Role of metal-responsive transcription factor-1 (MTF-1) in EGF-dependent DNA synthesis in primary hepatocytes
Abstract
Metal-responsive transcription factor-1 (MTF-1), which is involved in sensing heavy metal load, induces the transcription of several protective genes. The mouse Mtf-1 gene is essential, and Mtf-1−/− embryos die from liver degeneration. We showed that DNA synthesis induced in hepatocytes by epidermal growth factor (EGF) was delayed by inhibition of MTF-1. To inhibit MTF-1 activity, MTFΔC, a C-terminal deletion mutant of MTF-1, was expressed by infection with the virus Ad5MTFΔC. Lactate dehydrogenase (LDH) release and/or caspase-3/7 activation was not observed under our experimental conditions. The inhibitory effect of MTFΔC on EGF-dependent DNA synthesis in hepatocytes was not eliminated by zinc addition. EGF-dependent extracellular signal-related kinase (ERK) phosphorylation, an essential reaction for EGF-dependent DNA synthesis, was decreased in MTF-1-inhibited hepatocytes. Moreover, decrease of ERK phosphorylation was observed by using siRNA in MTF-1-downregulated hepatocytes. These results indicate that MTF-1 is particularly important for proper hepatocyte proliferation. This is the first report to suggest the function of MTF-1 in the ERK pathway. J. Cell. Biochem. 99: 485–494, 2006. © 2006 Wiley-Liss, Inc.
Metal-responsive transcription factor-1 (MTF-1) is involved in sensing heavy metal load and the induced transcription of several protective genes [Radtke et al., 1993; Otsuka et al., 1994. Its best-characterized targets are those that code for metallothioneins (MTs), small cysteine-rich proteins. Heavy metal-induced transcriptional activation of the genes coding for MTs is mediated by cis-acting DNA elements, the metal-responsive elements (MREs) [Stuart et al., 1985. The DNA motifs of the core consensus sequence TGCRCNC are present in multiple copies in the promoters of all Mt genes and other target genes of MTF-1 [Lichtlen et al., 2001. MTF-1 binds specifically via its zinc-finger to the MRE. Other targets of MTF-1 are ZnT-1 [Langmade et al., 2000, a zinc exporter pump, and γ-glutamylcysteine synthetase (γ-GCS), an essential enzyme in glutathione biosynthesis [Gunes et al., 1998. Because MT and γ-GCS have the potential to act as free radical scavengers, MTF-1 may help to control cellular redox status. Under hypoxic conditions, MTF-1 contributes to the expression of placental growth factor (PlGF), an angiogenic factor expressed in many tumors [Green et al., 2001. Loss of MTF-1 results in delayed tumor growth [Haroon et al., 2004. Molecular consequences of MTF-1 loss include increased production and activation of transforming growth factor-β1 (TGF-β1) and tissue transglutaminase, two proteins with documented roles in production and stabilization of the extracellular matrix. Phosphorylation is involved in the activation of MTF-1 [LaRochelle et al., 2001; Saydam et al., 2002. Protein kinase C (PKC), c-Jun N-terminal kinase (JNK), phosphoinositide 3-kinase (PI3K), and tyrosine-specific protein kinase are upstream components in the MTF-1 signal transduction cascade. MTF-1 null-mutant mice die in utero at approximately day 14 of gestation with impaired development of hepatocytes [Gunes et al., 1998.
