Regulatory processes affecting androgen receptor expression, stability, and function: Potential targets to treat hormone-refractory prostate cancer
Abstract
Prostate cancer cells rely on androgen receptor (AR) for proliferation and survival. Therefore, curing prostate cancer will require elimination of AR. Although androgen is the natural ligand that activates AR, AR activity is also subject to regulation by growth factor/growth factor receptor-stimulated signaling pathways that control the cell cycle. Cell cycle regulatory proteins and protein kinases in signaling pathways affected by growth factors can lead to AR activation in the absence of androgen. While downstream signaling proteins such as cyclins, cyclin-dependent kinases (CDKs), and pRB can modulate AR activity, upstream signaling pathways involving protein kinases such as mitogen-activated protein kinases, protein kinase A, and protein kinase B/Akt can affect post-translational modification of AR to affect not only AR function but also AR stability. Calcium and calmodulin (CaM), essential for proliferation and viability of a number of cells, including prostate cancer cells, play an important role in AR expression, stability, and function. CaM affects AR partly by interacting directly with AR and partly by activating protein kinases such as Akt and DNA-PK that can phosphorylate AR. The ubiquitin/26S proteasome pathway responsible for timely destruction of cell cycle regulatory proteins whose levels impede cell cycle progression also induces AR expression by activating NF-κB, and promotes AR activity by participating in the assembly of an AR transcription complex. Maspin, a serine protease inhibitor that is known mostly for its role as a tumor suppressor can also regulate AR intracellular localization and function by competing with AR for binding to the chaperone protein Hsp90 and co-repressor HDAC1, respectively. This perspective reviews the experimental evidence implicating these diverse cellular processes in AR expression, stability, and/or function, and presents a rationale for disrupting these cellular processes as a viable option for the treatment of both the hormone-sensitive and the hormone-insensitive prostate cancer. J. Cell. Biochem. © 2006 Wiley-Liss, Inc.
Prostate cancer is the most frequently diagnosed non-skin cancer in American men and the second leading cause of cancer deaths in this population [Jemal et al., 2005. Androgen (A), by activating androgen receptor (AR), plays an important role in both the development and progression of prostate cancer [Huggins, 1967; Huggins and Hodges, 1972; Bosland, 1996. Hence, for six decades, androgen-ablation has been the most commonly used frontline therapy for the treatment of disseminated prostate cancer. Most men with non-organ confined prostate cancer initially experience regression in response to androgen-ablation. Unfortunately, in almost all patients the disease eventually relapses to a hormone-refractory state that no longer responds to conventional anti-neoplastic therapy and becomes lethal [Gittes, 1991. AR is expressed in almost all prostate cancers regardless of their hormone sensitivity [Feldman and Feldman, 2001. In light of the observation that the growth of androgen-independent AR-positive prostate cancer cells can be inhibited by decreasing AR levels [Eder et al., 2000; Zegarra-Moro et al., 2002, it is logical to hypothesize that effective ways to inactivate and/or eliminate AR might yield a newcurative strategy for patients with hormone-insensitive prostate cancer. In this perspective, we identify processes that affect AR levels and AR function, and that may thereby influence proliferation and viability of prostate cancer cells.
Proliferation of most cell types depends on the sequential action of multiple peptide growth factors, such as platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and insulin-like growth factor (IGF-1). In response to growth factors, cells emerge from quiescence (G0 phase), progress through G1 phase, and enter S phase. Once they have entered S phase, cells no longer require external stimuli to complete S phase and undergo mitosis. By contrast, prostate epithelial cells require not only peptide growth factors but also androgen for proliferation and survival. Androgen, in combination with peptide growth factors, stimulates proliferation of prostate cancer cells by activating AR in an autocrine or paracrine fashion [Reddy et al., 2002. Inactivation of AR prevents prostate cancer cells from replicating DNA [Zegarra-Moro et al., 2002 and entering S phase [Cifuentes et al., 2003, and induces cell death [Zhan et al., 2002. Thus, as depicted in Figure 1, AR plays a critical role in maintaining a balance between life and death of prostate cancer cells—decreased AR activity compromises survival mechanisms and increased AR activity favors proliferation.
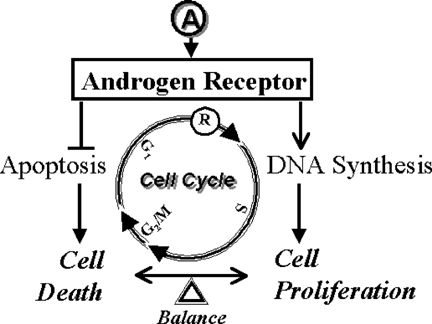
Cell cycle-dependent action of AR in regulating proliferation and viability of prostate cancer cells.
