Studies on the cell treatment conditions to elicit lipolytic responses from 3T3-L1 adipocytes to TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin
Abstract
Wasting syndrome is one of the hallmark symptoms of poisoning by TCDD (=dioxin), which is associated with the massive loss of adipose tissue and serum hyperlipidemia in vivo. Yet, the most widely used in vitro cell model 3T3-L1 adipocyte has not been useful for studying such an action of TCDD because of the difficulty of inducing their mature adipocytes to respond to TCDD to go through lipolysis. Here, we made efforts to find the right cell culture and treatment conditions to induce mature 3T3-L1 adipocytes to go through lipolysis, which is defined as events leading to reduction of lipids in adipocytes. The optimum condition was found to require 7-day differentiated adipocytes being subjected to DMEM medium containing TCDD (but without insulin) for 5 day incubation with two medium changes (the same composition) on incubation days 2 and 4. After 24 h, the early effect of TCDD on adipocytes was predominantly on inflammation, particularly induction of COX-2 and KC (IL-8), which is accompanied by upregulation of C/EBPβ and δ. The sign of TCDD-induced lipolysis starts slowly and by incubation day 3, a few markers showed modestly significant changes. By day 5 of incubation, however, many markers show highly significant signs of lipolytic changes. Although this process could take place without exogenous macrophages or their cytokines, addition of exogenous TNFα considerably synergized this action of TCDD. In conclusion, under a right condition, 3T3-L1 adipocytes were found to respond to TCDD to go through lipolysis. The early trigger of such a response appears to be activation of COX-2, which is amplified by TNFα. J. Cell. Biochem. 102: 389–402, 2007. © 2007 Wiley-Liss, Inc.
TCDD (2,3,7,8-tetrachlorodibenzo-p-dixon, or sometimes simply called “dioxin”) is a very toxic compound to many organisms [see reviews by Poland and Knutson, 1982; Whitlock, 1990]. One of the major toxic symptoms regarded as a hallmark effect of chronic exposure to TCDD, occurring in many species of animals, is wasting syndrome or cachexia, which involves general body weight loss, reduced appetite, and overall lethargy resulting in significant loss of adipose tissues and increased hyperlipidemia in serum. To study this phenomenon in vitro many scientists have relied on the 3T3-L1 adipocyte differentiation model. It was originally reported from this laboratory by Phillips et al. [1995] that TCDD at low concentrations causes inhibition of adipocyte differentiation of 3T3-L1 fibroblasts into adipocytes when it was given at the same time as the hormone inducers of adipocyte differentiation, consisting of insulin, dexamethasone (Dex), and IBMX, a standard method customary used by many scientists [e.g., MacDougald and Lane, 1995]. In the initial study of testing the effect of TCDD on this cell model, it was found that the effective timing of this action of TCDD is limited to the initial 48 h window after which the addition of TCDD did not appear to cause any significant effect on the final outcome of adipocyte differentiation [Phillips et al., 1995]. Subsequent studies [e.g., Brodie et al., 1996, 1997; Chen et al., 1997; Liu et al., 1996, 2002; Liu and Matsumura, 2006] confirmed this observation in this cell line. Subsequently, it was shown by Shimba et al. [1998, 2001] that upon full differentiation to mature adipocytes, the level of Ah receptor (AhR), which is known to mediate almost all of cellular actions of TCDD, in 3T3-L1 cells is drastically reduced. This observation was also confirmed in several lines of adipocyte differentiating mouse embryonic fibroblasts by Alexander et al. [1998]. Thus, it appeared at that time that this type of mouse cell models is suited only for the purpose of studying the initial anti-adipogenic action (i.e., inhibition of adipocyte differentiation) of TCDD, but not for its lipolytic action (i.e., reduction in lipid contents from fully differentiated adipocytes), which is known to occur in vivo or in vitro with ex-planted adipose tissues in culture [Enan and Matsumura, 1993].
More recently it has been reported by Kern et al. [2002a,b] in another 3T3-L1 related cell line, 3T3-F442A, the treatment during their adipocyte differentiation with 50 nM concentration of TCDD could induce a lipolysis-like phenomenon, which is accompanied with an increased production of TNFα as an autocrine and a decreased expression of LPL, simulating the case of adipose tissues treated with TCDD in vivo. This observation prompted us to re-examine the question on the perceived non-responsiveness of mature 3T3-L1 adipocytes to TCDD with respect to their lipolytic reactions. This is not a trivial question, since it has not been clarified whether the action of TCDD on adipose tissues in vivo is entirely due to its action to block pre-adipocyte differentiation, to induce lipolytic actions or a combination of both, since in vivo adipose tissues usually consist of significant percentages of immature pre-adipocytes, which differentiate into adipocytes. Furthermore, now that the action of TCDD in humans has been shown to cause diabetes in long runs [e.g., Longnecker and Michalek, 2000], an appropriate adipocyte model that is useful in studying such direct actions of TCDD on differentiated adipocytes is badly needed. In this study we investigated the influence of various cell culturing conditions as well as schemes of TCDD treatments on the expression of 3T3-L1 adipocytes, and found indeed that they can be made to become responsive to the lipolytic actions of TCDD, and now report the results.
MATERIALS AND METHODS
Chemicals
3-O-methyl-D-[1-3H] glucose (3H-Me-glu) was purchased from Amersham (Arlington Heights, IL). [y-32P]ATP (4000 Ci/mmol) was purchased from ICN (Costa Mesa, CA). TCDD (>99.9% purity) was originally obtained from Dow Chemicals Co. Dimethylsulfoxide (DMSO) was obtained from Aldrich Chemical Co. Antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). H89, Forskolin (FSK), SC-514, parthenolide, 3-isobutyl-1-methylxanthine (IBMX), and dexamethasone were purchased from Calbiochem. Insulin and TNFα were purchased from Sigma. Other molecular biological reagents for RT-PCR were purchased from Qiagen (Valencia, CA) and Roche Applied Science.
