Reduction of anabolic signals and alteration of osteoblast nuclear morphology in microgravity
Abstract
Bone loss has been repeatedly documented in astronauts after flight, yet little is known about the mechanism of bone loss in space flight. Osteoblasts were activated during space flight in microgravity (µg) with and without a 1 gravity (1 g) field and 24 genes were analyzed for early induction. Induction of proliferating cell nuclear antigen (PCNA), transforming growth factor β (TGFβ), cyclo-oxygenase-2 (cox-2), cpla2, osteocalcin (OC), c-myc, fibroblast growth factor-2 (fgf-2), bcl2, bax, and fgf-2 message as well as FGF-2 protein were significantly depressed in µg when compared to ground (gr). Artificial onboard gravity normalized the induction of c-myc, cox-2, TGFβ, bax, bcl2, and fgf-2 message as well as FGF-2 protein synthesis in spaceflight samples. In normal gravity, FGF-2 induces bcl2 expression; we found that bcl2 expression was significantly reduced in microgravity conditions. Since nuclear shape is known to elongate in the absence of mitogens like FGF-2, we used high-resolution image-based morphometry to characterize changes in osteoblast nuclear architecture under microgravity, 1 g flight, and ground conditions. Besides changes in cell shape (roundish/elliptic), other high-resolution analyses show clear influences of gravity on the inner nuclear structure. These changes occur in the texture, arrangement, and contrast of nuclear particles and mathematical modeling defines the single cell classification of the osteoblasts. Changes in nuclear structure were evident as early as 24 h after exposure to microgravity. This documented alteration in nuclear architecture may be a direct result of decreased expression of autocrine and cell cycle genes, suggesting an inhibition of anabolic response in µg. Life on this planet has evolved in a normal gravity field and these data suggest that gravity plays a significant role in regulation of osteoblast transcription. J. Cell. Biochem. © 2006 Wiley-Liss, Inc.
A projected bone loss of 20–30% in astronauts is one of the major physiological show stoppers in the proposed 30-month manned mission to Mars. For the first three decades of space flight, scientists have discovered systemic changes in the body, which included loss of bone and increases in urinary calcium. From these animal and human studies, investigators found that loss of weight bearing bone can be as high as 3% per month and this loss is primarily due to lack of new osteoblast growth in space flight [Wronski and Morey, 1983; Nicogossian et al., 1989; Marie et al., 2000; Vico et al., 2000. Although flight opportunities have been limited, the volume of data about microgravity effects on cell proliferation and differentiation is accumulating. It has been demonstrated that cell growth and differentiation of osteoblasts is inhibited or altered in a microgravity environment [Fitzgerald and Hughes-Fulford, 1996; Hughes-Fulford and Lewis, 1996; Carmeliet et al., 1998; Landis et al., 2000; Granet et al., 2001 suggesting that the osteoporosis seen in spaceflight is occurring at the cellular level. Altered gene expression, reduction in transcription factors, and reductions in growth factor related proteins have also been observed in other cells in microgravity [de Groot et al., 1990; Cubano and Lewis, 2000; Goodwin et al., 2000. In 2001, Lewis et al. found a 5.9-fold reduction in message for TGFα in space-flown lymphocytes [Lewis et al., 2001.
In this report, we found significant reduction in expression of several osteoblast genes including fibroblast growth factor-2 (fgf-2), cyclo-oxygenase-2 (cox-2), c-myc, transforming growth factor β (TGFβ), proliferating cell nuclear antigen (PCNA), osteocalcin (OC), and bax in osteoblasts grown in µg. Since fgf-2, TGFβ, and cox-2 are osteoblast autocrine/paracrine factors important in the G1-S phase transition [Canalis et al., 1991; Hughes-Fulford et al., 1992; Stachowiak et al., 1994; Coffin et al., 1995; Keresztes and Boonstra, 1999, it is possible that their decreased expression might be linked with inhibition of activation of bone growth in microgravity.
Since fgf-2 is a known osteoblast autocrine/paracrine factor important in the G1-S phase transition [Canalis et al., 1991; Stachowiak et al., 1994; Coffin et al., 1995; Keresztes and Boonstra, 1999, we hypothesized that changes in fgf-2 expression may be linked to inhibition of bone growth in µg. In this study, two groups of osteoblasts onboard the Space Shuttle were exposed to µg for 19 h before they were stimulated with fetal calf sera (FCS); one group was grown in microgravity, the other in an artificial 1 g environment. We analyzed expression of fgf-2, a mitogen, and transcription factor that promotes proliferation.
