Anti-Thy-1 antibody-induced neurite outgrowth in cultured dorsal root ganglionic neurons is mediated by the c-Src-MEK signaling pathway
Abstract
Our previous study has shown that anti-Thy-1 antibody promotes neurite outgrowth of cultured dorsal root ganglion (DRG) neurons in a protein kinase A (PKA)-dependent manner. The present study provided another intracellular signaling pathway for the neurotrophic effect of anti-Thy-1 antibody. In DMSO-treated control cells, Thy-1 was enriched in microdomain-like structures on cell membranes by immunofluorescence observation. Treatment of DRG neurons with anti-Thy-1 antibody not only stimulated neurite outgrowth, but also increased the branching complexity of the neurites in both small and large neurons. We have previously shown that anti-Thy-1 antibody causes a time-dependent activation of mitogen-activated protein kinase (MEK) and of cyclic AMP response-element binding protein (CREB). Here, anti-Thy-1 antibody elicited a transient activation of c-Src kinase, and the activation of c-Src kinase appeared occurring upstream of the activation of MEK and CREB, since pretreatment with the Src kinase inhibitor, PP2, effectively abolished the anti-Thy-1 antibody-induced neurite outgrowth and the phosphorylation of MEK and CREB. CREB phosphorylation might result in upregulation of certain neurite outgrowth-related proteins. We therefore conclude that anti-Thy-1 antibody activates the c-Src kinase-MEK-CREB cascade and overcomes the inhibitory effect of Thy-1 on neurite outgrowth in DRG neurons. J. Cell. Biochem. 103: 67–77, 2008. © 2007 Wiley-Liss, Inc.
Abbreviations used:
CREB, cyclic AMP response-element binding protein; DRG, dorsal root ganglion; ERK, extracellular signal-regulated kinase; GPI, glycosylphosphatidylinositol; HBSS, Hank's balanced salt solution; NF-L, neurofilament light chain subunit; MEK, mitogen activated protein kinase/extracellular signal-regulated kinase kinase; PBS, phosphate-buffered saline; PKA, protein kinase A; PKC, protein kinase C; TBS, Tris-buffered saline.
Thy-1, a member of the immunoglobulin superfamily, is a small glycoprotein abundantly expressed on the surface of many cell types, for example, thymocytes, T lymphocytes, and neurons [Morris and Grosveld, 1989]. Although its precise role in the nervous system is unknown, the fact that Thy-1 accounts for 2.5–7.5% of total surface proteins on axonal membranes suggests its importance in the nervous system [Beech et al., 1983]. Thy-1 expression is developmentally regulated in the early postnatal period [Morris, 1985] and it has therefore been suggested that it probably plays an important role in limiting axon growth in order to stabilize neural connections during postnatal development [Morris, 1992; Barlow and Huntley, 2000]. For example, in rat dorsal root ganglion (DRG) neurons, Thy-1 levels increase significantly after the second postnatal week, just after establishment of the central targeting of DRG neurons [Chen et al., 2005]. In mouse vestibular ganglion neurons, Thy-1 does not appear on the axonal surface until axon growth is complete [Xue et al., 1991]. In addition to acting as a negative regulator during axonal growth, Thy-1 is involved in the regulation of neurotransmitter release from presynaptic vesicles [Jeng et al., 1998] and in synaptic activity in the brain, as suggested by the phenotype of Thy-1 null mutant mice. These mice have an impairment of long-term potentiation in the dentate gyrus of the hippocampus [NostenBertrand et al., 1996] and fail to use socially transmitted cues to direct their choice of food [Mayeux-Portas et al., 2000]. Their neural development and neuronal architecture appear normal [Barlow et al., 2002], but the retina is thinner than in normal mice [Simon et al., 1999].