Cell Culture and Differentiation
3T3-L1 fibroblast cells [Green and Meuth, 1974] were obtained from American Type Culture Collection (ATCC). Cells were routinely cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS). Confluent cells were induced to differentiate by incubation for 2 days with differentiation medium containing 1 µM dexamethasone (Dex), 0.2 mM IBMX, 10 µg/ml insulin, and 10% FBS in DMEM. After this time, cells were kept in “maintenance medium” containing 10 µg/ml insulin and 10% FBS, and the medium was changed every 2–3 days [Moreno-Aliaga and Matsumura, 1999]. After 5 days differentiation in maintenance medium, colonies begin to show visible signs of mature adipocytes as attested by the appearance of rounded cells with numerous intracellular lipid droplets. To study the effects of TCDD or other chemicals thus differentiated adipocytes were treated with TCDD alone or together with other chemicals for specified period of time by addition of test compounds to fresh DMEM. Chemicals to be tested were prepared as 1000-fold concentrated stocks in DMSO, and treatment was renewed at the time of medium change (every 2 days). Control samples received an equal volume of the vehicle DMSO alone.
Quantitative Reverse Transcriptase-PCR (Real-Time RT-PCR)
Total RNA was isolated from 3T3 adipocyte cells using a high pure RNA isolation kit (Qiagen), and cDNA synthesis was carried out as described by Vogel and Matsumura [2003]. Briefly, DNA-free total RNA (1.0 µg) was reverse transcribed using 4 units of Omniscript reverse transcriptase (Qiagen, Valencia, CA) and 1 µg of oligo(dT)15 in a final volume of 40 µl. Mouse-specific primers of CYP1A1, C/EBPα, C/EBPβ, C/EBPδ, COX-2, KC(IL-8), IKKβ, SOCS-3, PPARγ, LPL, TNFα, NFκB, PKA, Glut4, and β-actin were selected from published sources and described in Table I. Quantitative detection of β-actin and above primers was performed with a LightCycler instrument (Roche Diagnostics) using the QuantiTect SYBR Green PCR Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. PCR amplification was carried out in a total volume of 20 µl, containing 2 µl of cDNA, 10 µl of 2 × QuantiTect SYBR Green PCR Master Mix, and 0.2 µM of each primer. The PCR cycling conditions were 95°C for 15 min followed by 40–50 cycles of 94°C for 15 s, 60°C for 20 s, and 72°C for 10 s. Detection of the fluorescent product was performed at the end of the 72°C extension period. Negative controls were run concomitantly to confirm that the samples were not cross-contaminated. A sample with DNase- and RNase-free water instead of RNA was concomitantly examined for each of the reaction units described above. To confirm the amplification specificity, the PCR products were subjected to melting curve analysis. All PCR assays were performed in duplicate or triplicate. The intra-assay variability was <7%. For quantification, data were analyzed with the LightCycler analysis software according to the manufacturer's instructions.
| Gene | FP | RP |
|---|---|---|
| CYP1A1 | GGC CAC TTT GAC CCT TAC AA | CAG GTA ACG GAG GAC AGG AA |
| C/EBPα | TTA CAA CAG GCC AGG TTT CC | CTC TGG GAT GGA TCG ATT GT |
| C/EBPβ | CAA GCT GAG CGA CGA GTA CA | ATC GCT GCA GCT TCC TAT GT |
| C/EBPδ | ATC GCT GCA GCT TCC TAT GT | AGT CAT GCT TTC CCG TGT TC |
| COX-2 | AGA AGG AAA TGG CTG CAG AA | GCT CGG CTT CCA GTA TTA AG |
| KC | CTT GAA GGF GTT GCC CTC AG | TGG GGA CAC CTT TTA GCA TC |
| IKKβ | TCA CTT GTC TCC TGG TGC TG | TGT GCA GCA GAT TTC CTT TG |
| SOCS-3 | GCG AGA AGA TTC CGC TGG TA | CCG TTG ACA GTC TTC CGA CAA |
| PPARγ | AAT CCT TGG CCC TCT GAG AT | TTT TCA AGG GTG CCA GTT TC |
| LPL | GCC CAG CAA CAT TAT CCA GT | GGT CAG ACT TCC TGT TAC GC |
| TNFα | ATG AGC ACA GAA AGC ATG ATC CGC | CCA AAG TAG ACC TGC CCG GAC TC |
| NFkB | CTG ACC TGA GCC TTC TGG AC | GCA GGC TAT TGC TCA TCA CA |
| PKA | CAG GAA AGC GCT CCA GAT AC | AAG GGA AGG TTG GCG TTA CT |
| Glut4 | GAT TCT GCT GCC CTT CTG TC | ATT GGA CGC TCT CTC TCC AA |
| IRS-1 | CCA GCC TGG CTA TTT AGC TG | CCC AAC TCA ACT CCA CCA CT |
| β-actin | AGC CAT GTA CGT AGC CAT CC | CTC TCA GCT GTG GTG GTG AA |
Glucose Uptake
Glucose uptake in differentiated 3T3-Ll cells was measured using a modification of the method described by Frost and Lane [1985]. Briefly, cells were grown and differentiated in 12-well culture plates, and incubated in 1 ml of DMEM containing 10% FBS ± test chemicals as indicated. Before each assay, cells were incubated in 1 ml of serum-free DMEM for 1.5 h at 37°C. Next, the cells were washed twice with 1 ml of Krebs–Ringer phosphate buffer (130 mM NaCl, 5 mM KCl, 1.3 mM CaCl2, 1.3 mM MgSO4, 10 mM Na2HPO4, pH 7.4) and incubated in 1 ml of the same buffer ± 100 nM insulin for 0.5 h at 37°C. Glucose transport assays were initiated by the addition of 1 µCi/ml 3H-Me-glu for 5 min at 37°C. Transport was terminated by the rapid removal of assay buffer from each well followed by two rapid washes of each well with 1 ml of ice-cold Krebs–Ringer phosphate buffer. Cells were removed from each well with 0.4 ml of 0.1% SDS and counted after the addition of 4 ml of Optifluor (Packard Instrument Co.). Non-specific glucose transport was defined as that occurred in the presence of 10 µM cytochalasin B. Under the conditions described, glucose transport was linear from 1 to 30 min. The results of glucose transport assays performed in 50 × 20-mm2 culture plates (Nunc) were in good agreement with those performed in the 12-well culture plates.