We speculated that MTF-1 could control hepatocyte proliferation. This hypothesis is supported by the above-mentioned study [Gunes et al., 1998 using MTF-1 null-mutant mice. It is well established that, in vivo, normal hepatocytes are largely unresponsive to growth factors and become competent only after “priming” induced by specific treatments, such as partial hepatectomy, necrosis following injury, metabolic stress, or any phenomenon leading to disruption of cell–cell contact or digestion of the extracellular matrix [Ikeda et al., 1989; Liu et al., 1994. These metabolic events trigger G0/G1 transition and increase the expression of hepatocyte growth factor, TGF-α, and epidermal growth factor (EGF) [Michalopoulos, 1990; Mullhaupt et al., 1994. The EGF level rapidly increases in the immediate early phase of liver regeneration. EGF activates the extracellular signal-regulated kinases (ERKs) cascade [Liu et al., 1996. ERKs are activated by phosphorylation of threonine and tyrosine residues [Seger and Krebs, 1995. There are two ERKs highly related mammalian mitogen-activated protein kinases (MAPKs), p44 and p42, also called ERK1 and ERK2. Their phosphorylation is catalyzed by protein kinases known as MEKs (MAPK-ERK kinases), which display extremely high substrate selectivity toward p42/p44 MAPKs. MEKs are also rapidly phosphorylated by Raf-1 on serine residues that are necessary and sufficient for MEK activation. The MEK/ERK cascade has been shown to be essential for the propagation of growth factors and differentiating signals. The fact that addition of PD98059, a MEK inhibitor, to EGF-stimulated hepatocytes inhibits DNA synthesis in hepatocytes indicates that MEK/ERK cascade activation is a key signaling pathway involved in G1-phase progression in proliferating hepatocytes [Talarmin et al., 1999. Here, we demonstrate the role of MTF-1 in EGF-dependent hepatocyte proliferation by using a dominant-negative mutant and siRNA of MTF-1.
MATERIALS AND METHODS
Preparation of Mouse Primary Hepatocytes and Cell Culture
Primary hepatocytes were isolated from the livers of ICR male (5- to 8-week-old) mice by a two-step perfusion procedure using 0.025% collagenase buffered with 0.1 M HEPES (pH 7.5), as described by Klaunig et al. 1981. They were plated at a density of 104 cells per cm2 in 12-well plates or 60-mm diameter dishes in a mixture of 75% minimal essential medium and 25% medium 199, supplemented with fetal bovine serum (10%) and insulin (10−7 M). After cell attachment, which occurred 4 h after plating, the medium was renewed with the same medium lacking FBS and supplemented with dexamethasone (10−9 M), sodium pyruvate (10 mM), and bovine serum albumin (0.1%). It was renewed every day thereafter. EGF (Promega, Madison, WI) was used at 50 ng/ml.
Adenovirus Transfection
A recombinant adenovirus, Ad5MTFΔC, which expresses a C-terminal deletion mutation of MTF-1, had been constructed previously [Kimura et al., 2004. To measure MTF-1 activity, an MREd-driven luciferase reporter adenovirus, AdHM4-MREd3L2, was constructed by an in vitro ligation method using I-CeuI/PI-SceI-digested pAdHM-RGD and pHMMREd3L. pHMMREd3L was constructed from XbaI/NotI-digested pAdHMMREd3 and from pCMVL1, which contains the luciferase gene, derived from pGL3-Control (Promega) in pHMCMV6. All recombinant adenoviruses were grown in HEK293 cells. Recombinant adenovirus stock supernatant was prepared from cell suspension by using a BD Adeno-X virus purification kit (BD Biosciences Clontech, Franklin Lakes, NJ). The recombinant adenovirus titer used was approximately TCID50/cell = 8 (TCID50 = ‘tissue culture infective dose,’ or the viral dose that induces cell lysis in at least 50% of HEK293 cell cultures). Recombinant adenovirus containing the GFP gene (Ad5GFP) was used at the equivalent titer as a control. To produce a zinc-finger protein, recombinant adenovirus harboring rat GATA-4 (AxCArGATA-4) was supplied by the RIKEN BioResource Center (Tsukuba, Japan). Recombinant adenovirus transfection was performed in serum-free medium. After a 2-h incubation, the medium containing the adenovirus was removed and replaced with virus-free medium.
Measurement of DNA Replication
DNA replication was estimated by measuring [3H]thymidine incorporation and 5-bromo-2′-deoxyuridine (BrdU) incorporation.
[3H]Thymidine incorporation was detected as follows: 10 µl of [3H]thymidine (2.89 kBq/well) was incubated for the periods indicated in the figure legends. After incubation, the medium was removed and the cells were washed with phosphate-buffered saline. [3H]Thymidine incorporation was measured after DNA precipitation with 5% trichloroacetic acid, washing with 95% ethanol, and dissolution with 1 N NaOH.