GROWTH FACTORS AND THE ANDROGEN RECEPTOR
Growth Factor/Cytokine Activation of AR
Growth factor activity can increase the activity of AR. For example, IGF-I, EGF, or keratinocyte growth factor (KGF) can stimulate AR-mediated reporter gene transcription to the same extent as the synthetic androgen methyltrienolone in androgen insensitive DU145 prostate cancer cells co-transfected with an AR expression vector and androgen-inducible reporter gene [Culig et al., 1994, 1995; Orio et al., 2002. Thus, elevated expression in prostate cancer of IGF-1 and -II, IGF-I receptor, and the EGF receptor family member ErbB2/Her-2/neu [Connolly and Rose, 1994; Arai et al., 1997; Grasso et al., 1997; Signoretti et al., 2000; Nickerson et al., 2001, might contribute to increased AR activity in prostate cancer. Similarly, IL-4 can enhance PSA expression in prostate cancer cells [Lee et al., 2003. In addition, androgen increases IL-6 expression [Okamoto et al., 1997a, and IL-6 signaling activates AR function by stimulating STAT3–AR interactions [Matsuda et al., 2001. IL-6 can stimulate proliferation of LNCaP cells [Okamoto et al., 1997a,b, and serum IL-6 levels are elevated in prostate cancer patients [George et al., 2005. These effects of growth factors on AR function suggest that therapeutic interference with growth factor signaling might decrease AR activity and benefit prostate cancer patients.
Growth Factor/Cytokines and AR Protein Interactions
The transcriptional activity of steroid receptors is modulated by interaction with other proteins acting as co-activators and co-repressors [Horwitz et al., 1996; Glass et al., 1997; Shibata et al., 1997; Yeh et al., 2000. In prostate cancer cells, growth factors such as EGF increase AR transcriptional activity via an increase in AR co-activators [Gregory et al., 2004. STAT3, which modulates gene expression induced by the IL-6 family of cytokines, interacts with AR and activates AR-mediated gene expression [Junicho et al., 2000; Gross et al., 2001. Furthermore, the protein inhibitor of STAT3 (PIAS3) may compete with AR for a STAT3 binding site thus inhibiting AR-induced STAT3-mediated gene expression [Yamamoto et al., 2003. These studies suggest that there is a direct interaction and cross-talk between cytokine-stimulated signaling pathway and AR, and that disruption of these pathways will affect AR activity in prostate cancer cells.
TRANSDUCTION OF MITOGENIC SIGNALS, CELL CYCLE REGULATORY PROTEINS AND THE ANDROGEN RECEPTOR
Role of Cell Cycle Regulatory Proteins in Regulation of AR Function
Cell cycle regulatory proteins, in addition to their role in the regulation of molecular events governing progression of cells from G1 to S phase, also regulate the transcriptional activity of the steroid hormone receptors AR and estrogen receptor (ER). For example, pRB, a key tumor suppressor protein, binds to and activates AR in an androgen-dependent manner [Lu and Danielsen, 1998; Yeh et al., 1998. Cyclin E can act as a co-activator, increasing AR transactivation in the presence of dihydrotestosterone [Yamamoto et al., 2000. This effect of cyclin E is resistant to inhibition by anti-androgen and is independent of interaction with Cdk2. Furthermore, this effect appears to be AR specific, since cyclin E does not show a similar effect on ER-, glucocorticoid receptor (GR)- or progesterone receptor (PR)-mediated gene transcription [Yamamoto et al., 2000. Conversely, cyclin D1 complexes with AR and inhibits AR transactivation [Knudsen et al., 1999; Reutens et al., 2001. This effect of cyclin D1 is androgen-dependent and independent of the role of cyclin D1 in cell cycle progression. The histone acetyltransferase activity of P/CAF (p300/CBP associated factor) rescues cyclin D1-mediated AR transrepression, suggesting that the cyclin D1 effect is mediated by a direct competition with AR for P/CAF binding. In contrast to the inhibitory effect of cyclin D1 on AR activity, Cdk6 (but not other Cdks) binds to AR and activates AR transcriptional activity. This effect of Cdk6 is independent of cyclin D1 and Cdk6 kinase activity. Interestingly, the binding of cyclin D1 to AR inhibits the ability of Cdk6 to stimulate the activity of AR. This suggests that overexpression of Cdk6 may contribute to the progression of prostate cancer to androgen-independence [Lim et al., 2005. It is possible that there are both common and unique binding sites in the regulatory domains on AR for cyclin E, cyclin D1, P/CAF, and Cdk6. Thus, mitogenic signals originating at the cell surface and transmitted through signal transduction pathways (Fig. 2) can alter the expression and activity of cell cycle regulatory proteins and thereby affect the transcriptional activity of steroid receptors.
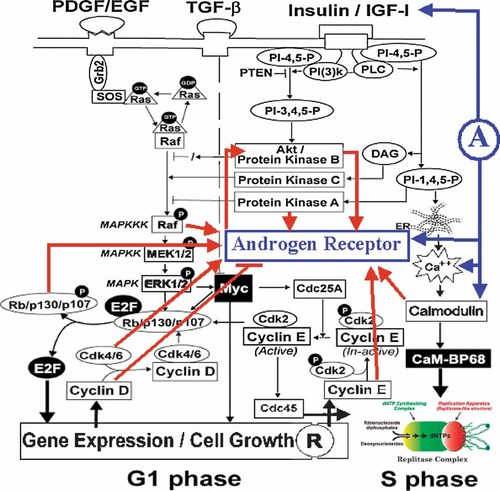
Cross-communication between AR and growth factor-stimulated signaling pathways in the cell cycle (modified from Reddy et al. 2002). R, cell cycle “Restriction Point”; A, androgen.