The importance of Thy-1 in the regulation of neurite outgrowth is supported by both in vivo and in vitro studies. After crush injury of sciatic nerves in young adult rats, there is an initial decline in Thy-1 expression on DRG neurons, followed by an increase, which correlates well with the recovery of sensory function during the course of regeneration [Chen et al., 2005]. When a neural cell line is transfected to express Thy-1, neurite outgrowth on a feeder layer of mature astrocytes is inhibited; furthermore, this effect can be reversed by soluble Thy-1 molecules or anti-Thy-1 antibodies [Tiveron et al., 1992]. The anti-Thy-1 antibodies presumably block the interaction between Thy-1 and astrocytes, thereby overcoming the inhibitory effect of Thy-1 on neurite outgrowth. Neuronal surface Thy-1 binds to αVβ3 integrin, a surface adhesion molecule on astrocytes [Leyton et al., 2001]. When Thy-1 binds to astrocyte integrin, it triggers tyrosine phosphorylation of focal adhesion proteins, increases RhoA activity, and promotes the attachment and spreading of astrocytes [Leyton et al., 2001; Avalos et al., 2002, 2004]. Whether binding of Thy-1 to integrin prevents neurite extension is not clear. In addition to interfering with the interaction between neurons and astrocytes, monoclonal anti-Thy-1 antibodies promote neurite outgrowth when applied to retinal ganglion cells [Leifer et al., 1984; Lipton et al., 1992], PC-12 cells [Mahanthappa and Patterson, 1992], or DRG neurons [Chen et al., 2005]. Divalent anti-Thy-1 antibody is effective in promoting neurite outgrowth, while the monovalent Fab fragment is not [Mahanthappa and Patterson, 1992]. The underlying mechanism might be due to the crosslinking of Thy-1 molecules by antibodies, followed by the shedding or internalization of Thy-1 from the cell surface, and the modulation of the signaling transduction pathway for neurite outgrowth [Mahanthappa and Patterson, 1992]. Thus, Thy-1 inhibits neurite growth by binding to a putative ligand on the mature astrocyte surface, and application of anti-Thy-1 antibodies overcomes this effect, reminiscent of the effects of another neurite growth regulator, F3, on the neuronal surface [Durbec et al., 1992; Pesheva et al., 1993].
Thy-1 is linked to the plasma membrane via a glycosylphosphatidylinositol (GPI) link, which is required for the control of neurite growth. For instance, when the GPI link is replaced by a transmembrane anchor, Thy-1 no longer inhibits neurite extension of neural cell lines grown on a mature astrocyte feeder layer [Tiveron et al., 1994]. Similarly, when Thy-1 is removed from the PC12 cell surface by phosphatidylinositol-specific phospholipase C, anti-Thy-1 antibody treatment no longer promotes neurite extension [Mahanthappa and Patterson, 1992; Doherty et al., 1993]. The GPI moiety contains saturated acyl/alkyl chains that associate preferentially with non-caveolar lipid microdomains in DRG neurons, retinal ganglion neurons, and PC12 cells [Lucero and Robbins, 2004]. Reggie/flotillin proteins, ubiquitous markers of lipid rafts in neurons, adipocytes, and macrophages, co-localize with Thy-1 in rat DRG neurons and regenerating goldfish optic nerves [Lang et al., 1998; Deininger et al., 2003]. Reggies function as protein scaffolds in the rafts, which contain adaptor molecules and signaling proteins and constitute platforms of signal transduction for GPI-anchored molecules [Stuermer and Plattner, 2005], such as Thy-1. Although Thy-1 lacks an intracellular domain, its activation can transduce signals by coupling to the non-receptor protein tyrosine kinases, Fyn, Lck, and Lyn, which are members of the Src kinase family [Stefanova et al., 1991; Thomas and Samelson, 1992; Narisawa-Saito et al., 1996]. In membrane fractions from neonatal chick brains, Thy-1 is linked to Fyn, tubulins, G protein subunit Gαi, and other phosphotyrosine-containing substrates, and anti-Thy-1 antibody treatment downregulates the kinase activity of the protein complex [Henke et al., 1997].
Although anti-Thy-1 antibodies effectively increase neurite outgrowth in several neuronal models, the mechanism might vary, depending on the cell type. DRG neurons have received less attention than other neuronal models. Our previous study showed that monoclonal anti-Thy-1 antibodies increase the branching number and total neurite length of cultured rat DRG neurons [Chen et al., 2005]. In a series of studies, we first reported that PKA activation is involved in neurite extension stimulated by anti-Thy-1 antibody [Chen et al., 2007]. The current study investigated other possible signaling pathways responsible for the neurotrophic effect of anti-Thy-1 antibody.