As part of our study of fgf-2 induction, we examined concomitant changes in nuclear morphology of osteoblasts grown in microgravity. Rijken [de Groot et al., 1991 first noticed changes in cell shape in response to changed gravity in sounding rocket experiments in 1991. Morphologic changes were also reported in parabolic flight with osteoblasts exhibiting cytoplasmic retraction and membrane ruffling in ROS/17/2.8 cells [Guignandon et al., 1995. Increased PGE2 was also found in media, accompanied by significant flight-induced changes that included a decrease in cell area and irregular shape in some cells [Guignandon et al., 1995. Indomethacin reduced the irregular shape changes, but not the loss of cell area. Osteoblasts grown in microgravity on STS-56 demonstrated a reduction of growth in osteoblasts activated in-flight, suggesting that microgravity caused a decrease in cell proliferation resulting in changes in cytoskeleton. A portion of the cells exhibited a spindle shape after 4 days in microgravity, with the most dramatic change observed being the elongation of nuclear shape [Fitzgerald and Hughes-Fulford, 1996.
Since it has been suggested that changes in nuclear architecture and control of gene expression are interrelated [Debnath et al., 1999, we analyzed message expression of other genes involved in cell growth and correlated morphological changes between osteoblasts grown in microgravity, 1 g or gr. In this study we report significant microgravity-induced alterations in transcription factor message and protein expression accompanied by dramatic changes in osteoblast cell morphology. The changes in gene expression observed in osteoblasts in µg were not observed in osteoblasts maintained in an in-flight 1 g field, implying a gravity requirement for normal osteoblast growth. These combined changes in osteoblast cell physiology suggest that the decrease in bone formation in astronauts may be in part due to microgravity-induced alterations in the osteoblast at the gene expression and signal transduction level.
MATERIALS AND METHODS
Preparation of Cells
The MC3T3-E1 osteoblast cell line clonally derived from embryonic mouse calvaria [Hiramatsu et al., 1983, was kindly provided to us by Dr. M. Kumegawa (Josai Dental University, Japan). The cell line was maintained at low passage number. Cells were grown in alpha minimal essential medium (αMEM) with 10% fetal calf serum (Hyclone Labs, Inc., Logan, UT) supplemented with 2 mM L-glutamine (Sigma, St. Louis, MO), 25 mM HEPES, and antibiotic–antimycotic solution (100 U penicillin/ml, 0.01 mg streptomycin/ml, 0.25 mg amphotericin B/ml). Cells were grown in a 37°C incubator with 5% CO2. They were fed three times a week and passaged at confluence. For spaceflight experimental samples, 120,000–200,000 cells were plated onto non-coated, sterile 11 × 22 mm glass coverslips (Thomas Scientific, Swedesboro, NJ), placed in 6-well plates, and grown in 10% serum-containing αMEM overnight. Cell-coated coverslips were then transferred into 2% serum-containing medium for spaceflight in plungerbox units. In accordance with NASA flight rules, the plungerbox units were held for 17 h in the Shuttle at mid-deck temperature prior to launch. This, combined with low serum-containing medium, placed the osteoblast in a quiescent state prior to Space Shuttle launch. In order to achieve our goal to study changes in nuclear morphology in microgravity, we launched cells in a quiescent condition. Osteoblasts were sera activated on-orbit and µg or 1 g samples collected in-flight (Fig. 1). There were four samples (n = 4) for each time point in µg, 1 g flight, and gr samples. NASA's LSLE (Life Sciences Laboratory Equipment) freezer, was used for sample storage on the Space Shuttle. Supplements, such as dexamethasone β-glycerol phosphate and ascorbic acid were not added to the media since these agents are known to directly effect gene expression and cell morphology of the osteoblast.
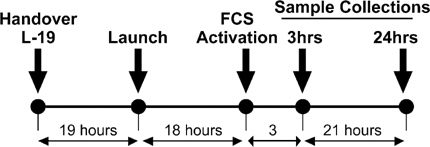
Time line of experiment from handover. 19 h prelaunch, activation in-flight, and termination of experiment in-flight.
Biorack Facility and Osteo Hardware
Cells were grown in the Biorack, a multiuser facility, which consists of incubators with variable gravity centrifuges, a cooler, a freezer, and a sealed glovebox. Two identical Biorack modules were used: one remained on earth (gr samples) and the other was integrated into SpaceHab and flown on each of the Space Shuttle flights STS-76, STS-81, and STS-84. Biorack has an important advantage over other microgravity facilities in that it provides a small radius (78 mm), slow rotating (107.0 ± 0.5 rpm) centrifuge for artificial 1 g in orbit. Because of their proximity, both the µg and 1 g samples experience identical launch vibrations, accelerations, cosmic radiation, and other unknown conditions of flight. In addition, an identical experiment was performed in the Biorack module on earth gr samples with a 2 h delay from in-flight procedures in order to control for any off-nominal operations during the flight. The Osteo (the name of this flight experiment) spaceflight hardware was designed according to European Space Agency (ESA) specifications for use in the Biorack facility and constructed by Centrum voor Constructie Mechatronica (CCM; Neuenen, Netherlands). The hardware consisted of the CCM plungerbox and its Type I container developed for spaceflight cell culture. The Type I container provided a second level of fluid containment. The plungerboxes were designed to provide a sterile environment for cell growth activation and fixation in a microgravity condition. The plungerbox is composed of two independent culture chambers that each hold two 11 mm × 22 mm glass coverslips. Each culture chamber was a separate sample. For each condition a sample size of n = 4 independent cultures was used. Each separate culture chamber has a series of reservoirs filled by either 10% serum-containing αMEM or fixative, which can be transferred into and out of the cell culture compartment by manually releasing a spring-loaded plunger.