Several cell cycle regulatory proteins that control proliferation and cell cycle arrest (Fig. 2), viz., c-Myc, p53, pRB, E2F-1, Akt/PKA, PKC, cyclins, cyclin-dependent kinases (CDKs), cyclin-dependent kinase inhibitors (CKIs), Bcl-2, and NF-κB, are also involved in signaling pathways leading to cell death [King and Cidlowski, 1998. Many morphological features of proliferating mitotic cells, such as cell rounding, shrinkage, substrate detachment, and chromosome condensation, resemble those of cells poised for programmed cell death (apoptosis). Furthermore, apoptotic stimuli that trigger cell death commonly affect cell-cycle progression. Therefore, a full understanding of cell cycle-dependent physical and functional interaction of AR with cell cycle regulatory proteins may provide insight into the role of AR not only in proliferation but also in preventing the death of prostate cancer cells.
Growth Factor-Stimulated Signaling Pathways That Affect AR
Growth factor-induced progression of cells from resting (G0) phase to S phase requires the sequential action of two sets of mitogenic stimuli (Fig. 2). The first set of stimuli acts early in G1 phase and involves activation of mitogen-activated protein kinases (MAPK) and extra-cellular signal-related kinase (ERK) 1/2 together with elevation of c-Myc levels. A second set of stimuli acts in mid- to late- G1 phase and activates phosphatidylinositol-3-OH kinase [PI(3)K] and diacylglycerol (DAG)-dependent forms of protein kinase C (PKC) [Jones and Kazlauskas, 2001. Several intermediary regulators and byproducts of these mitogenic stimuli, including Ras/Raf, Akt, PI(3)K, and protein kinase A, affect AR levels and function, as described in the following section.
The Ras-Raf-MAPK Pathway and Prostate Cancer
As depicted in Figure 2, growth factor activation of receptors in G1 phase leads to the activation of ERK 1/2 through a cascade of signal transduction kinase and phosphatase reactions involving small guanine nucleotide-binding proteins, such as Ras. The Ras-Raf-MAPK pathway phosphorylates and activates transcription factors required for the expression of cell cycle regulatory genes, such as cyclin D1. Ras activation occurs at multiple points during the progression of cells from G0/G1 to S phase [Dobrowolski et al., 1994, and plays a critical role in IGF-1-dependent transition of cells from G1 to S phase [Lu et al., 1989. In prostate cancer cells, overexpression of mutated Ras leads to increased growth, and treatment with phenylacetate, which interferes with Ras activity, reduces ERK2 phosphorylation and inhibits cell proliferation [Danesi et al., 1996. In human prostate tumors, a higher level of activated MAPK is associated with a higher Gleason score and tumor grade [Gioeli et al., 1999. In this regard, it is interesting to note that IGF-I stimulates ERK2 only in androgen-independent (DU145) cells but not in androgen-sensitive (LNCaP) cells [Putz et al., 1999. Thus, differences in androgen sensitivity may be associated with differences in sensitivity to growth factors that are responsible for the transition from G1 to S phase.
Protein Kinases That Directly Affect AR Activity
Protein kinase A (PKA), protein kinase C (PKC), and protein kinase B (PKB/Akt), are activated by some of the same growth factors that activate the ERK1/2 signaling pathway. Growth factor signal transduction pathways can also affect Ras and Raf activities, either positively or negatively, depending on the cell type and proliferative status. Activated Raf can regulate the transcriptional activity of AR in the presence or absence of ligand [Abreu-Martin et al., 1999. This link between Raf and AR signaling provides a molecular basis for androgen-independent growth of prostate cancer [Abreu-Martin et al., 1999. In prostate cancer cells, PKA modulates EGF- and IGF-I-induced activation of the ERK1/2 pathway [Putz et al., 1999 and the PKA signaling pathway can also lead to AR activation [Nazareth and Weigel, 1996. Therefore, PKA inactivation in prostate cancer cells might be an effective strategy for the treatment of prostate cancer.
The PI(3)K/Akt Pathway and AR Activity
Overexpression of Her-2/neu in prostate cancer cells causes an increase in protein kinase B (PKB)/Akt activity, and also promotes androgen-independent growth of prostate cancer cells [Craft et al., 1999. Akt is activated by Her2/neu and binds to and phosphorylates the AR, leading to androgen-independent growth of prostate cancer cells [Wen et al., 2000. Similarly, IL-4-induced activation of AR in prostate cancer cells is reported to be mediated by the activation of the PI(3)K/Akt pathway [Lee et al., 2005. These results suggest that signal transduction via PI(3)K/Akt/AR pathways may play an important role in progression of androgen independent prostate cancer cells (Fig. 3). Gregory et al. 2005 showed that heregulin-induced activation of Her-2 and Her-3 increased AR transactivation, leading to proliferation of CWR-R1 cells. Transactivation of AR could be inhibited by the Her-2/EGFR dual kinase inhibitor GW572016 through inactivation of Akt, suggesting the importance of Akt in the transactivation of AR. There is also a marked increase in Akt activity in androgen-independent LNA1 prostate cancer cells established from xenograft tumors of the androgen-dependent LNCaP cell line [Graff et al., 2000. The PTEN/MMAC1 tumor suppressor phosphatase, which exerts negative regulation on the PI(3)K/Akt pathway [Ramaswamy et al., 1999, is downregulated in hormone-refractory prostate cancer [Whang et al., 1998. Thus, there is a dynamic interaction between steroid hormones and growth factors in regulating the PI(3)K/Akt signaling pathway.
