Phenol red reduces ROSC mediated cell cycle arrest and apoptosis in human MCF-7 cells
Abstract
We reported recently that roscovitine (ROSC), a selective cyclin-dependent kinase (CDK) inhibitor, arrested human MCF-7 breast cancer cells in G2 phase of the cell cycle and concomitantly induced apoptosis. On the other hand, ROSC-induced G1 arrest observed by another group has not been accompanied by apoptosis. Therefore, we decided to prove to which extent components of tissue culture media could affect the primary action of ROSC. For this purpose we compared the efficacy of the ROSC treatment on MCF-7 cells cultivated in medium with and without phenol red. The kinetics of MCF-7 cell proliferation strongly depended on the presence of phenol red that has been recognized previously as a weak estrogen. Exposure of MCF-7 cells cultivated in phenol red-deprived medium to ROSC resulted in a strong G2 arrest and apoptosis. However, the anti-proliferative and pro-apoptotic action of ROSC was strongly diminished in cells maintained in medium containing phenol red. The ratio of the G2 cell population after 12 h ROSC was reduced by approximately 20% in the latter and correlated with the lack of CDK2 inactivation. Moreover, the kinetics of ROSC-induced apoptosis was delayed in the presence of phenol red. These results clearly evidence that the efficacy of the therapy of ER-positive breast cancers by CDK inhibitors is diminished in the presence of estrogen-mimicking compounds and indicate that phytoestrogens and xenoestrogens could interfere with the therapy. Therefore, the exposure of cancer patients to the estrogen mimics should be avoided at least during chemotherapy by CDK inhibitors. J. Cell. Biochem. © 2006 Wiley-Liss, Inc.
Abbreviations used:
AIF, apoptosis inducing factor; CAD, caspase activated DNase; CDKI, cyclin-dependent kinase inhibitor; CDK, cyclin-dependent kinase; MDM-2, mouse double minute-2; MCM7, minichromosome maintenance 7; p53AIP-1, p53 apoptosis inducing protein 1; PARP-1, poly(ADP-ribose) polymerase-1; PCNA, proliferating cell nuclear antigen; PD, Petri dish; PVDF, polyvinylidene difluoride; ROSC, roscovitine; WCL, whole cell lysate; WT, wild-type.
The proper control of the cell cycle progression is a prerequisite for growth and, if necessary, growth arrest of normal cells [Nurse, 1997]. Escape from the correct cell cycle regulation leads to malignant transformation [Vogelstein and Kinzler, 2004].
The malignant transformation of cells that occurs during the development of cancer [Hannah and Weinberg, 2000; Vogelstein and Kinzler, 2004; Blagosklonny, 2005] is characterized by inactivating mutations or alterations in the expression and activity of proteins involved in the proper regulation of the cell cycle and disturbances in the induction of apoptosis. Unfortunately, most changes that lead to an accelerated growth of a cell establish a selective evolutionary advantage for this particular cell. Therefore, a tight control of cellular growth and susceptibility of cells to respond to proapoptotic stimuli is of utmost importance for multicellular organisms to prevent the uncontrolled growth of mutated cells. For this purpose, cells have acquired a fine-tuned system of control mechanisms such as tumor suppressor genes and endogenous inhibitors of cyclin-dependent kinases (CDKs) that watch the cell cycle [Sherr and Roberts, 1999].