Electrophoretic Mobility Shift Assays (EMSA)
Nuclear extracts were isolated from treated 3T3 adipocyte cells according to Dennler et al. [1998]. In brief, 3T3 adipocyte cells were washed and harvested in Dulbecco's PBS containing 1 mM phenylmethylsulfonyl fluoride (PMSF) and 0.05 µg/µl aprotinin. After centrifugation the cell pellets were gently resuspended in 1 ml of hypotonic buffer (20 mM HEPES, 20 mM NaF, 1 mM Na3VO4, 1 mM Na4P2O7, 1 mM EDTA, 1 mM EGTA, 0.5 mM PMSF, 0.13 µM okadaic acid, 1 mM dithiothreitol, pH 7.9, and 1 µg/ml each leupeptin, aprotinin, and pepstatin). The cells were allowed to swell on ice for 15 min and then homogenized by 25 strokes of a Dounce homogenizer. After centrifugation for 1 min at 16,000g, nuclear pellets were resuspended in 300 µl of ice-cold high salt buffer (hypotonic buffer with 420 mM NaCl and 20% glycerol). The samples were passed through a 21-gauge needle and stirred for 30 min at 4°C. The nuclear lysates were microcentrifuged at 16,000g for 20 min, aliquoted, and stored at −70°C. Protein concentrations were determined by the method of Bradford.
For EMSA a double-stranded oligonucleotide corresponding to the C/EBP binding site or NFκB response element was end-labeled using [y-32P]ATP (Amersham Life Sciences) and T4 polynucleotide kinase (Promega, Madison, WI) according to the standard methods. DNA–protein-binding reactions were carried out in a total volume of 20 µl containing 15 µg of nuclear protein, 40,000 cpm of DNA oligonucleotide, 25 mM Tris buffer, pH 7.5, 50 mM NaCl, 1 mM MgCl2, 1 mM EDTA, 0.5 mM dithiothreitol, 5% glycerol, and 1 µg of poly (dI–dC). The samples were incubated at room temperature for 20 min. Supershift analyses were performed by adding 2 µg of polyclonal C/EBPα, C/EBPβ, C/EBPδ, NFκB-p50, and NFκB-p65 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) to the reaction mixtures. Competition experiments were performed in the presence of a 200-fold molar excess of unlabeled DNA fragments. Protein–DNA complexes were resolved on a 5% non-denaturating polyacrylamide gel and visualized by exposure of the dehydrated gels to x-ray films. In all cases each EMSA test was repeated more than two times to ascertain the reproducibility of the overall pattern of nuclear protein binding to the labeled oligonucleotides.
Statistical Analysis
All experiments were repeated a minimum of three times and results are expressed as means ± standard deviations. Data were evaluated statistically by one-way ANOVA followed by Student's t test at the significant level of P < 0.05.
RESULTS
Time-Dependent Effect of TCDD on the Expression of Inflammatory and Lypolytic Markers in 3T3 Adipocytes
To search for a method which will give us the lipolytic response of mature 3T3-L1 adipocytes, we first induced differentiation by treating confluent cultures of 3T3-L1 fibroblasts with a mixture of dexamethasone, insulin, and IBMX (DIX mixture) for 48 h, and further incubated in DMEM medium with insulin alone for additional 5 days (to day 7 of differentiation) with an intermediate medium change on day 5. On day 7 of differentiation (=post-differentiation day 0), the medium was changed once more to DMEM (without insulin) along with addition of TCDD and the experiment was terminated 24 h thereafter (see 1 day post-differentiation test, Table II). For tests requiring longer incubation tests, DMEM medium was changed on post-differentiation day 2 (for 3 day tests) or post-differentiation day 4 (for 5 day tests), each medium change being accompanied by fresh addition of TCDD. Samples of cells were harvested on day 10 or day 12 (i.e., always 24 h after the last medium change). The results of quantitative RT-PCR on cDNAs prepared from these samples are summarized in Table II.