METHODS
Cell Culture
Postnatal day 2 Wistar rats of both sexes were purchased from the Facility for Animal Research of the National Taiwan University. Animal care and procedures were performed as laid down in the “Guide for the Care and the Use of Laboratory Animals,” published by the U.S. National Institutes of Health (NIH publication No. 85–23, revised 1985). The animals were anesthetized with ether and DRG were dissected out and collected in Hank's balanced salt solution (HBSS), containing 10 mM sodium pyruvate and 10 mM HEPES. Cell dissociation was achieved by digestion with 0.05% trypsin (Gibco, Grand Island, NY) and 0.05% type I collagenase (Sigma, St. Louis, MO) in HBSS solution for 30 min at 37°C. After centrifugation at 800 × g for 10 min and aspiration of the enzyme solution, the pellets were washed twice with L-15 Leibovitz medium containing 1.176 g/L NaHCO3 and 1% penicillin and streptomycin (Gibco) by low-speed centrifugation. The cell pellets were then mechanically dissociated by trituration and the cells plated on coverslips at a density of 100 cells/mm2 or directly in 35 mm dishes at a density of 400 cells/mm2. Cultures were maintained in L-15 Leibovitz medium containing 5% fetal bovine serum and 100 IU/ml of penicillin and streptomycin (Gibco) at 37°C in an atmosphere of 95% air and 5% CO2.
Anti-Thy-1 Antibody Treatment
Ascites containing mouse monoclonal anti-Thy-1 antibody [Jeng et al., 1998] at a fina1 dilution of 1:20 in culture medium was applied for the indicated time to day 2 cultures as described by Chen et al. 2005. Either normal mouse serum or an irrelevant mouse monoclonal antibody was used as controls. After treatment, the neurons were immunostained with anti-neurofilament-L (NF-L) antibody (Sigma) and the neuronal morphology analyzed.
Inhibitors
The MEK inhibitor, PD98059 (Biomol, Plymouth Meeting, PA), and the Src family inhibitor, PP2 (Calbiochem, La Jolla, CA), were used.
Immunocytochemistry
DRG neurons on coverslips were briefly washed in cold phosphate-buffered saline (PBS; 136 mM NaCl, 2.5 mM KCl, 1.5 mM KH2PO4, 6.5 mM Na2HPO4, pH 7.4) and cold methanol, then fixed in −20°C methanol for 20 min. After a 15 min wash in PBS, the cells were permeabilized and blocked for 1 h at room temperature in blocking buffer (PBS containing 0.05% Triton X-100 and 5% normal goat serum). The neurons were then incubated overnight at 4°C with monoclonal mouse anti-NF-L antibodies (Sigma Chemical Co. 1:100) or anti-Thy-1 antibody (1: 100 diluted ascites diluted in PBS), then, after a PBS wash, with FITC-conjugated goat anti-mouse IgG (1: 50; Vector, Burlingame, CA), washed with PBS, and mounted with aqueous mounting medium (3% n-propyl gallate and 50% glycerol in PBS).
For neurite tracing, some neurons were incubated for 1 h at 37°C with a 1:50 dilution of biotinylated goat anti-mouse IgG antibodies or biotinylated goat anti-rabbit IgG antibodies (both from Vector Labs) in blocking buffer. After washes in 50 mM Tris buffer, pH 7.4, the neurons were incubated for 1 h at room temperature with a 1:100 dilution of ABC Reagent (avidin–biotin complex; Vector) in blocking buffer, washed with Tris buffer, and reacted with an SG substrate kit (3% chromogen, 3% H2O2 in PBS; Vector). After a buffer wash and serial dehydration in ethanols and xylene, the coverslips were mounted with Permount and immunofluorescent images taken at 100× magnification on a Zeiss Axiophot epifluorescence microscope (Carl Zeiss, Oberkocheu), equipped with a Nikon DIX digital camera (Nikon, Tokyo, Japan). Chromogen-stained neurons were sketched at 40× magnification under a light microscope (Nikon) and the drawing digitalized using a scanner for later analysis.