The p53 protein, a product of a tumor suppressor gene is a key molecule controlling the cell cycle machinery and apoptosis and is therefore a good target for anti-cancer therapy [for reviews, see Baronetti and Manfredi, 2002; Blagosklonny, 2002; Wesierska-Gadek et al., 2005a]. It has been recognized that the (re)activation of wt p53 in cancer cells may substantially contribute to the outcome of the chemotherapy [Wesierska-Gadek et al., 2002]. Wt p53, usually maintained in cells at low levels, is upregulated by a variety of anti-cancer drugs what is most frequently attributable to DNA damage generated by these agents [Baronetti and Manfredi, 2002; Blagosklonny, 2002]. Interestingly, a number of newly developed inhibitors of cyclin dependent kinases such as flavopiridol, UCN-01, or substituted purines, additionally to the CDK2 inhibition also positively affect the levels of p53 protein in cancer cells expressing wt p53 [David-Pfeuty, 1999; David-Pfeuty et al., 2001; Kotala et al., 2001; Blagosklonny et al., 2002a,b; Wojciechowski et al., 2003a; Wesierska-Gadek and Schmid, 2006]. Interestingly, substituted purines, for example roscovitine (ROSC) and olomoucine, mimicking the ATP molecule [De Azevedo et al., 1997; Meijer et al., 1997; Gray et al., 1999] exert very low, if any, direct cytoxicity and they activate p53 protein in a way independent of DNA damage [Ljungman and Paulsen, 2001; Lu et al., 2001]. ROSC is a very efficient drug towards human MCF-7 breast cancer cells and it inhibits proliferation of MCF-7 cells at lower concentrations than cisplatin [Wesierska Gadek et al., 2003]. ROSC arrested naive, asynchronously growing MCF-7 cells in the G2 phase of the cell cycle [Wojciechowski et al., 2003a; Wesierska Gadek et al., 2005], whereas the cells released from nocodazole-mediated mitotic block, were accumulated in G1 phase [David-Pfeuty, 1999; David-Pfeuty et al., 2001]. Moreover, longer exposure to ROSC resulted in the induction of apoptosis [Wojciechowski et al., 2003a; Wesierska Gadek et al., 2005]. Surprisingly, the induction of apoptosis occurred after exposure of asynchronously growing MCF-7 cells to ROSC treatment but not in cells released from nocodazole-mediated mitotic block [David-Pfeuty, 1999; David-Pfeuty et al., 2001]. Careful comparison of the experimental conditions revealed that the latter we cultivated in conventional medium. It raised the question whether the media components themselves could modulate the anti-proliferative and/or pro-apoptotic action of ROSC on MCF-7 cells.
In the present paper we addressed the question whether the action of ROSC may depend on the presence of phenol red in the culture medium. As previously reported [Berthois et al., 1986], phenol red promoted the proliferation of MCF-7 cells. However, within the first 24 h after cell plating the difference of the growth kinetics was relatively low implicating that this time window would be suitable for experiments designed to evaluate the efficacy of the therapy of the breast cancer cells by CDK inhibitors. Interestingly, ROSC action was negatively affected by supplementation of the medium with phenol red. ROSC inhibited proliferation of breast cancer cells more efficiently when cultivated in phenol red-deprived medium. In concordance with our previous observations, exposure of MCF-7 cells to ROSC resulted in a G2 arrest. However, the kinetics and the extent of the G2 block strongly depended on the content of the medium. Approximately 50% of cells cultivated in phenol red-deprived medium were G2 arrested after exposure to ROSC for 12 h, whereas only 30% cells cultivated in medium supplemented with phenol red was accumulated in G2. The clearly weaker effect of ROSC on the cell cycle in cells cultivated in the presence of phenol red coincided with the lack of CDK2 inhibition. Moreover, the number of cells undergoing apoptosis after longer exposure to ROSC was significantly reduced, if cells were cultivated in phenol red-supplemented medium. These results evidence that phenol red modulates the therapeutic effect of ROSC on MCF-7 cells by accelerating their proliferation and by reducing their susceptibility to proapoptotic stimuli.
MATERIALS AND METHODS
Cells
Human MCF-7 breast carcinoma cells were grown as a monolayer in Dulbecco's medium with and without phenol red supplemented with 10% FCS at 37°C in an atmosphere of 8% CO2 [Wojciechowski et al., 2003a; Wesierska Gadek et al., 2005]. Cells were grown up to 60% confluence and then treated with ROSC at a final concentration ranging from 1–40 µM for indicated periods of time. ROSC was dissolved as a stock solution in DMSO and stored at −20°C until used.