Experiment Time Line
Quiescent cells were stored in the Space Shuttle mid-deck locker until 19 h after launch. The astronauts then transferred the samples into a 37°C incubator for 1 h before stimulating the cells to grow in the 1g centrifuge or µg static rack by changing their media from 2% serum to 10% serum-containing medium (T = 0). µg and 1 g samples were fixed during flight at 24 h with 3.7% formaldehyde solution and were stored at +5°C for the remainder of the Space Shuttle mission, while the GITC samples were frozen until return. There were four separate independent samples for each gravity condition at each time point (Fig. 1).
BIOCHEMICAL MEASUREMENTS
FGF-2 Protein Analysis
Analysis of FGF-2 was completed with a Quantities ELISA Immunoassay (R&D Systems, Inc., Minneapolis, MN) according to manufacturer's instructions. Cellular protein was collected from the GITC subnatant as previously described [Fitzgerald and Hughes-Fulford, 1999.
Analysis of Gene Expression
Table I shows the overview of gene expression measured in these experiments. RNA was extracted and purified by a modified acid guanidium thiocyanate/phenol/chloroform extraction method previously described [Fitzgerald and Hughes-Fulford, 1996; Barry et al., 1999; Fitzgerald and Hughes-Fulford, 1999.
| GENE | STS-76, 81, and 84 composite | |||
|---|---|---|---|---|
| Time point | # Of cycles | Present | Significant change | |
| 18s | T = 0 | 18 | Yes | No |
| T = 3 | 18 | Yes | ||
| T = 24 | 18 | Yes | ||
| CPHI | T = 0 | 22 | Yes | No |
| T = 3 | 24 | Yes | ||
| T = 24 | 24 | Yes | ||
| IL-1a | T = 0 | 42 | No | — |
| T = 3 | 42 | No | ||
| T = 24 | 42 | No | ||
| cox-2 | T = 0 | 34 | Yes | Yes |
| T = 3 | 30 | Yes | ||
| T = 24 | 30 | Yes | ||
| cox-1 | T = 0 | 40 | No | — |
| T = 3 | 40 | No | ||
| T = 24 | 40 | No | ||
| cpla2 | T = 0 | 32 | Yes | Yes |
| T = 3 | 32 | Yes | ||
| T = 24 | 32 | No | ||
| EP1 | T = 0 | 37 | Yes | No |
| T = 3 | 37 | Yes | ||
| T = 24 | 37 | Yes | ||
| EP2 | T = 0 | 40 | No | — |
| T = 3 | 40 | No | ||
| T = 24 | 40 | No | ||
| EP3 | T = 0 | — | — | |
| T = 3 | 47 | No | ||
| T = 24 | 47 | No | ||
| LDLr | T = 0 | 38 | — | — |
| T = 3 | 38 | No | ||
| T = 24 | 38 | No | ||
| Cyclin A | T = 0 | 38 | Yes | No |
| T = 3 | 38 | Yes | ||
| T = 24 | 38 | Yes | ||
| Cyclin E | T = 0 | 38 | — | No |
| T = 3 | 38 | Yes | ||
| T = 24 | 38 | Yes | ||
| PCNA | T = 0 | 42 | Yes | No |
| T = 3 | 42 | Yes | ||
| T = 24 | 42 | — | ||
| c-myc | T = 0 | 38 | Yes | Yes |
| T = 3 | 38 | Yes | ||
| T = 24 | 38 | Yes | ||
| cjun | T = 0 | 35 | No | — |
| T = 3 | 35 | No | ||
| T = 24 | 35 | No | ||
| TGFβ1 | T = 0 | 38 | Faint | Yes |
| T = 3 | 38 | Yes | ||
| T = 24 | 38 | No | ||
| FGF-2 | T = 0 | 34 | — | Yes |
| T = 3 | 34 | No | ||
| T = 24 | 34 | Yes | ||
| Actin | T = 0 | 30 | Yes | No |
| T = 3 | 30 | Yes | ||
| T = 24 | 30 | Yes | ||
| Fibronectin | T = 0 | 34 | Yes | No |
| T = 3 | 34 | Yes | ||
| T = 24 | 34 | Yes | ||
| Collagen | T = 0 | 33 | No | — |
| T = 3 | 33 | No | ||
| T = 24 | 33 | No | ||
| Osteocalcin | T = 0 | — | Yes | |
| T = 3 | 38 | Yes | ||
| T = 24 | 38 | Yes | ||
| β1 integrin | T = 0 | 47 | No | — |
| T = 3 | 47 | No | ||
| T = 24 | 47 | No | ||
| Bax | T = 0 | 30 | Yes | Yes |
| T = 3 | 30 | Yes | ||
| T = 24 | 30 | Yes | ||
| bcl2 | T = 0 | 34 | Yes | Yes |
| T = 3 | 38 | Yes | ||
| T = 24 | 38 | Yes |
- Osteoblasts were flown on the Shuttle in a quiescent state; after insertion into orbit, the cultures were activated with FCS. Many of the genes that had significantly changed expression in microgravity are early pleiotopic genes that normally are increased by growth factors found in FCS. Significant changes at the collection timepoints (Fig. 1) are seen in Figures 2 and 3 (n = 4).