BrdU incorporation was analyzed by using a BrdU labeling and detection kit II (Roche Diagnostics, Penzberg, Germany). Briefly, hepatocytes were incubated with BrdU (10 µM) for 2 h. The hepatocytes were fixed with 15 mM glycin/70% ethanol (pH 2.0) and incubated with an anti-BrdU antibody (clone BMG 6H8) followed by an anti-mouse IgG antibody conjugated to alkaline phosphatase. Visualization of complexes was performed by color reaction of nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate. Between 200 and 300 cells were observed and their BrdU positivity judged.
Reverse Transcriptase/Polymerase Chain Reaction
Total RNA was extracted using Isogen (Nippon gene, Tokyo, Japan) according to the manual. RNA (10 µg) was reverse transcribed using oligo-dT with SuperScript™ II Reverse Transcriptase (Invitrogen, Carlsbad, CA). Aliquots of the reaction mix were then subjected to PCR. PCR was carried out using ExTaq (TakaRa, Ohtsu, Japan) and specific primers for MT-I and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; used as an internal RNA control) (MT-I: F 5′-TCTCGGAATGGACCCCAACTG-3′, R 5′-TTTACACGTGGTGGCAGCGC-3′; GAPDH: F 5′-CCAAGGTCATCCATGACAACT-3′, R 5′-TCCACCACCCTGTTGCTGTA-3′). PCR products were separated on a 1.5% agarose/Tris-acetate-EDTA gel and visualized with ethidium bromide staining. PCR conditions were as follows: MT-I, 30 s at 95°C, 30 s at 56°C, 45 s at 72°C; GAPDH, 30 s at 95°C, 30 s at 62°C, 45 s at 72°C for 26–35 cycle.
Cytotoxicity Assay
Lactate dehydrogenase (LDH) released in the culture supernatants was measured by using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega), a coupled enzymatic assay that results in conversion of the tetrazolium salt into a red formazan product. To determine the 100% LDH release, Triton X-100 was added to each well (final concentration 0.5%).
Caspase Activity Assay
Caspase-3/7 activity was measured by using a Caspase-Glo 3/7 Assay (Promega). The assay provided a proluminescent caspase-3/7 substrate that contained the tetrapeptide sequence DEVD. After caspase cleavage, the substrate for luciferase was released, resulting in a luciferase reaction and the production of light. The luminescence was measured by a luminometer (20/20n Luminometer, Promega)
Immunoblotting Analysis
Hepatocytes were washed three times with ice-cold PBS, scraped into PBS, and collected by centrifugation. Cell lysate of the hepatocytes was prepared by sonication in lysis buffer (20 mM Tris, pH 7.5; 150 mM NaCl; 1 mM EDTA; 1 mM EGTA; 1% Triton X-100; 2.5 mM sodium pyrophosphate; 1 mM β-glycerol phosphate; 1 mM Na3VO4; 1 mg/ml leupeptin; 1 mM PMSF). Loading buffer was added to the protein extracts, and the mixture was incubated at 65°C for 15 min. Extract volumes equivalent to 20 µg of protein were separated by using 10% SDS–PAGE gels, transferred to nitrocellulose membranes, and probed using 1:2,000–1:5,000 dilutions of primary antibody (Cell Signaling Technology, Inc., Beverly, MA) followed by a 1:10,000 dilution of anti-mouse or rabbit IgG antibody conjugated with horseradish peroxidase. Detection of complexes was performed with Western Lightning Chemiluminescence Reagent Plus (PerkinElmer Life Sciences, Boston, MA). These membranes were reprobed with β-actin antibody (clone AC-15; SIGMA-Aldrich, St. Louis, MO,) to demonstrate equal loading of proteins in each lane.
RNA Interference
Mouse MTF-1 (NM_008636)-specific siRNA was designed by QIAGEN (Hilden, Germany). MTF-1 siRNAs was purchased (Cat. No: SI01319731). The target sequence of the MTF-1 was CCGGTTCACAGTGAAGCACTA, which corresponded to nucleotides 2,311–2,331. ERK2 siRNA and non-silencing control siRNA were purchased commercially from QIAGEN as an RNAi Human/Mouse Control Kit. Hepatocytes were transfected with siRNA (final, 10 nM) using RNAiFect transfect reagent (QIAGEN), as described in the manufacturer's instructions.