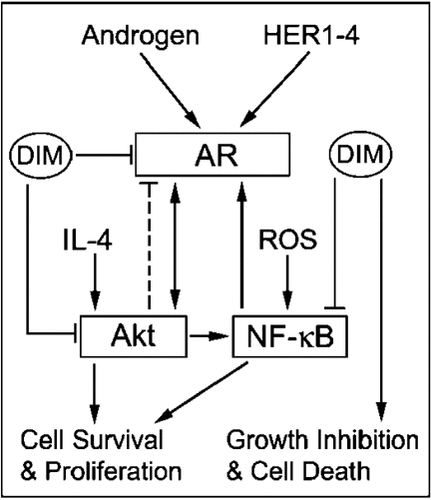
3-3′-Diindolemeythane (DIM) inhibition of Akt/NF-κB-mediated expression and function of AR. ROS, reactive oxygen species.
The exact mechanism by which Akt affects the AR signaling pathway remains obscure. It is reported that Akt enhances AR transactivation either directly through its interaction with AR or indirectly by regulating GSK3β and β-catenin [Wen et al., 2000; Sharma et al., 2002; Wang et al., 2004. Akt is also reported to be involved in signaling pathways that suppresses AR activity [Lin et al., 2001; Yang et al., 2003a,b. Yang et al. 2005 showed that activated FOXO3a could enhance AR gene expression and AR transactivation by binding to the AR 5′ promoter region. This indicates that inhibition of PI(3)K and Akt, the dominant survival factors in prostate cancer cells, may result in activation of the FOXO3a transcription factor, which in turn may induce AR gene expression to protect cells from apoptosis.
Akt, Diindolylmethane (DIM), and AR
Indole-3-carbinol (I3C), its in vivo dimeric product 3,3′-diindolylmethane (DIM), and formulated DIM (B-DIM), inhibit the growth of breast cancer cells and the PC3 prostate cancer cell line by blocking Akt signaling [Wattenberg and Loub, 1978; Rahman et al., 2000; Chinni et al., 2001; Chinni and Sarkar, 2002; Nachshon-Kedmi et al., 2003; Li et al., 2005. I3C, DIM, and B-DIM have very little effect on Akt activity or on proliferation or viability in non-tumorigenic CRL-2221 prostate epithelial cells. This suggests that I3C, DIM, and B-DIM may have a cancer specific effect [Chinni and Sarkar, 2002; Li et al., 2005. Thus, I3C, DIM, and B-DIM can block androgen-dependent or -independent growth of prostate cancer in a target-specific fashion by inhibiting the PI(3)K/Akt signaling pathway involved in AR expression and function.
CALCIUM AND CALMODULIN IN AR EXPRESSION AND FUNCTION
A Role for Calcium in Prostate Cancer
As shown in Figure 2, a final step in the G1 to S transition involves activation of membrane-bound phospholipase C (PLC) to generate diacyl glycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). The IP3 thus formed causes release of Ca++ from intracellular stores. In hormone-sensitive prostate cancer cells, intracellular Ca++ is a potent regulator of AR gene expression [Gong et al., 1995. Ca++ channel blockers, such as Verapamil or nifedipine, delay regression of androgen-dependent prostatic tissue by suppressing the induction of c-fos and testosterone-repressed prostate message-2 (TRPM-2), a gene expressed by dying prostate epithelial cells. This suggests a crucial role for Ca++ ion flux in the pathway leading to programmed prostate epithelial cell death [Connor et al., 1988. Androgen-deprivation induces an increase in intracellular Ca++ that could have a secondary effect on expression of AR-regulated genes. A23187, a Ca++-channel blocker, represses androgen-induced expression of PSA and hKLK2 mRNA, and rapidly induces c-fos and c-jun mRNA expression. AP-1 proteins (c-fos and c-jun) are suggested to interfere with AR transactivation activity [Murtha et al., 1997. Calcium activates several calcium-binding proteins, including calmodulin (CaM). Enhanced expression of CaM is associated with the induction of apoptosis in androgen-independent rat prostate cancer cells [Furuya et al., 1994 and androgen modulates CaM expression in rat prostate glandular cells [Furuya and Isaacs, 1994.
Calcium, Calmodulin (CaM), and AR
A specific cell-permeable CaM-antagonist, N- (6-aminohexyl)-5-chloro-1-naphthalene-sulfon-amide hydrochloride (W-7) [Hidaka et al., 1981, inhibits proliferation of androgen-sensitive LNCaP cells six to eight-times more effectively than it inhibits androgen-independent PC-3 cells [Cifuentes et al., 2004. Consistent with a possible role for CaM in AR action, AR in nuclear extracts of LNCaP cells binds to CaM [Cifuentes et al., 2004. AR binding to CaM is Ca++-dependent, and is inhibited by pre-treatment of the cell extracts with W-7. Furthermore, using immunofluorescence staining and confocal microscopy, AR can be shown to colocalize with CaM in the nucleus of LNCaP cells. The functional relevance of AR–CaM interactions in intact prostate cancer cells is demonstrated by the observation that W-7 is as effective as the anti-androgen Casodex in blocking AR-regulated expression of PSA in LNCaP cells. AR seems to interact with CaM directly since purified human AR binds to CaM-agarose, and CaM can be detected in AR-immunoprecipitates prepared from purified soluble proteins. Furthermore, the anti-CaM drug W-7 decreases AR levels in LNCaP cells [Cifuentes et al., 2004. Taken together, these observations suggest a direct interaction between CaM and AR that is essential for structural and functional stability of AR in prostate cancer cells. Anti-CaM drugs, such as W-7, may be useful for the treatment of prostate cancer by downregulating AR.