RT-PCR Analysis
The semi-quantitative RT-PCR analysis and primer sequences for many of the genes have been described [Fitzgerald and Hughes-Fulford, 1996; Barry et al., 1999; Fitzgerald and Hughes-Fulford, 1999. To correct for small variations between experiments, each PCR product was compared to an internal standard of cyclophilin or 18 S products derived from the same RT reaction. Since sample size was small (200,000 cells), RNA content was held constant and linear RT-PCR was accomplished by varying the number of PCR cycles. Primers were designed in this laboratory to span introns to eliminate the possibility of detection of DNA. PCR conditions were established so that amplification reaction was stopped in the linear range. Primers not previously described are 18s F-TCA AGA ACG AAA GTC GGA GG; R-GGA CAT CTA AGG GCA TCA CA; CPHI F-CGT CTC CTT TGAG CTG TTT GCA GAC R-CAT AAT CAT AAA C TAA CTC TGC AAT CCA GC; fgf-2 F-AAC TAC AAC TTC AAG CAG AAG AGA GAR-TTA AGA TCA GCT CTT AGC AGA CAT; bcl2 F ACTTGTG GCCCA GATAGGCACCCAG; R CGACTTC GCCGAGATG TCCAGC CAG; IL-1β F-GTC TCT GAA TCA GAA ATC CTT CTA TC R-ATG TCA AAT TTC ACT GCT TCA TCC; cpla2 F-GGA TTC TCT GGT GTG ATG AAG G, R-CCC AAT CTG CAA ACA TCA GC ep1 F-CTG GAG GGG CGT GTC ATT TC, R- GCT GCG GAG GGT GGC TGT GG ep2 F-CTA CGC CGC CTT CTC TTA CAT G, R- GAT GTC TTT CAC CAC GTT TGG C; ep3 CAT CAC CAT GAT GGT CAC TGG C, R-GTC TTC ATG TGG CTG GGA TAC C; LDLr F-CAA TGT CTC ACC AAG CTC T, R-TCT GTC TCG AGG GGT AGC T; cyclin A F AAA CAG CCT GCC TTC ACC ATT CA R-ATA TTC TTC TCC CAC CTC AAC CA; cyclin E, F-CAA GTG GCC TAT GTC AAC GA R- ACT GCT GGG TGG GGG TGT CA; PCNA F-CAC CAA ATC AAG AGA AAG TT, R-GAG AAT GGA GAG AAA AGA AA; c-myc F-CCA ACA GGA ACT ATG ACC TCG, R-CCA CAT ACA GTC CTG GAT G; cjun F-GGA AAC GAC CTT CTA TGA CGA TGC CCT CAA, R-GAA CCC CTC CTF CTC ATC TGT CAC GTT CTT; collagen, F-GTC CCC CTG GCT CTG CTG GTT, R- TTT GGG TTG TTC GTC TGT TTC; β1 Integrin, TGT TCA GTG CAG CCT TCA, R-CCT CAT ACT TCG GAT TGA CC; bax F-CAG CTC TGA GCA GAT CAT GAA GAC A, R-GCC CAT CTT CTT CCA GAT GGT GAG C.
Cell Morphology
Osteoblasts were fixed on coverslips with 3.7% formaldehyde in phosphate buffered saline during spaceflight and stored at 5°C for 1–2 weeks before staining. There was no visible difference between control samples that were immediately stained and samples stored in formaldehyde at 5°C. For nuclear visualization, coverslips were stained with Hoechst dye number 33258 and rinsed in distilled water. The dried osteoblast coverslips were mounted onto slides and photographed with a Zeiss Axioscope microscope at 40× and 100× magnification. Slides were processed at the same time under identical conditions. Photographic exposure was taken at identical exposure times and conditions (Tables VI and VII; Figs. 4-7, 9).