Statistical Analysis
Data were analyzed by ANOVA and Fisher's PLSD test. Differences between groups were considered significant at P < 0.05.
RESULTS
MTFΔC Delays EGF-Dependent Hepatocyte Proliferation
EGF-dependent DNA replication was estimated at various times after EGF treatment by measuring [3H]thymidine incorporation. Hepatocytes were plated in 60-mm diameter dishes. After cell attachment, adenovirus was added to the dishes. After a 2-h incubation, the medium containing the adenovirus was removed and treated with EGF. In the Ad5GFP-infected group, DNA replication began about 24–36 h after EGF treatment. Peak DNA replication was observed 36–48 h after EGF treatment (Fig. 1A). DNA replication of EGF non-treatment group was under 5,000 dpm/well/12 h during our experimental condition (data not shown). In contrast, DNA replication began about 36–48 h after EGF treatment in the Ad5MTFΔC-infected group. Peak DNA replication was observed at 48–60 h.
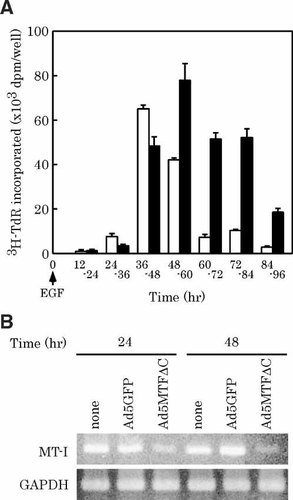
Effect of Ad5MTFΔC on EGF-induced [3H]-TdR incorporation and MT-I mRNA level. Hepatocytes were infected with Ad5GFP (white bars) or Ad5MTFΔC (black bars) 4 h after plating. After 2 h of incubation, the medium containing adenovirus was removed and treated with EGF. A: Cultures were incubated with [3H]thymidine (3H-TdR) for 12 h before harvest. Cultures were made in triplicate. Data represent means ± SD. The 3H-TdR incorporation in the Ad5MTFΔC group was significantly different from that in the Ad5GFP-group (P < 0.001). B: Total RNAs were isolated and reverse-transcribed. The resultant cDNA was amplified by PCR. The PCR products were electrophoresed on 1.5% agarose gels and visualized by ethidium bromide staining. PCR product sizes were MT-I = 227 bp, GAPDH = 496 bp. This experiment was repeated three times independently and similar results were obtained each time. Results from one representative experiment are shown.
We examined the effect of Ad5MTFΔC on gene expression. MTF-1 is essential for basal and heavy metal-induced MT gene expression [Heuchel et al., 1994. As shown in Figure 1B, MT-I expression was decreased in Ad5MTFΔC-infected cells. MRE-driven gene expression was monitored by using AdHM4-MREd3L2. AdHM4-MREd3L2 was infected at 10 MOI (multiplicity of infection) with Ad5GFP or Ad5MTFΔC. MREd-driven luciferase gene expression was measured as luciferase activity 36 h after infection. Luciferase activity in the Ad5GFP-infected cells was 116,590 ± 983 RLU and that in the Ad5MTFΔC-infected cells was 1,359 ± 93 RLU (P < 0.001).
The percentage of cells with DNA replication was determined by staining to determine BrdU incorporation. The percentage of BrdU-incorporated cells was 50.1 ± 13.4% in the Ad5GFP-infected group and 7.8 ± 7.0% in the Ad5MTFΔC-infected group (Fig. 2).
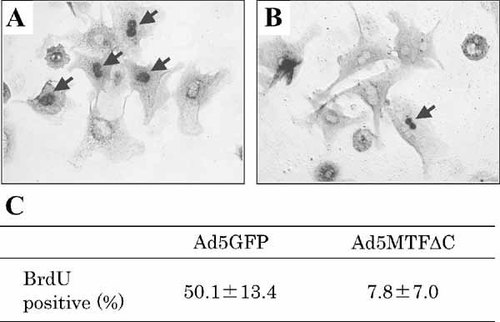
Effects of Ad5MTFΔC on EGF-induced BrdU incorporation. Hepatocytes were infected with Ad5GFP (A) or Ad5MTFΔC (B) 4 h after plating. After 2 h of incubation, the medium containing adenovirus was removed and treated with EGF. Thirty-four hours after incubation, hepatocytes were incubated with BrdU for 2 h. Arrows indicate BrdU-positive hepatocyte nuclei. C: Ratio of BrdU-positive hepatocytes was calculated as a percentage. The percentage of BrdU-positive hepatocytes in the Ad5MTFΔC group was significantly lower than that in the Ad5GFP-group (P < 0.01).