CaM is also known to activate a number of protein kinases, including death-associated protein kinase (DAPK) [Shohat et al., 2002, DNA-dependent protein kinase (DNA-PK) [Byrum et al., 2004, and Akt [Deb et al., 2004. DNA-PK catalytic subunits Ku70 and Ku80 [Mayeur et al., 2005, and Akt [Lin et al., 2001; Sun et al., 2003, form a complex with AR and affect phosphorylation, function, and stability of AR. CaM interacting with AR can engage CaM-dependent protein kinases, particularly DNA-PK and Akt that interact with AR [Lin et al., 2001, 2002c; Sun et al., 2003; Mayeur et al., 2005, to phosphorylate AR and influence AR nuclear localization and/or stability through the ubiquitin-proteasome pathway. Interestingly, CaM also binds to 26S proteasome subunits and deubiquitinating enzymes [Shen et al., 2005. Therefore, anti-CaM drugs may cause AR exclusion from nuclei and/or decrease the AR protein level by a mechanism involving post-translational modification of AR.
DIRECT AND INDIRECT ROLES FOR THE 26S PROTEASOME IN AR FUNCTION
Role of the 26S Proteasome and NF-κB in Apoptosis of Cancer Cells
Besides the signaling pathways that induce expression and activation of cell cycle regulatory proteins, endogenous proteases and protease inhibitors that control steady state levels of some of the cell cycle regulatory proteins also play an important role in proliferation and viability. The 26S proteasome pathway plays a critical role in regulating the level of a number of cell cycle regulatory proteins including cyclins (e.g., cyclins D and E) and CKIs (e.g., p21Cip, p27Kip) that interact with AR. Timely degradation of these proteins is essential for cells to progress from one phase of the cell cycle to the next [Reddy, 1999; Reddy et al., 2004. It is reported that the nuclear and cytosolic localization of the proteasome changes in a cell cycle-dependent manner [Palmer et al., 1994 and that the inhibition of chymotrypsin-like activity in the proteasome causes complete block of G1/S and metaphase transition and delays progression through S phase in cultured cells [Machiels et al., 1997. The ubiquitin/proteasome system involves two distinct steps: ubiquitination and degradation [Ciechanover, 1994; Hochstrasser, 1995. Ubiquitination, which requires several enzymes including E1, E2, and E3, is the step required for target protein recognition by the proteasome complex. Degradation of proteins then occurs on a large 26S-proteasome complex in an ATP-dependent manner [Seemuller et al., 1995; Voges et al., 1999. For example, AR is degraded by the proteasome pathway after AR is ubiquitinated by Mdm2 E3 ligase [Lin et al., 2002b. The importance of the proteasome is demonstrated by the induction of apoptosis by proteasome inhibitors, such as CEP1612 and Velcade. Tumor cell lines susceptible to proteasome inhibitor-induced programmed cell death include prostate, breast, leukemia, bone, brain, and head and neck; normal human cells are not affected [Imajoh-Ohmi et al., 1995; Shinohara et al., 1996; Drexler, 1997; Lopes et al., 1997; An et al., 1998; Adams et al., 1999.
Inhibition of tumor cell proteasome activity in various tumor culture models is generally associated with inactivation of NF-κB and the induction of apoptosis (Fig. 3) [Adams et al., 1999; An et al., 2000; Hideshima et al., 2001; Adams, 2002, 2003; Dou and Goldfarb, 2002. Transcription factors of the NF-κB family are constitutively activated in various human malignancies, including prostate tumors [Ditsworth and Zong, 2004; McCarty, 2004; Sarkar and Li, 2004. It has been suggested that NF-κB contributes to development and/or progression of malignancy by regulating the expression of genes involved in cell growth and proliferation, anti-apoptosis, angiogenesis, and metastasis [Aggarwal, 2004; Monks et al., 2004. Prostate cancer cells are reported to have constitutive NF-κB activity, partially due to increased activity of the IκB kinase complex [Suh et al., 2002. The ubiquitin/proteasome-dependent degradation pathway plays an essential role in activation of NF-κB by controlling the processing of the NF-κB precursor p105 to the DNA-binding component p50 and by degrading its cytosolic inhibitor IκB-α [Magnani et al., 2000; Tanaka et al., 2001; Beinke and Ley, 2004. It has been reported that inhibition of the proteasomal chymotrypsin-like activity is associated with induction of apoptosis in tumor cells [Lopes et al., 1997; An et al., 1998. This is consistent with increased proteasome activity in prostate cancer cells [Suh et al., 2002. Constitutive NF-κB activity and increased proteasome activity is also seen in primary prostate cancer tissue samples [Li and Dou, 2000; Suh et al., 2002.