IMAGING METHODS
Image Acquisition
Table II shows number of images at the total number of cells evaluated. Images were digitized from the color film slides with an Epson Expression 836XL scanner into Adobe Photoshop (Adobe Systems, Inc., Mountain View, CA) (Table III and Fig. 4). Images were stored as RGB (red, green, blue) TIF format image files (Fig. 5). From these RGB images (Fig. 5a) only the RED channel image (Fig. 5R) was used since the nuclear structure is best represented by this channel.
| Abbreviation | Number of images | Number of cells | Description |
|---|---|---|---|
| gr | 3 | 62 | Normal gravity |
| 1 g (flight) | 5 | 88 | Gravity by centrifuge |
| µg (flight) | 3 | 52 | Static in flight |
| % | gr | 1 g | µg | Feature | F-value | |
|---|---|---|---|---|---|---|
| gr | 75 | 47 | 13 | 2 | P2A | 49.7 |
| 1 g | 58.0 | 22 | 51 | 15 | HETERO | 28.4 |
| µg | 88.5 | 1 | 5 | 46 | GCV | 22.7 |
| Total | 71.3 | RHA | 11.3 |
Image Preprocessing and Segmentation
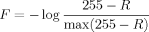
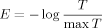
Feature Extraction
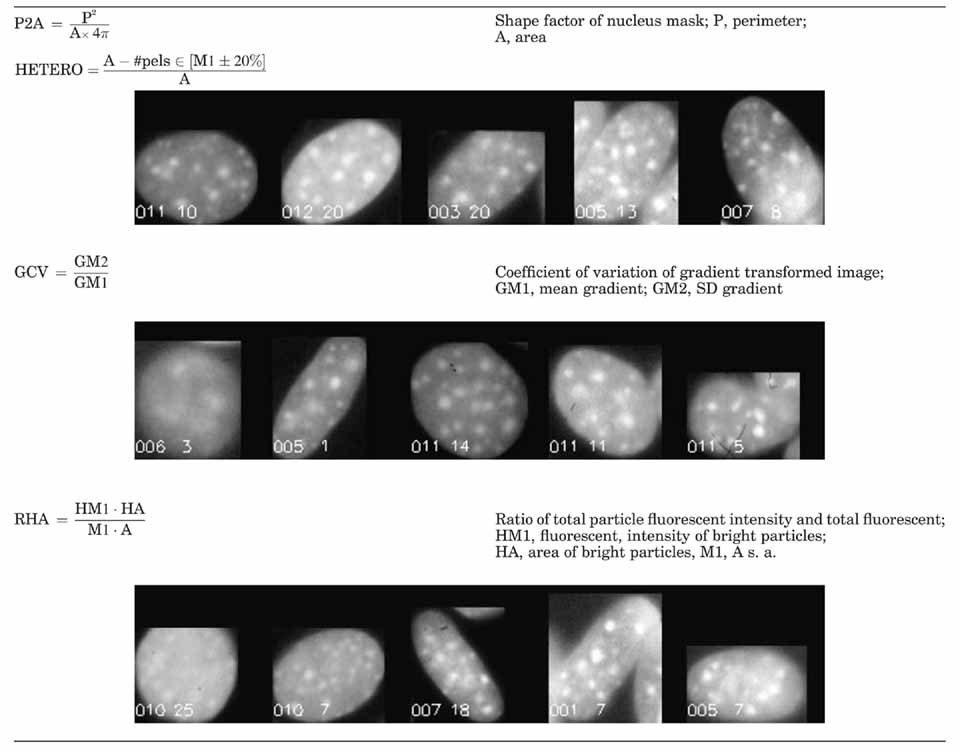
From each cell nucleus, represented by its mask M and the corresponding region in the fluorescent density image F, numerous quantitative features were calculated describing shape, photometric, and textural properties. The latter are understood as properties of the arrangement of fluorescent particles, which in this case is DNA, visualized by Hoechst 33528. These features are described in detail elsewhere [Rodenacker and Bengtsson, 2003 (Fig. 7). For classification, selected features are briefly described in Tables VI and VII. They are illustrated by image sequences showing the changing feature value with appropriate cell images.
Statistics and Classification

RESULTS
Gene Expression and Protein Levels in Microgravity
We analyzed induction of 24 osteoblast genes using linear semi-quantitative RT-PCR. We found reduced gene expression in 10 of these genes; no change in 9 of the genes and copy number was too low to measure in the remaining genes examined. Osteoblasts were activated during space flight in µg with and without a 1 g field and results compared to gr samples. Induction of PCNA, TGFβ, cox-2, cpla2, osteocalcin, c-myc, fgf-2, bcl2, and bax were significantly depressed in µg when compared to gr (Figs. 2 and 3). Artificial onboard gravity was able to normalize the induction of c-myc, cox-2, TGFβ, fgf-2, bax, and bcl2 as well as FGF-2 protein synthesis in spaceflight samples.