MTFΔC Does not Induce LDH Release or Caspase-3/7 Activation
To determine the cytotoxic effect of MTFΔC, we measured LDH release and caspase-3/7 activity. Notable LDH release (>20%) was not observed in Ad5GFP- or Ad5MTFΔC-infected cells (Fig. 3A). As a positive control of caspase-3/7 activated cells, TNF-α/actinomycin D (ActD)-treated cells were prepared. ActD (400 ng/ml) was added 30 min before recombinant human TNF-α (500 U/ml) treatment. Caspase-3/7 activity in Ad5GFP- and Ad5MTFΔC-infected cells was much lower than in TNF-α/ActD-treated cells. No difference in caspase-3/7 activation was observed between Ad5GFP- and Ad5MTFΔC-infected cells (Fig. 3B).
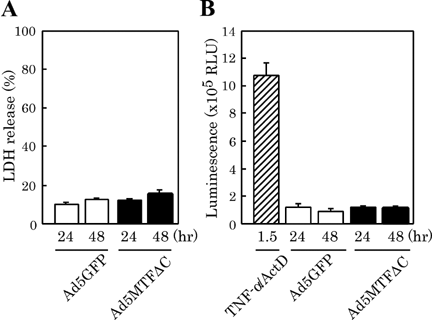
LDH release and caspase-3/7 activation in Ad5MTFΔC-infected hepatocytes. Hepatocytes were transfected with recombinant Ad5GFP or Ad5MTFΔC 4 h after plating. After 2 h of incubation the adenovirus was removed. LDH release into culture supernatants (A) and caspase-3/7 activity (B) were measured at the times indicated. rhTNF-α treatment was given 24 h after the start of incubation. Actinomycin D (ActD) was added 30 min before rhTNF-α treatment.
Zinc Does not Affect Ad5MTFΔC-Induced Delayed DNA Replication
Ad5MTFΔC expresses a zinc-finger protein; therefore, Ad5MTFΔC infection might cause depletion of intracellular zinc. To examine the zinc dependence of DNA replication, hepatocytes were treated with zinc (0, 5, 50 µM) and/or diethylenetriamine-N, N, N′, N″, N″-pentaacetic acid (DTPA; 50 µM), a zinc chelator. DTPA and/or zinc were added to the hepatocytes, together EGF. DNA replication was inhibited by DTPA addition (Fig. 4). The inhibitory effect of DTPA was eliminated by the addition of zinc. In contrast, the inhibitory effect of Ad5MTFΔC was not eliminated by zinc addition.
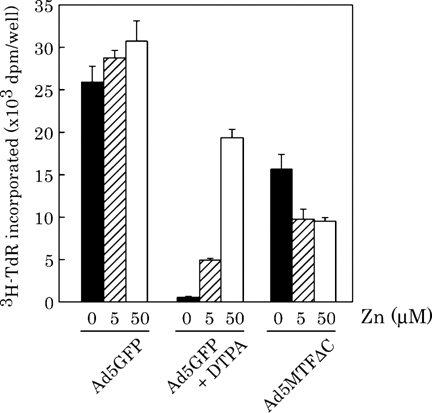
Effect of Zn on EGF-induced [3H]-TdR incorporation. Hepatocytes were infected with Ad5GFP or Ad5MTFΔC 4 h after plating. After 2 h of incubation, the medium containing adenovirus was removed and treated with EGF plus Zn (0, 5, 50 µM) (both Ad5GFP-infected and Ad5MTFΔC-infected hepatocytes) or with EGF plus Zn (0, 5, 50 µM) and DTPA (50 µM) (Ad5GFP-infected hepatocytes only). Thirty-four hours after the beginning of incubation, the hepatocytes were incubated with [3H]thymidine for 2 h. Cultures were made in triplicate. Data represent means ± SD.