| mRNA | 1d | 3d | 5d | |||
|---|---|---|---|---|---|---|
| Control | TCDD | Control | TCDD | Control | TCDD | |
| Inflammation Markers | ||||||
| COX-2 | 1.00 ± 0.19 | 2.87 ± 0.55** | 1.53 ± 0.13 | 3.59 ± 0.56** | 1.52 ± 0.34 | 2.97 ± 0.09** |
| KC | 1.00 ± 0.12 | 1.36 ± 0.16** | 1.62 ± 0.10 | 2.10 ± 0.16* | 2.23 ± 0.12 | 3.01 ± 0.17** |
| IKKβ | 1.00 ± 0.18 | 1.50 ± 0.17* | 1.88 ± 0.37 | 2.60 ± 0.10* | 1.51 ± 0.08 | 1.84 ± 0.10* |
| TNFα | 1.00 ± 0.10 | 1.21 ± 0.08* | 1.59 ± 0.27 | 1.84 ± 0.06 | 1.90 ± 0.21 | 1.88 ± 0.15 |
| NFκB | 1.00 ± 0.10 | 1.42 ± 0.24* | 1.59 ± 0.18 | 1.55 ± 0.22 | 1.39 ± 0.23 | 1.25 ± 0.11 |
| MCP-1 | 1.00 ± 0.20 | 0.99 ± 0.31 | 1.38 ± 0.17 | 0.97 ± 0.06** | 1.24 ± 0.15 | 1.36 ± 0.23 |
| SOCS-3 | 1.00 ± 0.11 | 1.35 ± 0.10** | 1.14 ± 0.04 | 1.36 ± 0.12* | 1.05 ± 0.11 | 1.27 ± 0.09* |
| PKA | 1.00 ± 0.07 | 1.43 ± 0.16** | 1.76 ± 0.05 | 1.65 ± 0.22 | 1.48 ± 0.19 | 1.15 ± 0.11 |
| Lipolytic Markers | ||||||
| PPARγ | 1.00 ± 0.10 | 1.08 ± 0.16 | 1.53 ± 0.05 | 1.32 ± 0.21 | 1.59 ± 0.21 | 0.95 ± 0.18** |
| C/EBPα | 1.00 ± 0.17 | 1.13 ± 0.05 | 2.22 ± 0.11 | 2.39 ± 0.39 | 1.52 ± 0.15 | 0.81 ± 0.07** |
| C/EBPβ | 1.00 ± 0.06 | 1.42 ± 0.09** | 1.26 ± 0.14 | 1.59 ± 0.23* | 1.54 ± 0.19 | 1.56 ± 0.14 |
| C/EBPδ | 1.00 ± 0.06 | 1.29 ± 0.18** | 1.70 ± 0.11 | 1.68 ± 0.28 | 1.74 ± 0.18 | 1.60 ± 0.07 |
| LPL | 1.00 ± 0.13 | 1.16 ± 0.11 | 1.46 ± 0.14 | 1.31 ± 0.15 | 1.05 ± 0.12 | 0.72 ± 0.09** |
| Glut4 | 1.00 ± 0.10 | 1.18 ± 0.12 | 1.64 ± 0.02 | 1.74 ± 0.32 | 1.08 ± 0.22 | 0.62 ± 0.04** |
| IRS-1 | 1.00 ± 0.13 | 1.09 ± 0.01 | 1.88 ± 0.19 | 1.49 ± 0.17* | 1.53 ± 0.14 | 1.04 ± 0.09** |
- The results are shown as means ± standard deviations from 3–5 independent tests. All P values were obtained through Student's-test analysis.
- Asterisk indicates the significant effect of TCDD as compared to the matched control in each agent test group (i.e., the values shown under the column, “Control” are significantly different from the corresponding values shown under “TCDD” within each testing day). The number of symbols indicates that the effect of TCDD is significant (as compared to control for each day) at *P < 0.05 and **P < 0.01, respectively.
As expected from the previous studies, the effect on the expression of selected lipolytic markers after 1 day of TCDD treatment was minimal. However, it was noticed that the expression of several inflammatory response marker expressions such as COX-2, followed by IKKβ and several markers of its down-stream signaling components were upregulated. In addition, PKA was found to be significantly elevated at this early stage of action of TCDD. At the same time, the expression of C/EBPβ and C/EBPδ increased significantly at this stage. By the end of 3 days TCDD treatment (i.e., 24 h after the second addition of TCDD with medium change on post-differentiation day 2), the increased expression of several inflammation markers especially COX-2, followed by KC, IKKβ, and SOCS-3, as well as that of C/EBPβ remained significant, but others like NFκB and TNFα subsided. However, the suppressive effect of TCDD on IRS-1 mRNA expression became significant at this time. After 5 days of treatment, all of the lipolysis markers such as PPARγ, C/EBPα, LPL, Glut4, and IRS-1 showed statistically significant downregulation in accordance with our previous observation in adipose tissue exposed to TCDD in vivo [Dunlap et al., 2002]. The inflammatory marker genes COX-2, KC, IKKβ, and SOCS-3 stayed elevated significantly throughout the 5-day treatment period.
In an effort to elucidate the mechanism and the involvement of PKA through which TCDD is causing these concerted changes in mRNA expressions, we have tested the effect of exogenously added TNFα (as an inflammation inducer), H89 (an inhibitor of cAMP-dependent protein kinase or PKA), and FSK (a stimulator of cAMP and hence PKA) on adipocytes treated for 5 days with TCDD. The results in Table III showed that (a) TNFα had a more pronounced effect on the expression of the selected genes but induced a similar pattern of changes compared to TCDD and its combined effects with TCDD were synergistic in the case of Cox-2 and KC, (b) H89, under this treatment condition, generally reduced the effect of TCDD, and (c) so did FSK, except for COX-2 and KC which were significantly upregulated by FSK alone. The effect of TCDD on PPARγ and LPL remained significant under co-treatment with FSK.