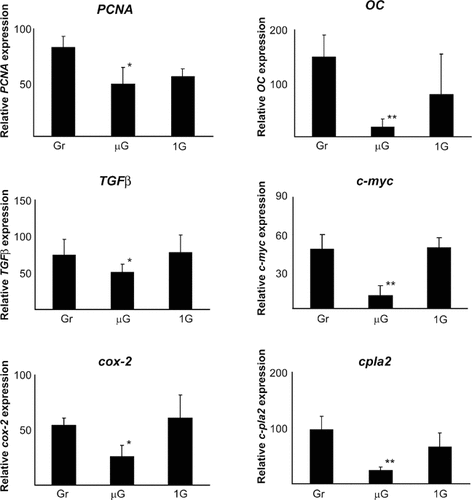
Analysis of gene expression at 3 h under 1 g flight, µg flight, and ground. N = 4, significance between 1 g and µg levels: P < 0.001, significance in ground and µg levels: P < 0.01 (bars denotes ± SD).
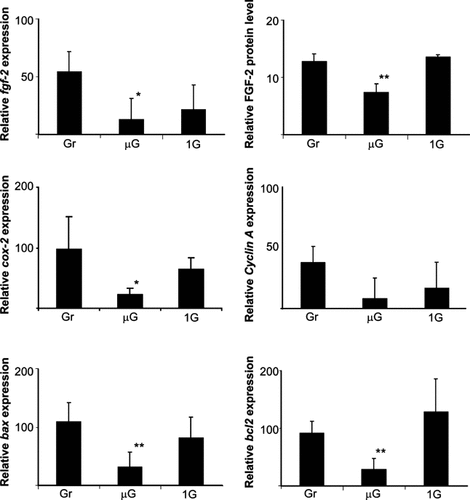
Analysis of gene expression at 24 h under 1 g flight, µg flight, and ground. N = 4, significance between 1 g and µg levels: P < 0.001, significance in ground and µg levels: P < 0.01 (bars denotes ± SD).
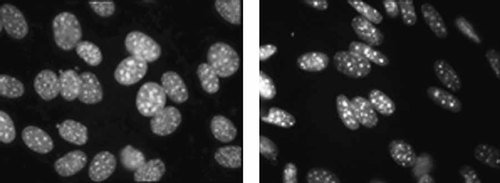
Original fluorescence image (gr) (left), µg (right).
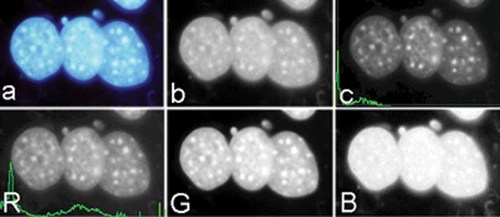
Effect of different filters on discrimination of features (a) original fluorescence image, (b) light intensity image, (c) fluorescent intensity F from RED channel with frequency distribution, (R) RED with frequency distribution, (G) GREEN, and (B) BLUE intensity channel. (Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.)
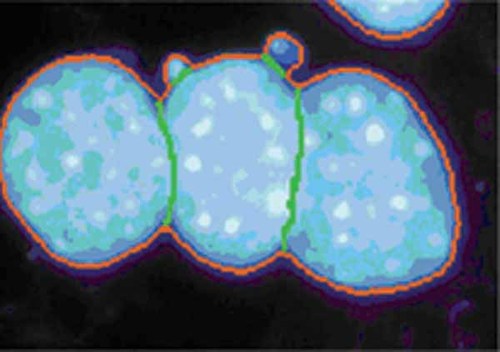
Segmentation with mask Mr by threshold (red) and manual cutting lines (green). (Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.)
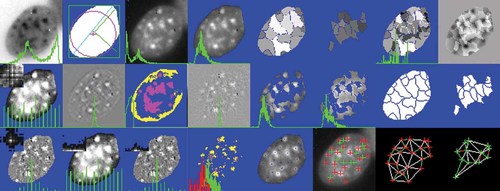
Illustrations of feature extraction for one nucleus.
There was no change in quiescent osteoblast PGE2 levels (gr 415 ± 163 vs. µg 453 ± 189). Even though the cox-2 message was reduced at 24 h, the PGE2 levels were high. However, in-flight at 24 h, both the 1 g and the µg samples had a significant rise in PGE2 levels: 1 g 6,065 ± 870 and µg 6,959 ± 1132 versus gr 3,904 ± 1673 (P < 0.005).
Since it has been previously demonstrated that a lack of mitogen causes elongation of nuclei [Foote et al., 1996, we measured endogenous levels of cellular fgf-2 message and protein in these microgravity grown samples. In our studies, we found that the fgf-2 message induction was depressed 24 h after activation (Fig. 3). Moreover, when the cellular protein levels were measured in these cells, we found that FGF-2 protein synthesis was also significantly reduced in µg flight, however, the presence of artificial gravity in-flight increased the ability of the cells to synthesize fgf-2 (Fig. 3) 24 h after serum activation. Finally, changes in bcl2 message (Fig. 3) induction parallel that of fgf-2 message. The bcl2 message was significantly decreased in microgravity compared to ground and 1 g samples.