Antibodies
We used the following antibodies: monoclonal anti-p53 antibody DO-1, a kind gift from Dr. B. Vojtesek (Masaryk Memorial Cancer Institute, Brno). The monoclonal anti-AIF antibody (E-1) and polyclonal anti-NF-κB (p65) antibody were from Santa Cruz Biotechnology (Santa Cruz, CA), monoclonal anti-MCM7 (clone DCS141.2) and anti-PCNA (PC-10) antibodies were from Oncogene Research Products (Cambridge, MA). Polyclonal anti-phospho-Thr14/Tyr15 CDK1, anti-phospho-Thr160 CDK2, anti-phospho-Ser46-p53, anti-phospho-Ser83 Myt1 antibodies and corresponding antibodies against the total antigen were from New England Biolabs (Beverly, MA). Rat monoclonal antibodies against APAF-1 (clone 2E12) and polyclonal anti-CAD (DFF40) were from Chemicon International, Inc. (Temecula, CA). Polyclonal anti-HtrA2/Omi antibodies were from R&D Systems, Inc. (Minneapolis, MN). Monoclonal anti-actin (Clone C4) antibodies were from ICN Biochemicals (Aurora, OH). Appropriate secondary antibodies linked to horseradish peroxidase (HRP) were from Amersham International (Little Chalfont, Buckinghamshire, UK).
Determination of the Number of Viable Cells
Proliferation of human MCF-7 breast cancer cells and their sensitivity to increasing concentrations of ROSC was determined by the CellTiter-Glo™ Luminescent Cell Viability Assay (Promega Corporation, Madison, WI). As described recently in more detail [Wojciechowski et al., 2003b; Wesierska-Gadek et al., 2005b], the CellTiter-Glo™ Luminescent Cell Viability Assay generating luminescent signals is based on quantification of the cellular ATP levels. Tests were performed at least in quadruplicates. Luminescence was measured in the Wallac 1420 Victor, a multilabel, multitask plate counter. Each point represents the mean ± SD (bars) of replicates from one representative experiment.
Measurement of DNA of Single Cells by Flow Cytometry
The measurement of DNA content was performed by flow cytometric analysis based on a slightly modified method [Wesierska-Gadek and Schmid, 2000] described previously by Vindelov et al. 1983. The cells were detached from substratum by trypsinization, then all cells were harvested by centrifugation and washed in PBS. Aliquots of 1 × 106 cells were used for further analysis. Cells were stained with propidium iodide as described previously and then the fluorescence was measured using the Becton Dickinson FACScan after at least 2 h incubation at +4°C in the dark.
Quantitative Analysis of the Mitochondrial Membrane Potential by Flow Cytometry
Mitochondrial depolarization was monitored using the cationic carbocyanine dye JC-1 (Molecular Probes Inc., Eugene, OR). Control and ROSC treated cells were trypsinized, washed and incubated with the dye at a final concentration of 10 µM for 15 min followed by extensive washing in PBS and immediate two-color analysis under fluorescence microscopy and by FACS [Kovar et al., 2000]. Alternatively, cells in Petri dishes were PBS-washed and directly incubated with JC-1. After washing samples were inspected under fluorescence microscopy using a band-pass filter (detects fluorescein and rhodamine). JC-1 exists as red-fluorescent aggregate (excitation or emission at 488 or 570 nm, respectively) in intact cells due to potential-driven mitochondrial accumulation and aggregation. Upon mitochondrial depolarization it occurs as green-fluorescent monomer (excitation or emission at 488 or 530 nm, respectively) [Reers et al., 1991].
Subcellular Fractionation of Cells
In experiments designed to examine the release of distinct proteins from mitochondria during the execution of apoptosis, a buffer containing 250 mM sucrose and a low concentration of digitonin was used for cell fractionation [Fiskum et al., 2000].
Electrophoretic Separation of Proteins and Immunblotting
Total cellular proteins or proteins of the distinct subcellular fractions dissolved in SDS sample buffer were separated on 10 or 15% SDS slab gels, transferred electrophoretically onto polyvinylidene difluoride membrane (PVDF) (Amersham International, Little Chalfont, Buckinghamshire, England) and immunoblotted as previously described [Wesierska-Gadek et al., 2000, 2002]. Equal protein loading was confirmed by Ponceau S staining. To determine the phosphorylation status of selected proteins, antibodies recognizing site specifically phosphorylated proteins were diluted to a final concentration of 1:1,000 in 1% BSA in Tris-saline-Tween-20 (TST) buffer [Wesierska-Gadek et al., 2004]. In some cases, blots were used for sequential incubations.