| mRNA | None | TNFα | H89 | FSK | ||||
|---|---|---|---|---|---|---|---|---|
| Control | TCDD | Control | TCDD | Control | TCDD | Control | TCDD | |
| Inflammation Markers | ||||||||
| COX-2 | 1.00 ± 0.15 | 1.97 ± 0.51** | 2.84 ± 0.45aaa | 28.0 ± 1.84***,aaa | 1.07 ± 0.12 | 1.81 ± 0.05* | 15.3 ± 2.80aaa | 11.8 ± 0.29aaa |
| IKKβ | 1.00 ± 0.13 | 1.57 ± 0.34* | 0.85 ± 0.25 | 1.53 ± 0.04** | 0.82 ± 0.05a | 0.82 ± 0.05 | 0.42 ± 0.20aa | 0.49 ± 0.18a |
| KC | 1.00 ± 0.06 | 1.39 ± 0.18** | 5.98 ± 0.75aaa | 15.7 ± 0.72***,aaa | 1.24 ± 0.41 | 1.27 ± 0.49 | 1.77 ± 0.03aa | 1.22 ± 0.48 |
| TNFα | 1.00 ± 0.03 | 1.26 ± 0.27 | 3.58 ± 0.28aaa | 2.27 ± 0.15*,aaa | 1.08 ± 0.32 | 1.04 ± 0.34 | 0.54 ± 0.24aa | 0.62 ± 0.18a |
| SOCS-3 | 1.00 ± 0.09 | 1.54 ± 0.21* | 1.24 ± 0.13aa | 1.83 ± 0.28* | 1.05 ± 0.06 | 1.46 ± 0.27* | 1.27 ± 0.65 | 1.61 ± 0.48 |
| NFκB | 1.00 ± 0.02 | 1.05 ± 0.17 | 1.78 ± 0.59a | 1.45 ± 0.12a | 1.02 ± 0.34 | 1.06 ± 0.21 | 0.69 ± 0.49 | 0.46 ± 0.21a |
| Lipolytic markers | ||||||||
| C/EBPα | 1.00 ± 0.14 | 0.62 ± 0.05*** | 0.10 ± 0.01aaa | 0.11 ± 0.03aaa | 0.97 ± 0.33 | 0.98 ± 0.32 | 0.65 ± 0.26a | 0.64 ± 0.20 |
| LPL | 1.00 ± 0.02 | 0.42 ± 0.19*** | 0.41 ± 0.13aaa | 0.15 ± 0.09**,a | 0.95 ± 0.14 | 0.74 ± 0.09 | 0.35 ± 0.12aa | 0.18 ± 0.06 |
| PPARγ | 1.00 ± 0.08 | 0.82 ± 0.11** | 0.35 ± 0.09aaa | 0.18 ± 0.05***,aaa | 1.00 ± 0.02 | 1.08 ± 0.03*,a | 0.70 ± 0.03aa | 0.36 ± 0.14*,aaa |
| Glut4 | 1.00 ± 0.09 | 0.65 ± 0.23* | 0.79 ± 0.23 | 0.55 ± 0.12a | 1.35 ± 1.14a | 0.96 ± 0.34 | 0.81 ± 0.42 | 0.85 ± 0.48 |
| IRS-1 | 1.00 ± 0.04 | 0.72 ± 0.19* | 0.50 ± 0.19aaa | 0.44 ± 0.06 | 1.13 ± 0.27 | 0.90 ± 0.10 | 0.39 ± 0.13aaa | 0.31 ± 0.18 |
- 3T3 L1 adipocyte cells were treated with TCDD (10 nM), TNF-a (10 ng/ml), H89 (1 µM), and FSK (10 µM) for 5 days. The results are shown as means ± standard deviations from 3–5 independent tests. All P values were obtained through Student's-test analysis.
- Asterisk indicates the significant effect of TCDD as compared to the matched control in each agent test group (i.e., the values shown under “control” column are significantly different from the corresponding values under “TCDD” within each treatment group such as none, TNFα, H89, and FSK).
- The asterisks and superscript letter indicate the significant effect of the test agent (e.g., TNFα, H89, and FSK) against the matched “none-control” or TCDD-treated sample (e.g., the value shown under the column “TNFα-control” is significantly higher than the corresponding value shown under “none-control”, indicating a significant effect of exogenous TNFα). The number of symbols indicates that the effect of TCDD is significant at *P < 0.05, **P < 0.01, ***P < 0.001, or the effect of TNFα, H89 or FSK is significant (as compared to Non/Control or None/TCDD) at aP < 0.05, aaP < 0.01 or aaaP < 0.001, respectively.
In view of the similarity of action of TNFα to that of TCDD, we tested next the influence of SC-514, an inhibitor of IKKβ (IKB kinase), which is an indispensable signal transducer of the TNFα signaling to activate NFκB, the nuclear transcription factor (Table IV). Surprisingly, SC-514 alone increased the expression of COX-2 and IKKβ, but reduced the mRNA level of SOCS-3. The effects of TCDD were no more significant in the presence of SC-514 to change the expression of markers of inflammation or lipolysis in this 5-day treatment test. To confirm the above results we have used another inhibitor, parthenolide, which is known to block both IKKβ and NFκB. The results (Table V) showed that the effect of this compound is similar to SC-514, except for COX-2, which was also still upregulated by TCDD under co-treatment with parthenolide.
| mRNA | None | Sc-514 | ||
|---|---|---|---|---|
| Control | TCDD | Control | TCDD | |
| Inflammation Markers | ||||
| COX-2 | 1.00 ± 0.07 | 1.61 ± 0.11** | 1.53 ± 0.16aa | 1.77 ± 0.04 |
| KC | 1.00 ± 0.11 | 1.34 ± 0.06* | 0.83 ± 0.08 | 0.96 ± 0.04aa |
| IKKβ | 1.00 ± 0.05 | 1.27 ± 0.07** | 1.63 ± 0.37aa | 1.66 ± 0.07aa |
| SOCS-3 | 1.00 ± 0.13 | 1.53 ± 0.26** | 0.65 ± 0.07a | 0.73 ± 0.06aa |
| Lipolytic markers | ||||
| PPARγ | 1.00 ± 0.06 | 0.69 ± 0.07** | 0.82 ± 0.07a | 0.78 ± 0.06 |
| CEBPα | 1.00 ± 0.09 | 0.61 ± 0.08** | 0.86 ± 0.03 | 0.79 ± 0.04 |
| LPL | 1.00 ± 0.08 | 0.64 ± 0.03** | 0.73 ± 0.04a | 0.62 ± 0.04 |
| Glut4 | 1.00 ± 0.13 | 0.86 ± 0.10 | 1.01 ± 0.07 | 1.21 ± 0.08* |
| IRS-1 | 1.00 ± 0.10 | 0.79 ± 0.13* | 1.05 ± 0.11 | 1.07 ± 0.08 |
- The results are shown as means ± standard deviations from 3–5 independent tests.
- Asterisk indicates the significant effect of TCDD as compared to the matched control in each test group (i.e., within “None” or “Sc514” group).