Three-Class Case
This results in the most discriminative features P2A, HETERO, GCV, RHA, RHUA, NR1, and HLM3. A short description of the features selected is given in Tables VI and VII. Cell nuclei show clear differences in shape and texture. The first selected feature (P2A) is shape, remaining features are all texture related. Variation of P2A is based on the deviation from circularity to ellipticity. Further differences are reflected by several features sensitive to the different arrangement of bright fluorescent particles (HETERO) inside the darker nucleus surrounding and the less pronounced change between background inside the nucleus and bright particle (GCV).
The classification result is listed in Table III and illustrated in Figure 8 using the 1st and 2nd canonical variables (CAN1, CAN2) with the class discrimination lines. Using three-class discrimination analysis, it is clear that the gr and µg cell populations are significantly different from one another. The first canonical variable CAN1 alone permits a nearly complete discrimination at about CAN1 = 0.8 of the µg cells from the others (see Fig. 8). A sequence of cell images is displayed in Figure 9 using the first canonical variable as order criteria. Note the change of particle structure from left to right.
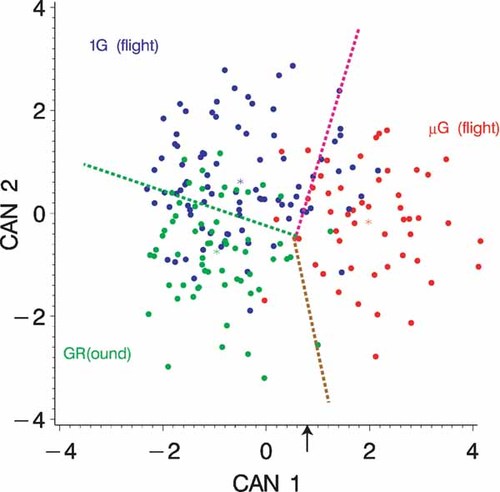
Three-class discrimination result using the 1st and 2nd canonical variables with discrimination lines between respective classes.
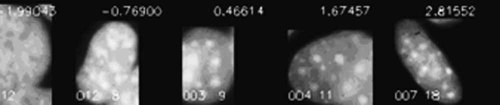
Transition of gr, 1 g cells to µg cells ordered by the first canonical variable CAN1 from the three-class discrimination (see Fig. 6) in the interval CAN1 ∈ [−2,3]. Notice the changes of the bright ‘particle’ structures from left to right. The corresponding CAN1 values are outlined in the upper image regions.
Two-Class Cases
They were analyzed to find out more about the changes from one experimental condition to another in terms of most selective features.
From ground (gr) to 1 g (in-flight) (Table IV): The selected features HETERO, ELNENL, RHA, and NR4 represent pure textural changes.
| % | gr | 1 g | Feature | F-value | |
|---|---|---|---|---|---|
| gr | 71.6 | 55 | 7 | HETERO | 20.3 |
| 1 g | 88.7 | 25 | 63 | ELNENL | 16.5 |
| Total | 78.7 | RHA | 10.6 |
From 1 g (in-flight) to µg (Table VA): The sequence of selected features starts with P2A, a shape factor showing the influence of gravity on growth form, followed by texture features HLM3 and RHUA.
| % | µg | 1 g | Feature | F-value | |
|---|---|---|---|---|---|
| µg | 90.4 | 47 | 5 | P2A | 69.5 |
| 1 g | 81.8 | 16 | 72 | HLM3 | 32.6 |
| Total | 85.0 | RHUA | 11.6 |
From ground (gr) to µg (Table VB): For completeness the direct transition from ground (GR) to µg is also outlined. Chosen features are HETERO, RHUA, and RATIO. The first two are pure textural and the last is a shape feature similar to P2A.