RESULTS
Phenol Red Promotes the Proliferation of Asynchronously Growing MCF-7 Cells
In the first step we determined the effect of phenol red on the proliferation of MCF-7 cells at concentrations routinely used in Dulbecco's medium. The cells (8 × 104/well) were plated into 6-well microtiter plates in medium with and without phenol red and the number of viable cells was measured in 12 h intervals. After cultivation for 24 h the number of viable cells increased 3.5- and 2.8-fold in medium with or without phenol red, respectively (Fig. 1A). After longer cultivation of MCF-7 cells (60 h) the difference resulting from the phenol red content became more pronounced. These results showed not only the promoting effect of phenol red on the proliferation of MCF-7 cells, but also revealed that within the first 24 h after cell plating, the difference in the proliferation kinetics is negligible and this time window is suitable for comparative investigations of the status of the cell cycle parameters.
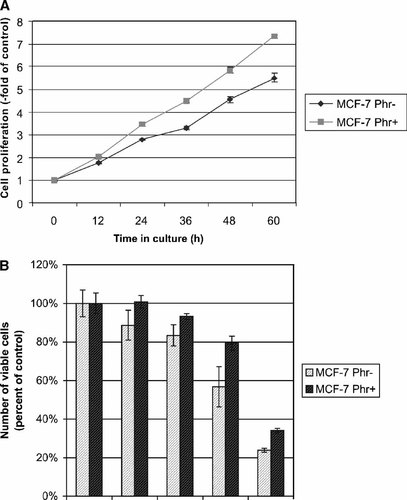
Phenol red diminishes the ROSC-mediated mitotic block. A: Phenol red increases the rate of the proliferation of MCF-7 cells. Human MCF-7 breast cancer cells growing in normal or phenol-deprived medium were plated in 6-well microtiter plates (8 × 104 cells/well) and the number of viable cells was determined in 12 h intervals by the CellTiterLumiGlo Assay (Promega). A representative experiment is shown. Each value represents the mean of nine replicates ± SD. B: ROSC more strongly inhibits the proliferation of human MCF-7 cells cultivated in phenol red-deprived medium. MCF-7 cells were plated in 96-well microtiter plates (5 × 103 cells/well) and the number of viable cells was determined after treatment for 24 h by the CellTiterLumiGlo Assay (Promega). A representative experiment is shown. Each value represents the mean of four replicates ± SD.
A Lower ROSC Dose is Sufficient to Inhibit the Proliferation of MCF-7 Cells Cultivated in Phenol Red-Deprived Medium
The continuous exposure of MCF-7 cells to ROSC resulted in the reduction of the numbers of viable cells in a time- and concentration-dependent manner (Fig. 1B). However, a higher ROSC dose was necessary to reduce the number of viable cells by 50%, if cells were cultivated in medium supplemented with phenol red (IC50 = 12.4 and 20.5 µM, respectively).
The ROSC-Mediated G2 Arrest is Markedly Reduced in Cells Cultivated in Phenol Red-Supplemented Medium
As previously shown, continuous exposure of MCF-7 cells cultivated in phenol red-deprived medium to 20 µM ROSC resulted in a rapid accumulation of G2 arrested cells (Fig. 2A and B). After 12 h approximately 50% of the cell population accumulated in G2/M phase. In phenol red-supplemented medium the frequency of G2 arrested cells was reduced by approximately 20%. The comparison of the G2/S ratio showed a great difference (Fig. 2C).
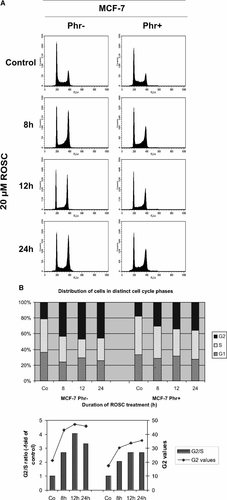
Phenol red reduces the ROSC-mediated G2 arrest of MCF-7 cells. Cells cultivated in normal and phenol red-deprived medium were exposed to 20 µM ROSC for indicated periods of time, harvested and stained with propidium iodide. DNA histograms depicting a representative experiment performed in duplicate were prepared using the ModFIT software. Comparison of the ROSC effect on the distribution of cells in distinct cell cycle phases. Upper panel: Values of cells in distinct cell cycle phases. Lower panel: Bars: G2/S ratio. Values calculated for each sample were normalized to the control; Curve: Percentage of cells arrested in G2.