Western Blotting
After treatment, the cultured DRG neurons were homogenized in ice-cold lysis buffer solution (10 mM EGTA, 2 mM MgCl2, 0.15% Triton X-100, 60 mM PIPES, 25 mM HEPES, 1 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 1 µg/ml of leupeptin, 1 µg/ml of pepstatin A, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM NaVO4, pH 6.9) using a glass homogenizer and sonicated. An equal volume of 2X concentrated reducing SDS sample buffer was added to the lysates and the mixture boiled at 95°C for 5 min. Protein concentrations were determined using a Bio-Rad protein Kit (Bio-Rad Lab, Hercules, CA), then samples (50 µg protein/gel lane) were separated on 10% polyacrylamide–SDS gels and electrotransferred to a nitrocellulose membrane (Schleicher and Schuell, Inc., Keene, NH). The membrane was then blocked with Tris-buffered saline (TBS: 50 mM Tris-Base, 150 mM NaCl, pH8.2) containing 5% bovine serum albumin, 0.1% Tween-20, then incubated overnight at 4°C with the monoclonal mouse antibodies [anti-phosphorylated MEK1/2 (p-MEK) (1:500; Cell Signaling Technology, Beverly, MA), anti-MEK1/2 (1:500; Santa Crutz), anti-c-Src kinase (1: 1,000, Upstates, Beverly, MA), anti-β actin (1:1,000; Sigma,)] or with polyclonal rabbit antibodies [anti-phosphorylated cyclic AMP response-element binding protein (p-CREB), anti-Tyr416-phospho-Src kinase (p-Src) (1:500; both from Cell Signaling)]. Following washes with TBST (TBS containing 0.1% Tween-20), alkaline phosphatase-conjugated secondary antibodies (Promega Corporation, Madison, WI) at a 1:7,500 dilution were added, and the bound antibodies visualized using substrate solution (3.3 mg/ml nitro blue tetrazolium and 1.65 mg/ml 5-bromo-4-chloro-3-indolyl phosphate in 100 mM NaCl, 5 mM MgCl2, 100 mM Tris-base, pH 9.5).
Enhanced chemiluminescence was used to probe the blotted membrane for other proteins. Membranes strips were soaked for 30 min at room temperature in stripping buffer (25 mM glycine-HCl, 1% SDS, pH 2), washed with TBST for 30 min, then incubated for 1 h at room temperature in blocking buffer 5% bovine serum albumin, 0.1% Tween-20, 150 mM NaCl, 50 mM Tris-base, pH 8.2. The strips were then incubated overnight at 4°C with different primary antibodies diluted in blocking buffer. After washing with TBST, the strips were incubated for 2 h at room temperature with horseradish peroxidase-conjugated goat anti-mouse IgG antibodies (1:7,500 in TBST) or horseradish peroxidase-conjugated goat anti-rabbit IgG antibodies (1:5,000) (both from BD Biosciences), washed with TBST for 30 min, and bound antibodies detected by chemiluminescence with chemiluminescence kits (Santa Cruz Biotechnology, Santa Cruz, CA). The blots were digitalized and the positive bands analyzed.
Immunoprecipitation
DRG cultures were treated with normal mouse serum or mouse anti-Thy-1 antibody at 37°C for 15 min and then extracted for 30 min on ice with immunoprecipitation buffer (150 mM NaCl, 1% NP-40, 0.5% DOC, 0.1% SDS, 1mM PMSF, 1 µg/ml of pepstatin A, 1 µg/ml of leupeptin in 50 mM Tris-HCl, pH 7.5). The cell suspensions were briefly ultrasonicated and centrifuged at 13,000×g for 30 min, then the supernatants were collected and their protein concentrations determined. A sample containing 800 µg of protein was incubated with 5 µl of anti-Tyr416-phospho-Src kinase antibodies (Cell Singaling) incubated at 4°C for 3 h before reacting at 4°C for 30 min with 100 µl of protein G-Sepharose beads (Pharmacia, Uppsala, Sweden). The Sepharose beads were extensively washed with immunoprecipitation buffer, and the adsorbed proteins dissolved by Laemmli sample buffer. The immunoprecipitates were further analyzed for c-Src or Fyn by Western blotting.