The Proteasome and AR
Several lines of experimental evidence suggest involvement of the proteasome in regulating AR expression and function. First, the proteasome inhibitor MG132 inhibits AR protein expression, which results in reduced transcriptional activity of AR, and suppression of PSA expression [Lin et al., 2002a. Second, the proteasome inhibitor PS-341 downregulates AR basal levels and induces growth arrest and apoptosis of androgen-dependent human prostate cancer LNCaP cells. In addition, PS-341 downregulates both 5α-dihydrotestosterone (DHT)- and IL-6-induced PSA expression [Ikezoe et al., 2004. Third, hyperthermia-induced apoptosis and radiosensitization [Pajonk et al., 2005 are associated with decreased 26S proteasome activity and accumulation of IκB (which inhibits constitutive and radiation-induced activation of NF-κB). This is accompanied by dramatically decreased AR protein levels in LNCaP cells [Pajonk et al., 2005. These studies taken together suggest that inhibition of proteasome function and proteasome-dependent signal transduction pathways may be a major molecular mechanism of AR downregulation and induction of apoptosis and radiosensitization. Lastly, under certain experimental conditions, NF-κB binding to the AR promoter region also results in repression of AR expression [Supakar et al., 1995. Thus, NF-κB can affect AR expression either positively or negatively in prostate cancer cells.
Potential Mechanisms of the AR–Proteasome Interaction
Although proteasome inhibition is associated with downregulation of AR expression [Lin et al., 2002a; Ikezoe et al., 2004; Pajonk et al., 2005, the molecular mechanism in this process is not clear. However, three potential mechanisms have been suggested (Fig. 4). First, proteasome inhibition could lead to the inhibition of AR transcription. It is known that expression of the AR gene is mediated by NF-κB activity [Delfino et al., 2003; Zhang et al., 2004. Consistently, three NF-κB-binding sites have been identified in the promoter region of rat, mouse, and human AR genes, and overexpression of the NF-κB subunits stimulates AR promoter activity [Delfino et al., 2003; Zhang et al., 2004. Since NF-κB activation requires the ubiquitin/proteasome-dependent processing of its precursor p105 to the DNA-binding component p50 and degradation of its cytosolic inhibitor IκB-α [Magnani et al., 2000; Tanaka et al., 2001; Beinke and Ley, 2004, proteasome inhibition may lead to downregulation of AR expression by blocking the NF-κB pathway.
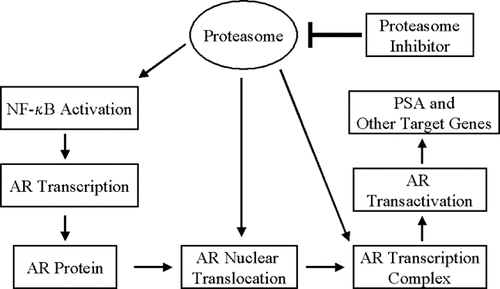
Proteasome inhibitors target multiple pathways essential for AR expression and function in human prostate cancer cells.
Second, proteasome inhibition could cause inhibition of AR nuclear translocation (Fig. 4). Mechanistic studies indicate that the proteasome inhibitor MG132 suppresses AR transactivation by inhibiting nuclear translocation of AR [Lin et al., 2002a. Another proteasome inhibitor PS-341 was shown to decrease nuclear AR protein levels and inhibit PSA promoter-driven gene expression [Ikezoe et al., 2004.
Third, proteasome inhibitors may inhibit interaction between AR and its co-regulators (Fig. 4). The proteasome inhibitor MG132 suppresses AR transactivation by inhibiting AR interaction with the co-regulators ARA70 or TIF2 [Lin et al., 2002a. Indeed, proteasome activity is required for formation of the AR transcription complex that contains AR, RNA polymerase II, and co-activators such as GRIP1 and CREB-binding protein [Kang et al., 2002. It appears that the loading of AR protein onto, and the recruitment of RNA polymerase II to, the PSA and kallikrein 2 promoters occur transiently in LNCaP cells. The proteasome inhibitor MG-132 prevents release of AR from the PSA promoter and blocks androgen-induced PSA mRNA accumulation. Finally, AR and a 19S proteasome subcomplex both bind to the PSA promoter [Kang et al., 2002, consistent with the hypothesis that they are part of the same complex. Thus, inhibition of 26S proteasome not only detrimental to prostate cancer cells but also suppresses the expression and stability of AR. Therefore, targeting 26S proteasome to eliminate AR expression and function could be an effective strategy for the treatment of prostate cancer.