Persistence of Activating Phosphorylation of CDK2 at Thr160 in Cells Cultivated in Phenol Red-Supplemented Medium Despite Exposure to ROSC
The activity of cyclin/CDK complexes is regulated by phosphorylation of the threonine residue localized in the catalytic pocket. As expected, ROSC inhibited the phosphorylation of threonine 160 of CDK2 in MCF-7 cells cultivated in phenol red-deprived medium (Fig. 3). Surprisingly, phenol red supplementation reduced the ROSC mediated CDK2 inactivation (Fig. 3). This result being in concordance with the DNA profiles obtained by flow cytometry explains the mechanisms of the lower efficacy of ROSC in the latter. Interestingly, Myt1, another cell cycle-related protein was stronger modified in MCF-7 cells cultivated in the presence of phenol red (Fig. 4). Phosphorylation of Myt1 kinase at serine 83 is necessary for its activation. Exposure of MCF-7 cells to ROSC also affected the cellular levels of cyclin D3 (Fig. 3). Consistently with our previous observations, expression of cyclin D3 was down-regulated upon ROSC treatment of MCF-7 cells [Wojciechowski et al., 2003a]. Also, other pharmacological inhibitors of CDKs negatively affected cyclin D expression [Carlson et al., 1999]. Moreover, the basal levels of cyclin D3 and PCNA were markedly reduced in MCF-7 cells cultivated in phenol-red deprived medium (Fig. 3). To examine whether the proliferative state of MCF-7 cells has any effect on the basal cyclin D3 level, we compared the expression of cyclin D3 in exponentially growing and confluent MCF-7 cells. Interestingly, changes of the cyclin D3 levels that were linked to the proliferative status, were observed only in cells cultivated in medium with phenol red (data not shown). Higher cyclin D3 expression was detected in confluent MCF-7 cells.
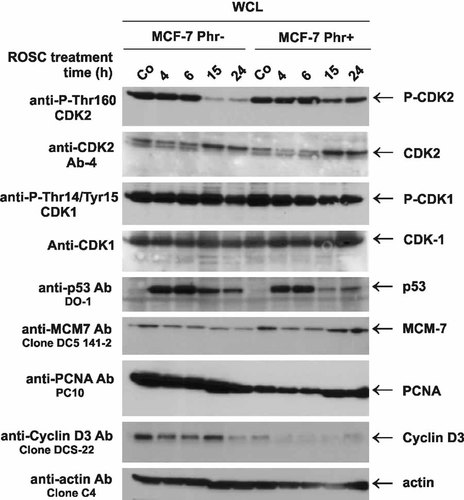
ROSC inhibits the activating, site-specific phosphorylation of CDK2 to a lesser extent in the presence of phenol red. Whole cell extracts (WCLs) prepared from human MCF-7 control cells and cells exposed to 20 µM ROSC were separated on 10 and 12% SDS gels (30 µg/lane) and transferred onto a PVDF membrane. The blots were incubated with indicated primary antibodies in the appropriate concentration. After incubation with secondary antibodies linked to HRP, immune complexes were detected by chemiluminescence using the ECL+ detection system. Equal protein loading was confirmed by Ponceau S staining of the membrane and, additionally, by incubation with anti-actin antibodies.