Effect of MTF-1 Inhibition on EGF-Dependent ERK Phosphorylation
We examined the effect of Ad5MTFΔC on EGF-dependent ERK phosphorylation. ERK phosphorylation was monitored after EGF treatment by immunoblotting with an antibody that recognizes the active, phosphorylated forms of ERK1 and ERK2. Phosphorylation of ERK was observed 24 h after EGF treatment (Fig. 5). The amount of phosphorylated ERK increased 48 h after EGF treatment. Phosphorylation of ERK1 and ERK2 requires active, phosphorylated MEK1 and MEK2. Phosphorylated MEK was observed 24 h after EGF treatment, but the amount of MEK phosphorylation was decreased 48 h after EGF treatment. Infection with Ad5MTFΔC decreased ERK and MEK phosphorylation (Fig. 5).
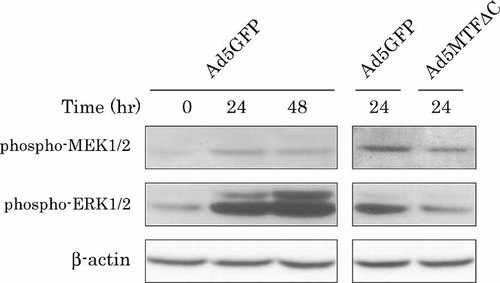
Effects of Ad5MTFΔC on EGF-dependent ERK phosphorylation. Hepatocytes were infected with Ad5GFP or Ad5MTFΔC 4 h after plating. After 2 h of incubation, the medium containing adenovirus was removed and treated with EGF. Cell lysate was prepared at the times indicated, and then immunoblotting of phospho-MEK1/2 and phospho-ERK1/2 was performed. These membranes were reprobed with β-actin antibody to demonstrate equal loading of proteins per lane.
Next, to confirm the hypothesis that MTF-1 modulates EGF-dependent ERK phosphorylation, we examined the effect of downregulation of MTF-1 on EGF-dependent ERK phosphorylation by using siRNA. MTF-1 siRNA decreased MTF-1 levels within 2 days, as detected by MRE-driven luciferase gene expression (control siRNA: 707,466 ± 110,052 RLU, MTF-1 siRNA: 167,533 ± 24,875 RLU;P < 0.01), and strongly reduced mRNA levels as detected by RT-PCR (data not shown). Control siRNA had no effect on MTF-1 mRNA levels. ERK2 protein levels were downregulated by ERK2 siRNA (Fig. 6). MTF-1 siRNA had no effect on ERK2 protein levels. Phosphorylated ERK1/2 levels were increased by EGF treatment of control siRNA-transfected cells. This increase was partially suppressed by downregulation of MTF-1 with siRNA. The other siRNA for MTF-1 (Cat. No. SI01319738; target sequence: 521ACGGAAAGAAGTAAAGCGGTA541) also partially suppressed the phosphorylation of ERK (data not shown). We examined whether suppression was present in Ad5MTFΔC-infected cells. Partial suppression induced by Ad5MTFΔC was observed. AxCArGATA-4 did not suppress ERK phosphorylation.
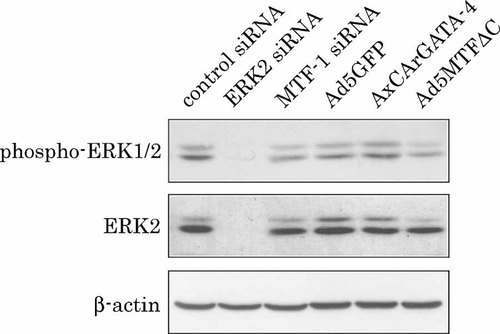
Effects of MTF-1 siRNA on EGF-dependent ERK phosphorylation. Hepatocytes were transfected with siRNAs (control siRNA, ERK2 siRNA, or MTF-1 siRNA) or infected with recombinant adenovirus vectors (Ad5GFP for control, AxCArGATA-4 for 4 h after plating. After 2 h of incubation the adenovirus was removed. Sixty hours after transfection/infection, hepatocytes were stimulated with EGF for 10 min, and then cell lysate was prepared. The membrane was probed with anti-phospho-ERK1/2 or anti-ERK1. These membranes were reprobed with β-actin antibody to demonstrate equal loading of proteins per lane.