Quantification and Statistics
The neuritogenic effect of various treatments on DRG neurons was analyzed based on the scanned images of anti-NF-L antibody-stained cells. The total neurite length, longest neurite length, and total tip number of neurite branches were measured from the somata using a PC-based image analyzer software, Image Pro 3.0 Plus (Media Cybernetics, Silver Spring, MD). For Western blotting, the intensity of kinase protein bands was quantified using Gel pro 3.1 (Media Cybernetics). The Western blots used for statistical analysis were repeated at least three times for each group. Student's t-test was used to evaluate statistical differences between the means for different groups, a P value less than 0.05 being considered significant.
RESULTS
Enhanced Neuritogenesis of Cultured DRG Neurons by Anti-Thy-1 Antibody
For convenience of description, we classified DRG neurons into small and large neurons according to the criteria reported by Obata et al. 2004. Small neurons have a diameter less than 13.8 µm (area less than 600 µm2) and large neurons a diameter larger than 13.8 µm (area greater than 600 µm2).
Thy-1 molecules were distributed in a punctate pattern, mainly on the cell membrane of both large and small-sized neurons in untreated control group (data not shown) and in normal mouse, serum-treated group (Fig. 1A,B). To confirm the role of Thy-1 in neuritogenesis, mouse ascites containing anti-Thy-1 antibody or normal mouse serum (both at a 1:20 dilution) or an irrelevant monoclonal antibody was applied to rat DRG neuronal cultures. No toxic effect was detected with any of the preparations, as judged by propidium iodide vital staining and DAPI staining (data not shown). Anti-Thy-1 antibody (Fig. 2C,D) induced neuritogenesis, while normal mouse serum did not (Fig. 2A,B). Both large DRG neurons (Fig. 2C) and small DRG neurons (Fig. 2D) showed an increase in neurite length and branching complexity after treatment with anti-Thy-1 antibody. Total neurite length, tip number of neurite branches, and longest neurite length were all greater in cultures treated with anti-Thy-1 antibody than in those treated with normal mouse serum. As shown in Figure 3, both small and large neurons exhibited prominent neuritogenesis defined by all these parameters after anti-Thy-1 antibody treatment.
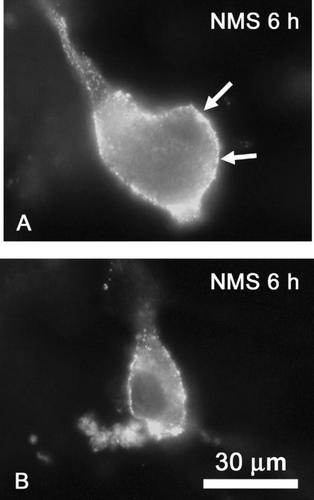
Distribution of Thy-1 in DRG neurons. DRG neurons were incubated in growth medium containing 5% normal mouse serum (NMS) for 6 h (NMS 6 h) and then immunostained for Thy-1. A, large DRG neuron. B, small DRG neuron. Bar = 30 µm.
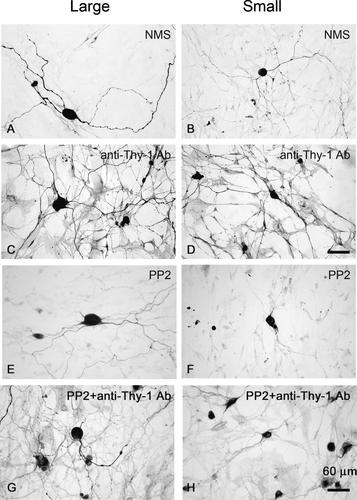
Induction of neurite outgrowth of DRG neurons by anti-Thy-1 antibody and effect of Src kinase inhibitor PP2 on anti-Thy-1 antibody-induced neurite outgrowth. DRG cultures were incubated for 6 h with normal mouse serum (NMS) (A, B), mouse anti-Thy-1 antibody (anti-Thy-1 Ab) (C, D), PP2 (E, F) or pretreatment with 30 µM PP2 for 30 min prior to incubation with anti-Thy-1 antibody (G, H), then immunostained for NF-L. Bar = 60 µm.

Quantitative analyses of the effect of Src kinase inhibitor PP2 on the anti-Thy-1 antibody-induced neurite outgrowth. A: camera lucida images of neurons. B: DRG neurons were treated as above and subjected to quantitative analyses for total neurite length and tip number of neurite branches. Three independent experiments were performed. Six neurons were chosen from each group in one representative experiment for such analysis. **, p < 0.01.