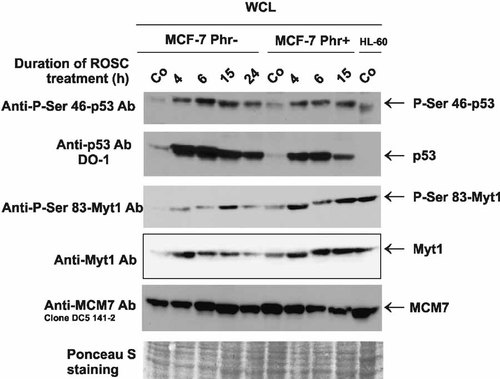
ROSC-mediated activation of wt p53 protein is diminished in MCF-7 cells cultivated in the presence of phenol red. Total cellular proteins (WCLs) (30 µg/lane) were loaded on 10% SDS-gels and electroblotted. The site-specific phosphorylation of p53 was determined using affinity-purified anti-P-Ser46-p53 antibodies. Total p53 protein irrespective of modification was detected after incubation with monoclonal anti-p53 DO-1 antibodies. The site-specific phosphorylation of Myt1 was determined on twin blots using affinity-purified anti-P-Ser83-Myt1 antibodies and sequentially total Myt1 protein irrespective of modification was detected after incubation with monoclonal anti-Myt1 antibodies.
Stronger Activation of wt p53 Protein in Cells Cultivated in Phenol Red-Deprived Medium
As predicted, ROSC strongly activated wt p53 protein in MCF-7 cells. However, the extent and kinetics of p53 upregulation differed depending on the presence of phenol red in the medium. ROSC induced specific phosphorylation of p53 protein at serine 46 (Fig. 4). Stronger site-specific phosphorylation of p53 was observed in the absence of phenol red in medium. Moreover, an increase of the p53 level was diminished in cells cultivated in medium containing phenol red (Fig. 4) and after exposure of cells to ROSC for 24 h p53 protein declined to a barely detectable level.
The Presence of Phenol Red in the Medium Abolishes the Pro-Apoptotic Action of ROSC
Additionally to the cell cycle block, ROSC induces apoptosis in MCF-7 cells by changing the potential of the mitochondrial membrane and by release of mitochondrial proteins into the cytosol. To prove the effect of phenol red on the kinetics of the dissipation of the mitochondrial membrane, we determined the capability of mitochondria to form J-aggregates and the accumulation of mitochondrial protein in the cytosol under both conditions (Fig. 5). As previously shown, mitochondria of MCF-7 cells cultivated in phenol red-free medium lost the capability to form J-aggregates upon exposure to ROSC. Supplementation of tissue medium with phenol red diminished and delayed the depolarization of mitochondrial membrane (not shown). These observations were substantiated by the analysis of subcellular fractions prepared according to the procedure described by Fiskum et al. 2000. As shown in Figure 5, apoptosis inducing factor (AIF) was released into the cytosol already after 6 h of ROSC treatment in cells maintained in phenol red-deprived medium. In cells cultivated in phenol red-supplemented medium, the onset of release of mitochondrial proteins was markedly delayed. A first weak AIF signal was detected in the cytosol after 15 h ROSC treatment and it reached the intensity comparable with that observed in cells maintained in phenol red-deprived medium at 24 h post-treatment. This finding correlated with the early activation of caspase-activated DNase (CAD) in MCF-7 cells in Phr-medium. As expected a CAD signal was detected in the cytosol obtained from control cells. After exposure of MCF-7 cells maintained in phenol red-deprived medium to ROSC, CAD disappeared from the cytosol in a time-dependent manner. This coincided with the appearance of a CAD signal in nuclei. Within the first 6 h the intensity of the signal in the cytosol was reduced and at 15 and 24 h post-treatment the protein signal was found in the isolated nuclei (Fig. 5). In cells cultivated in medium with phenol-red CAD was detectable solely in the cytosol and was found even at 15 h post-treatment. On the other hand APAF-1 protein was detectable in cytosol after longer treatment time thereby substantiating the specificity of CAD translocation.