DISCUSSION
We showed here that EGF-dependent hepatocyte proliferation was delayed by inhibition of MTF-1, and inhibition of MTF-1 decreased EGF-dependent ERK phosphorylation. The hepatocytes were isolated by a collagenase perfusion procedure. Collagenase perfusion of the liver triggers G0/G1 transition of quiescent normal hepatocytes [Liu et al., 1994. The MEK/ERK cascade activation is a key signaling pathway involved in the regulation of G1-phase progression following EGF stimulation [Talarmin et al., 1999. Our results suggest that MTF-1 causes progression from early G1 to mid-late G1. Gunes et al. generated MTF-1 null-mutant mice and reported that these mice consistently died on day 14 of gestation from liver degeneration [Gunes et al., 1998. These results imply that MTF-1 is particularly important for proper liver growth.
We first examined whether inhibition of MTF-1 would affect EGF-dependent DNA replication. To inhibit MTF-1, we used a dominant-negative MTF-1 (MTFΔC), a C-terminal deletion mutant of MTF-1. MTF-1 binds directly and specifically to MRE [Radtke et al., 1993; Koizumi et al., 1999. MTFΔC can bind MRE and competes with MTF-1 for MTF–MRE complex formation [Kimura et al., 2004. Expression of MTFΔC in cells reduced MRE-driven gene expression. Ad5MTFΔC-infection delayed EGF-dependent hepatocyte DNA replication (Fig. 1). Several lines of evidence support the likelihood that MTF-1 plays a role in the oxidative stress response [Heuchel et al., 1994; Gunes et al., 1998; Kimura et al., 2004, and we considered possible that the delay in DNA replication is caused by cytotoxicity. However, LDH release and/or caspase-3/7 activation were not observed Ad5MTFΔC infection (Fig. 3). It has been reported that adult Mtf-1 deletion mice, generated by conditional knockout using the Cre-loxP system, are viable under laboratory conditions, our results shown in Figure 3 are consistent with those in Mtf-1 deletion mice [Wang et al., 2004. Moreover, the amount of incorporated [3H]thymidine in the Ad5MTFΔC-infected group was higher than in the Ad5GFP-infected group (Fig. 1). Loyer et al. have reported that increases in the amount of incorporated [3H]thymidine can be caused by differences in the culture time at which EGF is added [Loyer et al., 1996: this follows from differences in cyclin expression levels. Cyclin D1 and D3 expression vary according to the cell culture conditions. Expression of cyclin D3 is increased before the addition of EGF. Peak cyclin D1 expression occurs after EGF stimulation, and the peak value increases with increasing duration of time before stimulation. Expression of MTFΔC was transient because MTFΔC was expressed by the adenovirus system. We considered that EGF signaling would be blocked by MTFΔC and would increase with elimination of MTFΔC expression. If MTFΔC blocks EGF signaling, then a difference in the percentage of cells with DNA replication should have been observed between the Ad5GFP-infected group and the Ad5MTFΔC-infected group. The percentage of DNA-replicated cells was decreased among those infected with Ad5MTFΔC (Fig. 2). These results suggest that MTFΔC blocks EGF signaling.
Several studies have examined the function of MT in hepatocyte proliferation or development. MTF-1 is essential for basal and heavy metal-induced Mt gene expression [Heuchel et al., 1994. MT is induced in the regenerating liver in response to the stimuli evoked by partial hepatectomy. The MT is translocated into the nuclei from the cytoplasm [Tohyama et al., 1993; Tsujikawa et al., 1994. MT-I/II null-mutant mice are viable under laboratory conditions [Michalska and Choo, 1993; Masters et al., 1994, but hepatic regeneration after partial hepatectomy is impaired in these mice [Oliver et al., 2005. Blocking of EGF signaling by MTFΔC might occur through a decrease in MT levels.