CONTROL OF PROSTATE CELL GROWTH BY REGULATION OF AR TRAFFICKING
Growth factor/cytokine-dependent progression of cells from one phase to the next is also tightly coupled to transient changes in a number of proteins that regulate gene expression. Some of these proteins include transcription factors (e.g., NF-κB, E2F1, pRB), co-activators (e.g., p300/CBP, P/CAF), co-repressors (e.g., HDAC1), and chaperone proteins (e.g., Hsp70, Hsp90) that interact with AR to influence its expression, stability, and function [Horwitz et al., 1996; Glass et al., 1997; Shibata et al., 1997; Yeh et al., 2000. Transcription-regulating factors, such as Hsp90 and HDAC1, are regulated not only by growth factors that promote proliferation [Meares et al., 2004; Joshi et al., 2005; Peng et al., 2005 but also by AR [Georget et al., 2002; Hong et al., 2005. In addition to the regulation of their expression by AR, both Hsp90 and HDAC1 bind directly to AR [Georget et al., 2002; Marks et al., 2004. These two proteins also bind to a serine protease inhibitor (serpin), maspin [Lockett et al., 2006 whose expression interestingly is repressed by AR [Zhang et al., 1997a.
Maspin—An Epithelial Serpin With Tumor Suppressor Activity
In general, serpins are endogenous anti-proteases some of which may be tumor suppressors. Maspin is a 42-kDa protein with overall sequence homology to serpins [Zou et al., 1994. Maspin's protein sequence is highly conserved among rats, mice, and humans [Umekita et al., 1997; Zhang et al., 1997b. Maspin expression predicts a better prognosis in breast, prostate, colon, and oral carcinomas [Sheng, 2004. For example, in breast tissues, maspin is highly expressed in normal epithelial cells (especially in myoepithelial cells) and it is downregulated in invasive and metastatic breast carcinoma [Zou et al., 1994. In oral squamous carcinoma, maspin expression correlates with better prognosis [Xia et al., 2000. In prostate cancer, evaluation of maspin expression in tumor foci from whole mounted prostate specimen revealed that maspin was not expressed in a significant proportion of tumors. Prostate cancer patients whose tumors expressed maspin had a significantly longer recurrence-free survival [Zou et al., 2002. Thus, loss of maspin expression correlates with tumor aggressiveness [Pierson et al., 2002.
Regulation of Maspin Expression
Maspin expression is regulated by a promoter with two response elements—Ets and a promiscuous hormone response element that binds GR and PR [Zhang et al., 1997a. In normal prostate epithelia and normal prostate cell lines, maspin is highly expressed [Pierson et al., 2002 particularly by basal cells [Pierson et al., 2002. Maspin expression is upregulated in high-grade prostate intraepithelial neoplasia, a presumed precursor of prostate carcinoma [Pierson et al., 2002. In prostate carcinoma, maspin expression is progressively downregulated in a manner that correlates with tumor grade [Pierson et al., 2002 and maspin expression is almost totally suppressed in the prostate cancer cell lines LNCaP, DU145, and PC-3 [Zhang et al., 1997a.
The role of AR in regulating maspin expression is complex since the hormone response element of maspin's promoter appears to be a negative hormone response element [Zhang et al., 1997a. When patients are treated with androgen-ablation therapy before radical prostatectomy, maspin expression is elevated [Zou et al., 2002. Similarly, LNCaP cells cultured in androgen-depleted medium show induction of maspin promoter activity in a promoter luciferase reporter assay [Zhang et al., 1997a. Furthermore, castration of nude mice induces maspin expression in LNCaP xenograft tumors. These data indicate that maspin may be transiently upregulated in early stages of prostate tumor development and remains sensitive to AR repression.
What do prostate cancer cells gain by AR-mediated maspin suppression? Maspin downregulation correlates with increased tumor growth and metastasis. Conversely, experimental evidence demonstrates a tumor suppressive effect of maspin in terms of tumor cell motility and invasion in vitro, tumor growth, tumor-induced angiogenesis, and tumor metastasis in vivo. Maspin has also been shown to induce prostate tumor cell dedifferentiation and to increase tumor cell sensitivity to drug-induced apoptosis [Sheng, 2004; Lockett et al., 2006. The role of AR both in tumorigenesis and tumor progression may be, at least in part, facilitated by downregulation of tumor suppressive maspin. The evidence that maspin exerts a tumor suppressive effect in various prostate cancer cell lines regardless of their AR status does not exclude the possibility that maspin may be of potential therapeutic value in treating hormone-refractory prostate cancer.
Regulation of AR by Maspin
Screening studies identified a short list of candidate intracellular partners of maspin [Lockett et al., 2006. These included heat shock proteins (Hsp90 and Hsp70), glutathione s-transferase (GST), interferon regulatory factor 6 (IRF6), and histone deacetylase 1 (HDAC1) [Lockett et al., 2006. These findings shift our understanding of the role of maspin from that of a serine protease inhibitory serpin to that of a stress-responsive chaperone, and provide a new framework for understanding the mechanism by which maspin operates in tumor suppression [Wade, 2001; Mai et al., 2005. HDAC1, the most abundant HDAC in mammalian cells, is a nuclear protein [Ruden et al., 2005, whereas Hsp90, one of the most abundant stress-responsive chaperones, may be shuttled between cytoplasm and nucleus to protect its client proteins from degradation [Lockett et al., 2006. While the molecular interactions of maspin with HDAC1 and/or Hsp90 may underlie the positive correlation between nuclear maspin and better prognosis of cancer [Edwards and Bartlett, 2005a,b, as summarized below, both Hsp90 and HDAC have been implicated as key regulators of AR activity and stability.
