidence that skeletal muscle is a target tissue for estrogens. Decreased muscle strength and sarcopenia are observed in postmenopausal osteoporosis [Sirola and Rikkonen, 2005]. Although it has been shown that estrogens promote proliferation and differentiation of skeletal muscle myoblasts [Kahlert et al., 1997], the exact mechanism of estrogen-dependent sarcopenia remains to be clarified. Characterization of the relative roles of the muscle cell ERα-like proteins with non-classical intracellular distribution detected in the present study may provide basis on estrogen modulation of skeletal muscle development.