Zinc is important to cell proliferation. It has been reported that, when cells are cultured in media containing an extracellular chelator, such as EDTA or DTPA, growth factor-induced DNA synthesis is impaired [MacDonald et al., 1998; Lefebvre et al., 1999. There are six zinc-fingers of the C2H2 type in the structure of MTFΔC. We considered that MTFΔC might act as a zinc chelator. We examined whether MTFΔC acted as a zinc chelator under our experimental conditions. When DTPA was added together with zinc to cells infected with Ad5GFP, DNA synthesis was restored (Fig. 4). In contrast, the inhibitory effect of MTFΔC was not eliminated by addition of zinc. These results suggest that the inhibitory effect of MTFΔC was not caused by chelation of zinc. The mechanisms of zinc chelator-induced inhibition of cell proliferation might instead act through the inhibition of MTF-1.
To clarify the mechanisms of MTFΔC-induced DNA synthesis inhibition, we examined EGF-dependent ERK1/2 phosphorylation. It has been reported that phosphorylation of ERK1/2, which is crucial for the cell proliferation induced by IGF-I in Rat-1 cells, is partly blunted by DTPA [Lefebvre et al., 1999. MTFΔC partly blunted ERK1/2 phosphorylation (Fig. 5B). EGF-induced ERK1/2 phosphorylation occurred within 10 min. MTFΔC suppressed rapid ERK1/2 phosphorylation (Fig. 6). Moreover, partial suppression was observed by using siRNA in MTF-1 downregulated hepatocytes. GATA-4 did not modulate EGF-induced ERK phosphorylation (Fig. 6). GATA-4 (also named GATA-GT2) is a DNA binding protein and contains a C4 class zinc-finger motif [Arceci et al., 1993. Suppression of ERK phosphorylation therefore did not occur by overexpression of a zinc-finger protein. These results suggest that MTF-1 might modulate the signaling of the ERK1/2 pathway. Some signal pathway factors, including ALK-6 (TGF-β receptor type I), inhibin/activin bc subunit, FGF receptor, Gas3/PMP22, and Notch-1 are potential target genes of MTF-1 [Lichtlen et al., 2001. In 2004, Haroon et al. reported that loss of MTF-1 suppressed tumor growth through enhanced matrix deposition [Haroon et al., 2004. They showed that production and activation of TGF-β and tissue transglutaminase were increased in MTF-1 null cells. TGF-β is well known as a growth inhibitory factor and inhibits DNA synthesis of hepatocytes in primary culture [Nakamura et al., 1985. Inhibition of hepatocyte proliferation by TGF-β is associated with inhibition of ERK1/2 [Dixon et al., 1999. Hepatocytes in primary culture have the ability to express TGF-β [Gao et al., 1996. It is possible that inhibition of ERK1/2 phosphorylation is caused by enhanced TGF-β expression in MTF-1 suppressed cells.
In conclusion, we have shown here that EGF-dependent hepatocyte proliferation was delayed by inhibition of MTF-1 and that inhibition of MTF-1 decreased EGF-dependent ERK phosphorylation. These results imply that MTF-1 is particularly important for proper liver growth. Wang et al. reported that hepatocytes from Mtf-1 null-mutant embryos were lost within a few days [Wang et al., 2004. They thought that this consumption resulted from deficient expression of carbamoyl phosphate synthetase. The function of MTF-1 on EGF-dependent ERK phosphorylation might be involved in the consumption of embryonic hepatocytes. This is also the first report to suggest the function of MTF-1 in the MEK/ERK cascade. It has been reported that phosphorylation is involved in the activation of MTF-1 [LaRochelle et al., 2001; Saydam et al., 2002. PKC, JNK, PI3K, and tyrosine-specific protein kinase are upstream components of the MTF-1 signal transduction cascade, and these phosphorylation cascades are concerned with cell function. Experiments investigating the role of MTF-1 in cell function will be followed with great interest.
Acknowledgements
We thank Dr. Hiroyuki Mizuguchi (National Institute of Health Sciences, Osaka Branch, Japan) for technical support and advice on the construction of adenovirus AdHM4-MREd3L2. We also thank RIKEN BioResource Center for providing us with recombinant adenovirus harboring rat GATA-4.