Hsp90 binds and protects the native conformation of AR [Georget et al., 2002. Ligand binding, mutation of AR, or Her-2/neu-mediated Akt induced phosphorylation of AR results in an active AR conformation. The active AR translocates into the nucleus where it recruits co-activators, dissociates from co-repressors, and binds to the target promoter sequence to activate expression of ARE controlled genes [Georget et al., 2002; Orio et al., 2002; Solit et al., 2003. Inhibition of Hsp90 destabilizes both wild type and mutant AR, presumably by releasing AR from the chaperone complex, to be subjected to degradation by the proteasome [Gaughan et al., 2002; Fu et al., 2004. Once in the nucleus, AR is acetylated, which may be mediated by its co-activators such as p300 [Gaughan et al., 2002. Acetylated AR has been shown to specifically interact with and be deacetylated by HDAC1 [Marks et al., 2004. Inhibition of HDAC specifically upregulates genes that promote cell differentiation, cell cycle arrest, or cell death, and downregulates genes that promote tumor survival and epithelial–mysenchymal transition [Mai et al., 2005. Pharmacological inhibitors of HDAC show clinical activity with objective tumor regression [Kelly et al., 2005; Ryan et al., 2005.
In light of the tumor suppressive effects of maspin and new evidence for direct interactions of maspin with both Hsp90 and HDAC1, we propose a working model (Fig. 5) in which maspin plays an important role in the regulation of AR stability and function. We propose that maspin, by binding to the ADP-bound Hsp90, dissociates AR from the Hsp90 complex. The dissociated AR may subsequently be degraded by the proteasome, thereby reducing the cytosolic pool of AR for ligand binding or kinase-mediated activation. Furthermore, maspin may interact with nuclear HDAC1 to regulate the acetylation of both histone and AR, and ultimately suppress AR-mediated gene expression. According to this model, maspin may negatively regulate AR-dependent survival/proliferation of both hormone-sensitive and hormone-refractory prostate epithelial cells. Therefore, genetic engineering approaches to induce maspin expression may prove to be effective in blocking Hsp90-mediated stability, and/or HDAC1-mediated transcriptional activation, of AR in prostate cancer cells.
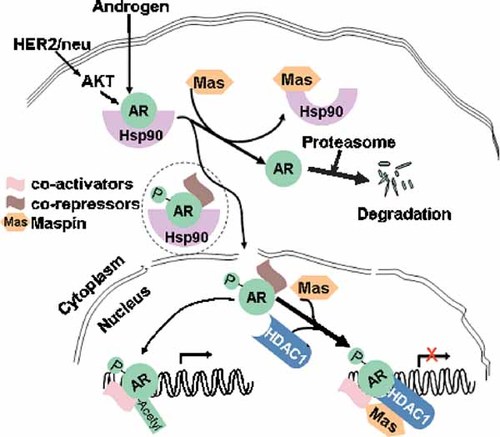
A hypothetical role of maspin in intracellular localization, stability, and function of AR.
CONCLUSION
Centrally involved in the process of prostate carcinogenesis, AR appears to be required for the growth of adenocarcinoma of the prostate even when androgen ablation therapy has rendered the prostate cancer hormone refractory. Blocking AR activity in cells that have become hormone refractory has been a daunting task in the treatment of prostate cancer. Recent advances in prostate cancer research have identified a number of cellular processes that control the growth and survival of prostate cancer cells; these pathways can regulate the expression, stability, and function of AR, and may even circumvent the androgen requirement for AR activation. Among the cellular processes that may contribute to ligand-independent AR activity are the signaling molecules and pathways activated by growth factors (e.g., IGF-I, EGF, ErbB2, and KGF) and cytokines (e.g., IL-6 and IL-4), and their cognate receptors, whose expression is elevated in prostate cancer patients. Protein kinase pathways, such as the PI(3)K/Akt, stimulated by growth factors and cytokines can activate AR. Similarly, Ca++ released from intracellular stores by growth factor/cytokine activation of phospholipase C can induce AR expression and activate calmodulin, which in turn binds to AR and regulates its stability and function. Studies of the effect of inhibitors of the 26S proteasome pathway suggest that it activates NF-kB required for AR expression and that it is involved in the assembly of the AR transcription complex. Finally, recent screening studies discovered that a chaperone protein Hsp90 and a deacetylase enzyme HDAC1 that regulate AR intracellular location and function, respectively, bind directly to the serpin maspin. This novel finding raises the intriguing possibility that maspin, by binding to AR interacting proteins Hsp90 and HDAC1, may play an important role in the regulation of AR stability and function. These inter-relationships and unexpected regulatory interactions between some of the signaling molecules and pathways that control cell fate and the AR open up a new world of potential sites for pharmacological intervention aimed at inhibiting AR expression, stability, and function. Frontline therapy using agents that disrupt the interaction of AR with pathways involving PI(3)K/Akt, Ca++/calmodulin, ubiquitin/26S proteasome, and maspin/Hsp90/HDAC1, in combination with androgen-ablation, rather than androgen-ablation alone, may prove to be an effective strategy not only to prevent the development, but also to treat hormone-refractory prostate cancer that often is fatal.
Acknowledgements
We gratefully acknowledge the support of NIH grants DK57864 (GPVR), CA083695, CA101870, and CA108535 (FHS), and DOD grant PC41004 (GPVR).