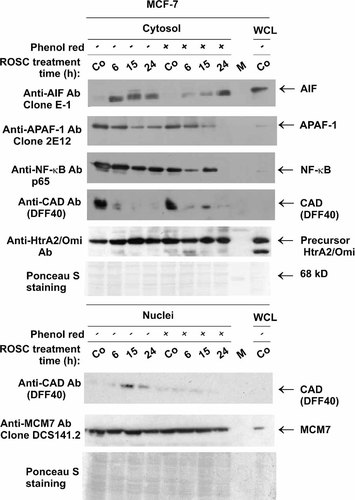
Dissipation of mitochondrial membrane and release of proteins into the cytosol. Proteins of the cytosol (S-100 fraction) isolated according to Fiskum et al. 2000 and proteins of nuclei prepared from untreated controls and cells treated with 20 µM ROSC for indicated periods of time were resolved on 10 or 15% SDS gels and transferred onto a PVDF membrane. The blots were incubated with indicated primary antibodies in the appropriate concentration. After incubation with secondary antibodies linked to HRP, immune complexes were detected by chemiluminescence using ECL+. Equal protein loading was confirmed by Ponceau S staining of the membrane and, additionally, by incubation with NF-κB antibodies and HtrA2/Omi for cytosol samples and by incubation with anti-MCM-7 antibodies for nucleus samples. The detection of actin is suitable for control of equal loading of WCLs proteins but not of proteins of different subcellular fractions. Due to the isolation procedure, cytosol (S-100) and mitochondria are devoid of actin.
DISCUSSION
CDK inhibitors are very efficient anti-cancer agents [Cohen, 2002; Fischer and Gianella-Borradori, 2005; Wesierska-Gadek and Schmid, 2006]. There are some reasons why distinct CDK inhibitors seem to be a promising weapon against cancer. First, a number of CDK inhibitors, especially those belonging to the family of purine/pyrimidine analogs exhibit a low direct cytotoxicity and negligible, if any, mutagenic side effects. Secondly, the CDK inhibitors are relatively selective and target primarily the activity of distinct CDKs and most of them inhibit highly specific CDK2 complexes [De Azevedo et al., 1997; Meijer et al., 1997; Gray et al., 1999]. Thirdly, a number of CDK inhibitors exert a dual action [Wesierska-Gadek and Schmid, 2006]. They not only inhibit CDK2 activity, but also activate p53 tumor suppressor protein in cancer cells, thereby amplifying their anti-proliferative and pro-apoptotic action [David-Pfeuty, 1999; David-Pfeuty et al., 2001; Kotala et al., 2001; Blagosklonny et al., 2002b; Wojciechowski et al., 2003a; Wesierska-Gadek and Schmid, 2006]. This feature of CDK inhibitors seems to be of importance for treatment of CDK2-independent cancers or malignancies characterized by disturbances of the apoptotic pathway. Finally, the effective action of CDK inhibitors is not restricted to rapidly proliferating cancer cells. They also efficiently target malignant cells resting in Go phase of the cell cycle [Wesierska-Gadek and Schmid, 2006]. The accumulation of quiescent B-type lymphocytes in B-CLL leukaemia is not a consequence of their escape of the cell cycle control but is primarily attributable to their decreased elimination by apoptosis [Granziero et al., 2001]. The dysfunction of apoptotic processes occurring in some types of malignant diseases such as lymphoma or B-CLL leukaemia is closely related to the overexpression of pro-survival proteins such as Bcl-2, survivin or Mcl-1 [Kitada et al., 1998; Hahntow et al., 2004; Stankovic et al., 2004; Alvi et al., 2005]. These observations illustrate the importance of the pro-apoptotic action of ROSC. Interestingly, ROSC is also able to induce apoptosis in cycling cells. We recently reported that ROSC initiated apoptosis in human MCF-7 breast cancer cells [Wojciechowski et al., 2003a; Wesierska Gadek et al., 2005] that are known to be resistant to pro-apoptotic stimuli [Jänicke et al., 1998]. The reduced susceptibility of human MCF-7 cells to apoptosis-inducing factors is attributable to the lack of caspase-3 expression due to a 47 bp deletion within exon 3 of the caspase-3 gene [Jänicke et al., 1998]. Thus, it became evident that ROSC is a more efficient drug towards MCF-7 cells than cisplatin [Wesierska Gadek et al., 2003]. As previously reported, ROSC arrested naive, asynchronously growing MCF-7 cells in the G2 phase of the cell cycle in a time- and concentration-dependent manner [Wojciechowski et al., 2003a; Wesierska Gadek et al., 2005]. Moreover, longer exposure of asynchronously growing human MCF-7 cancer cells to ROSC resulted in the induction of apoptosis [Wojciechowski et al., 2003a; Wesierska Gadek et al., 2005]. Interestingly, ROSC strongly activated and stabilized wt p53. Considering this fact and the impact of the p53 protein on the regulation of apoptosis, we assumed that it could be involved in the initiation and in the execution of