- a Indicates the significant effect of the test agent against the matched “none” control or TCDD-treated sample (see Table III captions for the convention of the presentation of statistical symbols).
| mRNA | None | Parthenolide | ||
|---|---|---|---|---|
| Control | TCDD | Control | TCDD | |
| Inflammation Markers | ||||
| COX-2 | 1.00 ± 0.10 | 1.77 ± 0.24*** | 1.13 ± 0.11 | 1.56 ± 0.12* |
| KC | 1.00 ± 0.06 | 1.39 ± 0.17** | 0.99 ± 0.11 | 1.09 ± 0.08a |
| SOCS-3 | 1.00 ± 0.03 | 1.34 ± 0.21* | 0.64 ± 0.05aa | 0.73 ± 0.06aa |
| IKKβ | 1.00 ± 0.04 | 1.23 ± 0.12* | 0.75 ± 0.03aa | 0.82 ± 0.07aa |
| Lipolytic Markers | ||||
| PPARγ | 1.00 ± 0.13 | 0.77 ± 0.13* | 1.11 ± 0.14 | 1.06 ± 0.31 |
| CEBPα | 1.00 ± 0.06 | 0.65 ± 0.08** | 1.22 ± 0.12 | 1.21 ± 0.15aa |
| LPL | 1.00 ± 0.04 | 0.72 ± 0.09** | 1.12 ± 0.06 | 1.07 ± 0.03aa |
| Glut4 | 1.00 ± 0.03 | 0.86 ± 0.05* | 0.79 ± 0.15 | 0.87 ± 0.11 |
| IRS-1 | 1.00 ± 0.08 | 0.75 ± 0.03** | 0.88 ± 0.06 | 0.81 ± 0.09 |
- On the expression of inflammation and lipolysis markers by 3T3-L1 adipocytes. Cells were treated with TCDD (10 nM) and parthenolide (10 µM) for 5 days.
- Asterisk indicates the significant effect of TCDD as compared to the matched control in each agent test group.
- a Indicates the significant effect of the test agent against the matched “none-control” or TCDD-treated sample (see Table III captions for the convention of the presentation of statistical symbols).
One interesting observation is that TCDD consistently caused upregulation of SOCS-3 at all time points (Tables II–V). The increase in SOCS-3 protein/function has been implicated to the phenomenon of insulin resistance particularly that is induced by increased TNFα production as seen in obesity. In view of the similarity between the action of TCDD and TNFα in this regard, we conducted a 3H-Me-glu uptake assay according to the protocol of Frost and Lane [1985]. The results summarized in Figure 1 show that both TNFα (Fig. 1A) and TCDD (Fig. 1B) treatments reduced the insulin response of 3T3-L1 adipocytes to stimulate 3H-Me-glu uptake, so that in their presence, the stimulation action of insulin became statistically insignificant. In contrast to TCDD, TNFα also reduced the non-stimulated glucose uptake.
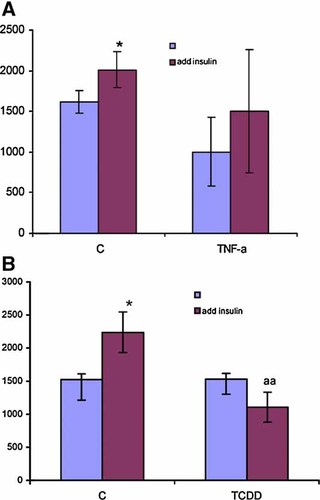
Assessment of “Insulin-resistance” induced by TNFα (A) or TCDD (B) in 3T3-L1 adipocytes, using 3H-labeled 3-Me-glucose uptake (shown on the Y axis in 3H DPM) assay in the presence (right column) and the absence (left column) of insulin. 3T3-L1 adipocytes were treated with TCDD (10 nM) and TNFα (10ng/ml) for 3 days. The results are shown as means ± standard deviations from three independent tests. * and a indicate the significant effect of insulin as compared to the non-insulin in each control group and agent (TNFα or TCDD) test group, respectively. The number of symbols indicates: one, P < 0.05, two <0.01, respectively. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Next, an effort was made to understand the mechanism through which TCDD causes these changes in 3T3-L1 adipocytes, by conducting electrophoretic gel mobility shift assays (EMSA). In the first test we asked the question which of the nucleotide sequences representing the C/EBP binding region of the promoter from GLUT4 or COX-2 would be more responsive to both TCDD and TNFα. Results from EMSA (Fig. 2A) testing DNA binding activities of nuclear proteins from 3T3 adipocytes treated with TCDD, TNFα, and parthenolide for 5 days (Fig. 2A) clearly indicate that the C/EBP binding sequence from the COX-2 gene promoter is a better choice for this purpose. The C/EBP binding activity was increased more by TCDD than by TNFα treatment at day 5 and this effect of TCDD was significantly reduced by parthenolide. As expected, TCDD treatment increased protein binding also to the dioxin response element (DRE) at day 5 of treatment. Next we examined the effects of the same agents at an earlier time point at 24 h on protein binding to the C/EBP response element from the COX-2 promoter (Fig. 2B). In contrast to day 5, the results showed (Fig. 2B) that the effect of TCDD is less pronounced at day 1 compared to effect of TNFα. Nevertheless, judging by the intensity of the bands remaining after supershifting C/EBPβ proteins, it was deduced that the action of TCDD likely results in a decrease of the binding action of lower molecular weight C/EBP isoforms (non-beta isoforms). Together with supershift analysis of the 5-day treated samples (Fig. 2C) the results indicate that the effect of TCDD is more pronounced and longer lasting than that of TNFα. Parthenolide clearly reduced the action of TCDD to increase C/EBP protein binding to this site. Furthermore, the results of supershift assay showed that C/EBPβ is the main type of protein bound to this site.