Blocking of Anti-Thy-1 Antibody-Induced Neuritogenesis by Src Kinase Inhibitor
Thy-1 is associated with non-caveolar lipid microdomains consisting of dynamic assemblies of cholesterol, sphingolipids, caveolins, and Src kinases (Fyn, Lck, Lyn, c-Src) [Draberova and Draber, 1993; Draberova et al., 1996; Stuermer et al., 2001], and these microdomains play important roles in many cellular responses to growth factors and in immune responses. We asked whether Src family kinases were involved in mediating the neuritogenic activity induced by anti-Thy-1 antibody. In the present study, we used PP2 (30 µM) to inhibit Src kinase activity. Treatment with PP2 alone for a short-term treatment (total 45 min) did not affect cell viability and effectively abolished the phosphorylation of Src kinase in control groups (data not shown). When we pretreated cultured DRG neurons with, the neuritogenic effect induced by anti-Thy-1 antibody was largely abolished by PP2 (Fig. 2G,H), the cells showing a morphology similar to that of PP2-treated group (Fig. 2E,F) and to neurons treated with normal mouse serum (Fig. 2A,B). Quantitative analyses confirmed that pretreatment with PP2 had a negative impact on total neurite length and tip number of neurite branches in both small and large neurons (Fig. 3). These results support the involvement of Src kinase in the anti-Thy-1 antibody-induced signaling pathway.
Activation of Kinases and CREB After Anti-Thy-1 Antibody Treatment
We next examined whether there was a change in Src kinase activity after anti-Thy-1 antibody treatment. To study the extent of kinase activation caused by the binding of anti-Thy-1 antibody to Thy-1, the level of phosphorylation of active Src kinase was examined by Western blotting. Cell lysates from DRG cultures at several time points (0 to 60 min) of anti-Thy-1 antibody treatment were subjected to analysis. This treatment induced a rapid phosphorylation of Src kinase (Fig. 4A). To further identify the member of Src kinases activated by anti-Thy-1 antibody, we performed immunoprecipitation with anti-p-Src antibodies and analyzed the immunoprecipitates by Western blotting with anti-c-Src antibodies. The protein levels of c-Src kinase increased by 50% after incubation with anti-Thy-1 antibodies (Fig. 4B).
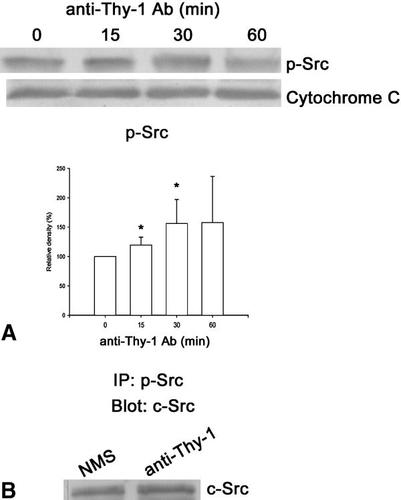
Phosphorylation of Src kinase induced by anti-Thy-1 antibody. DRG cultures were incubated with anti-Thy-1 antibodies for 0, 15, 30, or 60 min, then the cell homogenates were analyzed for phosphorylated Src (p-Src). Cytochrome c, loading control. A representative blot from one experiment and densitometric scans of five blots obtained from five independent experiments are shown. (A) *, p < 0.05, compared to the 0 min group. (B) Increase in c-Src phosphorylation by anti-Thy-1 Ab. DRG neurons were incubated for 30 min in NMS or anti-Thy-1 Ab, then the whole cell extracts were precipitated with anti-p-Src antibodies and subjected to immunoblotting with anti-c-Src antibodies. The experiments were repeated twice and one representative blot is shown.
Anti-Thy-1 antibody treatment induced activation of MEK and CREB at 15 min after addition of the antibodies (Fig. 5A,B). We further proved that c-Src is the upstream regulator of the MEK cascade by the observation that inhibition of Src activity by PP2 abolished anti-Thy-1 antibody-induced MEK activation (Fig. 5A).