apoptosis. Indeed, the ROSC-mediated induction of p53 protein preceded the onset of apoptotic changes. Moreover, ROSC induced site-specific phosphorylation of the p53 protein [Wesierska Gadek et al., 2005]. The P-Ser46-modified p53 induced p53AIP1 protein, which is its transcriptional target [Oda et al., 2000; Matsuda et al., 2002]. After de novo synthesis p53AIP1 protein was translocated into the mitochondria, thereby contributing to the dissipation of the mitochondrial membrane. However, the apoptosis was induced by ROSC in G2 [Wojciechowski et al., 2003a; Wesierska Gadek et al., 2005] but not in G1 arrested MCF-7 cells [David-Pfeuty, 1999; David-Pfeuty et al., 2001]. This was surprising, because CDK inhibitors are known to induce apoptosis in cells in any phase of the cell cycle. Careful comparison of the experimental conditions revealed that the latter we cultivated in conventional medium containing phenol red. Therefore, it raised the question whether the medium composition, especially the presence of phenol red, could modulate the anti-proliferative and/or pro-apoptotic action of ROSC on MCF-7 cells. Phenol red, routinely used in tissue culture media as a pH indicator, has been recognized previously as a weak estrogen [Berthois et al., 1986]. Phenol red, bearing some structural similarity to certain nonsteroidal estrogens, has been shown to enhance the proliferation of ER-positive breast cancer cells [Berthois et al., 1986]. Considering this observation, we decided to investigate whether the efficacy of the therapeutic treatment of ER-positive MCF-7 breast cancer cells could depend on the presence of phenol red in tissue culture media. Indeed, phenol red significantly abolished the anti-mitogenic and pro-apoptotic action of ROSC on MCF-7 cells. Apparently, the basal expression of distinct cell cycle regulators in MCF-7 cells depends on the content of the cell culture media. Thus, cells exponentially growing in the presence of phenol red express lower levels of cyclin D, PCNA, p27, and p21waf1 (Wesierska-Gadek unpublished data).
These results bring the explanation for the different outcomes, when human MCF-7 cells are exposed to ROSC in culture media differing in their composition. Moreover, they unequivocally evidence that the efficacy of chemotherapy with CDK inhibitors of estrogen-responsive human breast cancers can depend on the presence and concentration of the estrogen-mimicking compounds such as phytoestrogens or xenoestrogens.
How could we explain this observation? Breast cancer is a multifactorial disease arising from a complex interplay between genetic changes and environmental factors. In the mid-1990s the breast cancer susceptibility genes BRCA1 and BRCA2 were discovered [Miki et al., 1994; Wooster et al., 1994]. Intense efforts to discover further breast cancer susceptibility genes have been unsuccessful. It is likely that low and moderate penetrant mutations combined with increased hormonal exposure could be a risk factor for breast cancer [Martin and Weber, 2000].
Estrogen, playing a key role in the normal development of the mammary gland, putatively promotes cancer progression by increasing the rate of cell proliferation in human breast epithelium [Platet et al., 2004]. Estrogen exerts its physiological effects through binding to and activation of the cognate nuclear receptors, either estrogen receptor alpha (ERα) [Green et al., 1986; Greene et al., 1986] or the more recently discovered estrogen receptor beta (ERβ) [Kuiper et al., 1996; Mosselman et al., 1996]. Once bound to the receptor(s), the activated complex is able to induce gene transcription of the responsive genes by either directly binding estrogen responsive elements within the promoter [McDonnel, 2004] or by complexing with DNA bound transcription factors [Shang and Brown, 2002].
A number of breast cancers depend on estrogen for progression and invasiveness. Interestingly, some compounds, the so-called xenoestrogens present in the environment, mimic the hormone action and activate the estrogen receptors also in the absence of genuine ligand. Considering the pivotal role of the activation of ER in the proliferation of breast cancer cells, elucidation of the effects of xenoestrogens on the mitogenic potential of breast cancer cells and on their susceptibility to the therapy is of great importance.
Acknowledgements
We thank Ms. M. Eisenbauer and Ms. A. Maderner for cell cultivation.