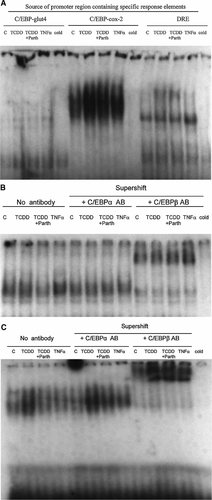
A: EMSA (gel mobility shift assay) on binding of nuclear proteins from 3T3-L1 adipocytes treated with TCDD, parthenolide (Parth), and TNFα to the C/EBP response elements on Glut4 and Cox-2 gene promoters, and to the consensus DRE. Nuclear protein extracts were prepared from 7-day differentiated 3T3 L1 adipocytes treated with TCDD (10 nM), TNFα (10 ng/ml), and parthenolide (Parth) (10 µM) for 5 days. B: EMSA and supershift assay on nuclear protein binding activity to the C/EBP response element from the promoter of Cox-2 gene. Nuclear protein extracts were prepared from 7-day differentiated 3T3 L1 adipocytes treated with TCDD (10 nM), TNFα (10 ng/ml), and parthenolide (Parth) (10 µM) for 24 h. C: EMSA and supershift assay study with specific antibodies (AB) against C/EBPα and C/EBPβ on the binding of nuclear proteins from TCDD-, parthenolide (Parth)-, and TNFα-treated 3T3-L1 adipocytes to the C/EBP response element of the promoter of Cox-2 gene. Nuclear protein extracts were prepared from 7-day differentiated 3T3 L1 adipocytes treated with TCDD (10 nM), TNFα (10 ng/ml), and parthenolide (Parth) (10 µM) for 5 days.
We then studied the effect of these same agents on protein binding to the specific NFκB response element. At an early stage (24 h), the stimulatory effect of TNFα on this nuclear transcription factor was more pronounced than that of TCDD (Fig. 3A). As a consequence of the weak action of TCDD at this early stage of its action the effect of parthenolide on the action of TCDD to protein binding to this NFκB site was not apparent (Fig. 3A). In contrast, by day 5 the difference between TCDD and TNFα narrowed considerably, indicating again that TCDD is a slow activator of NFκB as compared to TNFα (Fig. 3B). Such a significant action of TCDD on protein binding to the NFκB site also made it easy to recognize the suppressive action of parthenolide at this late stage of action of TCDD. We examined the same nuclear protein samples by using supershift assays with either an antibody against p65 (=RelA) or that against the AhR. The results showed that p65 is the main protein of the upper complex binding to the NFκB consensus site and that the AhR protein is a component of the lower complex. To test whether binding of p50 proteins is involved in binding of nuclear proteins from 3T3-L1 adipocytes to NFκB site, we also run a supershift assay using an antibody against p50 (Fig. 3C). The result shows that this antibody specifically shifted the proteins in the lower band position and to some extent of the upper complex, which is likely consisting of p65/p50 heterodimers. Parthenolide appears to selectively reduce the effect of TCDD on p50 and p65 (Fig. 3B and C). By comparing the results of p50 supershift to that of p65 (i.e., Fig. 3B vs. C), we could determine that the ratio of p65 to p50 of actively binding proteins was higher in TNFα-treated cells than in the corresponding TCDD. This result indicates the clear effect of TNFα to activate RelA via the classical activation pathway of NFκB. Finally we assessed the level of the AhR expression in 7-day differentiated 3T3-L1 adipocytes (i.e., the study material we used in this study) in comparison to that expressed in the corresponding undifferentiated fibroblasts using qRT-PCR in view of a report on its virtual absence in fully matured 3T3-L1 adipocytes [Shimba et al., 2001]. The result showed that under our experimental condition the 7-day differentiated adipocyte preparation showed approximately 38% of AhR compared to the level found in the original culture of fibroblasts (Fig. 4).
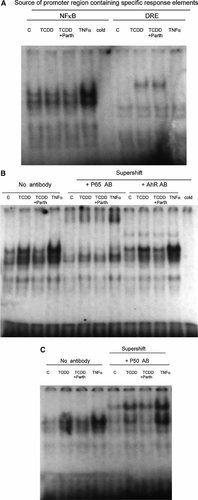
A: EMSA study on the effect of TCDD, parthenolide (Parth), and TNFα on binding of nuclear proteins from treated 3T3-L1 adipocytes to the consensus NFκB response element or DRE. Nuclear protein extracts were prepared from 7-day differentiated 3T3 L1 adipocytes treated with TCDD (10 nM), TNFα (10 ng/ml), and parthenolide (Parth) (10 µM) for 24 h. B: EMSA and supershift assay with specific antibodies against P65 (an NFκB protein) and AhR to study binding of nuclear proteins extracted from treated 3T3-L1 adipocytes to the consensus NFκB response element. Nuclear protein extracts were prepared from 7-day differentiated 3T3 L1 adipocytes treated with TCDD (10 nM), TNFα (10 ng/ml), and parthenolide (Parth) (10 µM) for 5 days. C: EMSA-supershift assay study with a specific antibody against P50 NFκB protein on the binding of nuclear proteins from treated 3T3-L1 adipocytes to the consensus NFκB response element. Nuclear protein extracts were prepared from 7-day differentiated 3T3 L1 adipocytes treated with TCDD (10 nM), TNFα (10 ng/ml), and parthenolide (Parth) (10 µM) for 5 days.
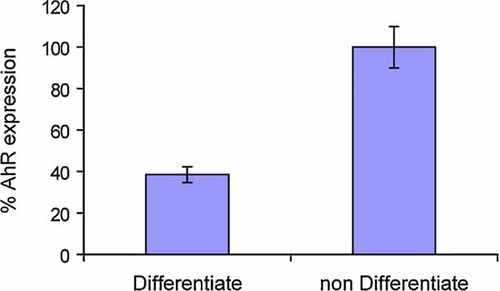
Quantitative RT-PCR analysis on mRNA expression of AhR in 7-day differentiated or non-differentiated (fibroblasts) 3T3 L1 cells. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
In the current study, we have established that mature 3T3-L1 adipocytes could be made to show lipolytic responses to exogenously added TCDD by selecting specific treatment conditions. The first question we must address is why these conditions are effective in inducing this response, while our previous efforts failed. The main reason considered previously to be the cause for the loss in TCDD sensitivity is the decline of the AhR expression in mature 3T3-L1 adipocytes [Shimba et al., 1998]. However, the level of expression of the AhR at day 7 of the DIX-induced differentiation, under our test condition, is not zero. As shown in Figure 4, the level of expression of AhR at 7-day differentiated 3T3 adipocytes is 38% of non-differentiated control. Therefore, the selection of this timing of adipocyte differentiation for TCDD treatment may have been important. Second, the importance of a series of medium change at 2 day intervals following the day 7 differentiation using insulin-free 10% FBS–DMEM must have also been important, since we have noted that without this medium changing process, the lipolytic effect of TCDD was not significant. The fact that it took 5 days of post-differentiation treatment to manifest lipolytic responses supports may suggest that, in addition to the basic nature of TCDD to act slowly, some step-wise sequence of events following the initial activation of inflammatory responses take place during this time period, which may have been assisted by those medium changes. Finally, the addition of TCDD at the time of each medium change appears to help this action of TCDD which suggest a direct action of TCDD to maintain a sustained increase of inflammatory markers such as SOCS-3 and COX-2. It must be pointed out that none of the above treatments is considered to be artificial manipulation of in vitro cultured adipocytes or unusual treatments with TCDD as there are precedents for those culturing and TCDD treatment schemes in the open literature. What we have done is to select the right sequence and timing of these treatments.