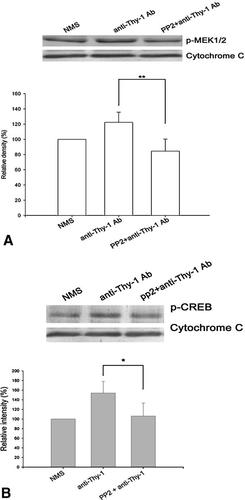
Effects of PP2 on anti-Thy-1 antibody-induced phosphorylation of MEK and CREB. DRG cultures were treated for 15 min with NMS or anti-Thy-1 Ab or were first incubated for 30 min with 30 µM PP2, then for 15 min with anti-Thy-1 Ab. The cell homogenates were analyzed for phosphorylated MEK (A) and phosphorylated CREB (B). Cytochrome c, loading control. A representative blot from one experiment and densitometric scans of four blots obtained from four independent experiments are shown. n = 4. *, p < 0.05; **, p < 0.01, compared to the anti-Thy-1 antibody-treated group.
Effect of a MEK or a Src Kinase Inhibitor on the Degree of CREB Activation Induced by Anti-Thy-1 Antibody
CREB-mediated gene transcription is required for various neuronal functions, such as growth, survival, differentiation, and neuroplasticity [Lonze and Ginty, 2002]. To test the hypothesis that CREB might be a downstream target of the c-Src-MEK signaling pathway in this DRG culture system, we checked whether CREB phosphorylation triggered by anti-Thy-1 antibody treatment was affected by kinase inhibitors. Pretreatment with PP2 resulted in decreased CREB phosphorylation (Fig. 5B). These data suggest that the neuritogenic response caused by anti-Thy-1 antibody might be due to its binding to Thy-1 molecules on the neuronal plasma membrane and the subsequent activation of c-Src and MEK signaling pathways leading to phosphorylation of the transcription factor, CREB, and finally to neurite extension and branching. We have previously shown that PKA-MEK-CREB is one of the signaling pathways stimulated by anti-Thy-1 antibody treatment [Chen et al., 2007]. We further investigated if there was a crosstalk between c-Src and PKA. Pretreatment with PP2 did not prevent anti-Thy-1 antibody-induced activation of PKA, which was detected by phosphorylation of VASP (data not shown). Further, pretreatment with a PKA inhibitor, PKI, did not affect the anti-Thy-1 antibody-induced phosphorylation of Src kinase (data not shown). Thus, PKA-MEK-CREB and c-Src-MEK-CREB are two distinct pathways elicited by anti-Thy-1 antibody treatment.
DISCUSSION
In the present study, we investigated the signaling pathways activated by anti-Thy-1 antibody treatment, which induced enhanced neuritogenesis in rat DRG neurons. Our results showed that Thy-1 was localized to microdomains in the cell membrane of DRG neurons. Anti-Thy-1 antibody treatment effectively promoted neurite outgrowth of DRG neurons via the c-Src kinase–MEK-CREB signaling pathway.
It has been known for several years that perturbation of Thy-1 function with anti-Thy-1 antibodies overcomes the inhibitory effects of Thy-1 on axonal growth. For example, neurite outgrowth of retinal ganglionic cells is promoted by culturing the cells on glass coated with monoclonal anti-Thy-1 antibody [Leifer et al., 1984; Lipton et al., 1992]. Likewise, anti-Thy-1 antibody treatment induces neurite outgrowth of PC12 cells in the absence of nerve growth factor [Mahanthappa and Patterson, 1992]. Anti-Thy-1 antibodies, by binding to the ganglionic cell membrane, increase the immunoreactivity of microtubule-associated protein 2 and tau in both the somatodendritic and axonal compartments [Leifer et al., 1991]. Consistent with previous findings in other cell types, we found that the presence of surface Thy-1 molecules inhibited neurite extension in the DRG neuron model. Moreover, we observed clusters of Thy-1 molecules in microdomain-like punctuate structures, probably lipid rafts, on the plasma membrane. These microdomains might be important in mediating the signaling mechanism for anti-Thy-1 antibody-induced neuritogenesis, since we observed an increase in cytoplasmic Thy-1-containing vesicles, representing the internalization of surface Thy-1 molecules after anti-Thy-1 antibody treatment (data not shown).
In the present study, we detected phosphorylation of c-Src kinase shortly after anti-Thy-1 antibody treatment. Since Thy-1 does not possess classical transmembrane or cytoplasmic segments, it is unclear how Thy-1 molecules induce the activation of intracellular signaling pathways. The localization of Thy-1 in lipid rafts raises the possibility that it may mediate biological responses through direct interactions with neighboring molecules on the cell surface. For example, in thymocytes, Thy-1 has been shown to interact with an 85–90 kD transmembrane phosphorylated protein containing binding sites for SH2 domain-containing proteins, such as Fyn and PI3K [Durrheim et al., 2001]. Biochemical studies on C32 and HeLa cells have also suggested a direct interaction between the GPI anchor of Thy-1 and palmitylated and myristylated cysteines in the N-terminal region of Src family kinases within lipid rafts [Stefanova et al., 1991; Shenoyscaria et al., 1993]. In addition, several lines of evidence support the idea that Src kinases and Thy-1 co-localize in the same microdomains [Thomas and Samelson, 1992; Narisawa-Saito et al., 1996; Deininger et al., 2003; Rajendran and Simons, 2005]. Cross-linking of Thy-1 has been shown to induce the aggregation of Src kinases located in the same microdomains, resulting in trans-phosphorylation of both the soluble and membrane-bound substrates of the kinase [Draberova et al., 1996; Akhand et al., 1999]. Src kinases may therefore serve as a direct signaling partner of Thy-1 and thus as an upstream regulator in the signaling cascade. Our result, however, conflict with those of Henke et al. 1997, who showed that anti-Thy-1 antibodies decrease c-Src kinase activity in isolated lipid rafts. This discrepancy may be explained by the different experimental models used in the two studies.
Our data suggest that anti-Thy-1 antibody treatment leads to MEK activation, which is required for the neurotrophic effects of the antibodies. Furthermore, we present evidence showing that c-Src kinase is upstream regulator of MEK activation by anti-Thy-1 antibody. The role of MEK-ERK in neurite outgrowth is well established. When constitutively active ERK is injected into PC12 cells, significant neurite outgrowth is induced [Fukuda et al., 1995; Robinson et al., 1998]. When ERK is phosphorylated by MEK, the phosphorylated ERK activates various cytosolic effector proteins and the nuclear transcription factor, CREB, leading to altered transcription of genes, such as those controlling cell differentiation and proliferation [Impey et al., 1998; Tojima et al., 2003]. For example, induction of neurite outgrowth in human neuroblastoma Paju cell lines by interferon-γ is mediated by ERK-dependent upregulation of cyclin-dependent kinase 5 (Cdk5) [Song et al., 2005]. Cdk5 also plays a role in cytoskeleton dynamics by regulating the phosphorylation of neurofilament protein neurofilament heavy chain subunit, microtubule-associated protein 2, and p27 kinase [Kesavapany et al., 2003; Kawauchi et al., 2006]. In addition, ERK upregulates the expression of growth-associated protein 43 (GAP-43) [Kim et al., 1997; Olsson and Nanberg, 2001; Johansson et al., 2005]. It is possible that ERK directly affects Cdk5 and GAP-43 gene expression to regulate the neurotrophic response in DRG neurons induced by anti-Thy-1 antibody. Activation of the c-Src-MEK cascade leads to activation of CREB, which can upregulate the expression of several neurite outgrowth-related genes, including NF-L, nerve growth factor, and brain-derived neurotrophic factor [Lonze and Ginty, 2002]. The expression of these genes might therefore be responsible for the anti-Thy-1 antibody-induced neurite outgrowth. The present study supports the notion that Thy-1 signaling in DRG neurons involves different kinase signaling pathways. We propose that the signaling cascade of c-Src kinase → MEK → CREB is probably responsible for the neurotrophic effect of anti-Thy-1 antibody. Our results shed new light on the mechanisms of Thy-1 function in rodent sensory neurons and provide useful information on the potential use of anti-Thy-1 antibody in stimulating nerve regeneration in the peripheral sensory system.
Acknowledgements
We thank Dr. Thomas Barkas for his critical reading and correction of this manuscript.