It is important to point out that despite the low expression of the AhR in mature 3T3-L1 adipocytes is not totally refractory to other types of lipolysis inducers. For instance, it has been shown to respond to the lipolytic action of PKA inducers [Kaestner et al., 1991] as well as TNFα (Table III), indicating that they are not terminally committed. In this regard, the observation that the actions of TCDD to induce PKA as well as inflammation, particularly that is mediated by COX-2 and the TNFα-IKKβ-NFκB axis are already significantly expressed at day 1 of treatment (Table II). These effects of TCDD were accompanied by significant upregulation of both C/EBPβ and C/EBPδ on day 1 of treatment, agreeing with the observation made in mouse adipose tissues exposed to TCDD in vivo [Liu et al., 1998]. However, the effect of TCDD to downregulate PPARγ did not take place until day 5, unlike the case observed in vivo. This observation points to the resiliency of 3T3-L1 adipocytes against induced lipolysis due probably to their expression of a high titer of C/EBPα and low titers of C/EBPβ and C/EBPδ after 7–8 days of differentiation (e.g., MacDougald and Lane, 1995].
As for the relative importance of the contribution of the inflammatory responses versus PKA activation as the main cause for this action of TCDD, it appears that both are important as judged by the data summarized in Table III. The activation of COX-2, which lasts throughout the 5 day treatment period (Table II), is highly responsive to the stimulatory action of FSK (see FSK-treated control sample, Table III), and yet in its presence, TCDD-induced downregulation of PPARγ as well as LPL is even more pronounced. In a similar manner, H89, a specific inhibitor of PKA, could not totally abrogate the action of TCDD to increase COX-2 or SOCS-3. Judging by the observation that TNFα alone could upregulate COX-2 and other inflammation markers and at the same time downregulate most of the lipolytic markers (see TNFα-treated control values, Table III), the effect of inflammation is likely to be also prominent in the action of TCDD, although the importance of PKA should not be underestimated. The above reasoning has led us to test the effect of parthenolide, a naturally occurring substance which is well known for its inhibitory action on the inflammatory responses of cells through blocking the activation of the TNFα–IKKβ–NFκB axis. The results of a series of EMSA studies have shown that parthenolide is indeed effective in suppressing the action of TCDD to activate NFκB and reducing the activity of the C/EBP protein binding to the binding site of the COX-2 promoter (Figs. 2 and 3). COX-2 has been shown to impair adipocyte differentiation [Fajas et al., 2003; Petersen et al., 2003] and has been implicated in body fat regulation [Fain et al., 2001; Yan et al., 2003]. These reports confirm our notion that inflammatory responses such as COX-2 induced by TCDD are likely to be the primary cause for the lipolytic action of TCDD in this model system. In this regard the finding by Kern et al. [2002], that TCDD induced the production of TNFα in F442A adipocytes was instrumental in coming up with our experimental design (e.g., test on the effects of exogenous TNFα, Table III). Indeed there is a good possibility that induction of TNFα plays a significant role in the lipolytic action of TCDD in 3T3-L1 adipocytes as well, particularly in inducing inflammation at its early stage of action. Our observation that the expression of TNFα as well as NFκB increase after 24 h of action of TCDD (Table II) attests to this view. On the other hand the action pattern of TCDD is not totally identical to that of TNFα alone, particularly at later stages of their actions (Table III). While both agents clearly increased the expression of Cox-2 and KC, and their actions were synergistic, not all of the markers followed this pattern (e.g., see IKKβ expression, Table III). Thus, judging by the results of this study (e.g., Tables II and III and Figs. 2 and 3), the likely sequence of events taking place is that TNFα plays an important role in triggering the cascade of events by evoking adipocyte responses to activate the axis of NFκB-mediate inflammatory signaling, which is gradually supplemented by the action of TCDD to set a more permanent arrangement to induce and maintain the chronic lipolytic status. However, much more work would be needed to elucidate the actual mechanism through which TCDD causes such late changes in adipocyte responses to induce persistent lipolysis in this model.
Finally, we have noted that this action of TCDD is accompanied with significant upregulation of SOCS-3 and downregulation of IRS-1 mRNA expressions (Table II). Since these are the changes known to be involved in the development of insulin resistance [Olsen et al., 1998; Nagashima and Matsumura, 2002], we examined the effect of TCDD on insulin-stimulated glucose uptake according to the method developed by Frost and Lane [1985]. The result showed that indeed cells treated with TCDD lose insulin sensitivity with respect to their glucose uptake activities.
In conclusion, we have clearly established that under the right cell culture and treatment condition, it is possible to use 3T3-L1 adipocytes as a model system to study the molecular mechanism of lipolytic action of TCDD. This finding is significant in view of the prospect of using this well-studied cell material for the purpose of future investigations of the mechanisms of diabetogenic action of TCDD, which has been shown to occur in human populations exposed to TCDD by a number of epidemiological studies [Henriksen et al. 1997].
Acknowledgements
Supported by research grants R01-ES05233 and P01-ES05707 from the National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina.