| % | gr | µg | Feature | F-value | |
|---|---|---|---|---|---|
| gr | 96.2 | 58 | 4 | HETERO | 98.9 |
| µg | 93.5 | 2 | 50 | RHUA | 43.2 |
| Total | 94.7 | RATIO | 30.1 |
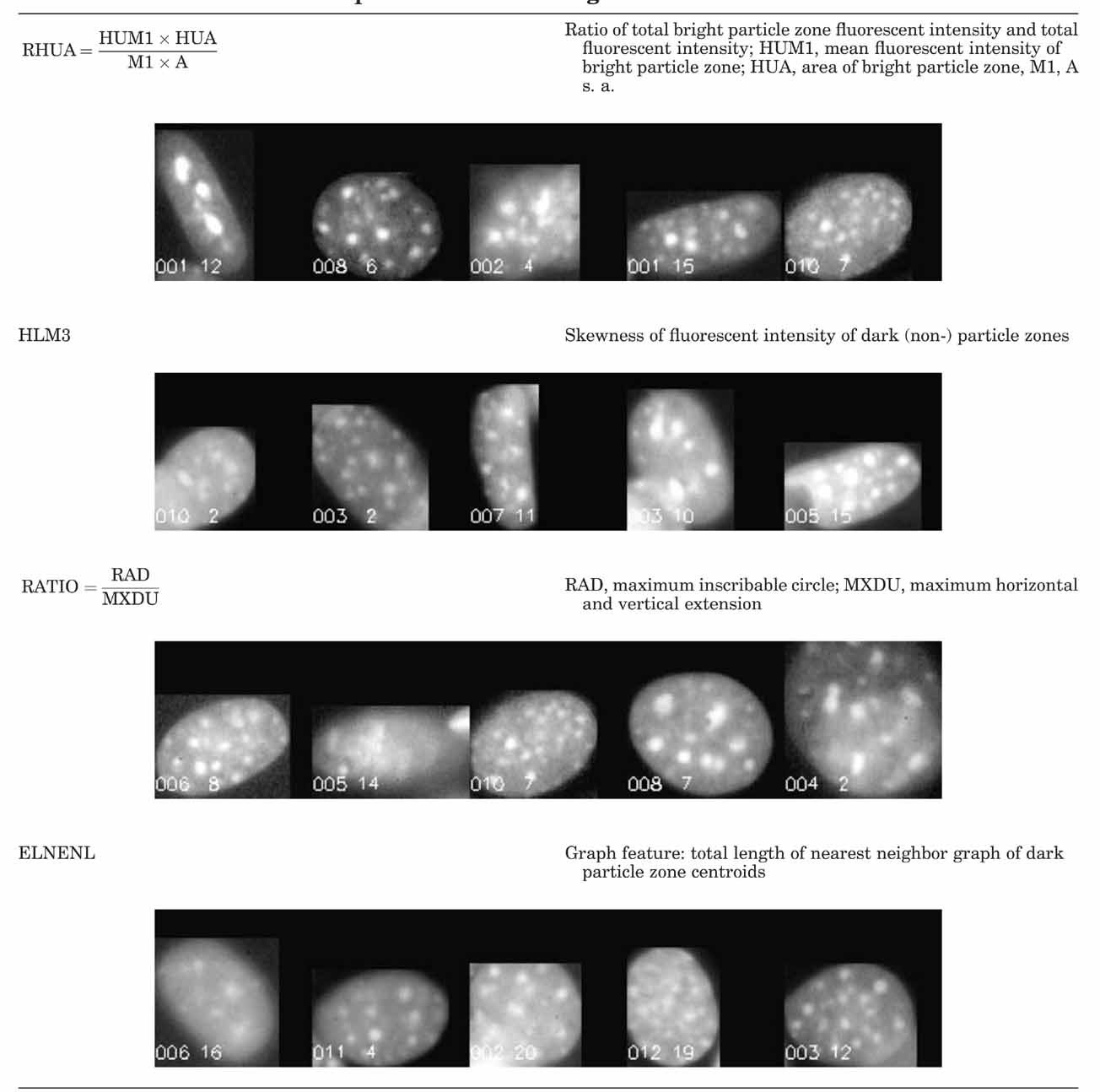
The two-class discrimination analysis provided a comparable result with the heterogeneity of nuclear texture (HETERO) showing the greatest difference between gr and µg (correct class 94.7%). A similar difference, with a lower correlation was observed for 1 g and µg (correct class 86.4%). Furthermore, the 1 g cells have very similar morphology to the ground cells (correct class 78.7%). In other words, the ground and 1 g samples are more similar to each other than they are to µg cells. The morphological deviation of µg cells may be due to the influence of gravity on the arrangement of nuclear particles as a result of altered gene expression. All other selected features (HLM3, RHUA, NR2, NC9) except the last are features of particle arrangement. RATIO reflects an additional shape influence.
DISCUSSION
Combating space osteoporosis is a key factor in planning long-range space missions of greater than 6 months. In order to understand underlying cellular mechanisms of disuse osteoporosis, osteoblasts were grown in µg and 1 g on the Space Shuttle and were analyzed for changes in gene expression and morphology after growth stimulation. We noted visual differences in nuclear shape in cells growth in µg. Since changes in autocrine, growth, cytoskeletal, and transcription factors may be important in osteoblast physiology in spaceflight [Cubano and Lewis, 2000; Goodwin et al., 2000, we analyzed 24 osteoblast genes under µg, 1 g, and gr conditions. Of the genes with significantly altered expression, there were four cell cycle related, one osteoblast specific, two immediate early, and two growth factor genes reduced at one or both of the timepoints. Many genes had no significant change in message level, these included 18 S, H4 histone, fibronectin, β-actin, cyclophilin, and prostaglandin E2 receptor 1 (EP1). In contrast, there were nine genes that had a significant reduction in message induction in µg, these included cox-2, cpla2, PCNA, bcl2, bax, c-myc, TGFβ, OC, and fgf-2 suggesting a change in anabolic response in the absence of gravity.
Of particular interest was the reduction in cox-2 message in the presence of high levels of PGE2. Cox-2 is a key enzyme in the maintenance of bone in response to stress and exercise [Forwood, 1996; Forwood et al., 1998. Moreover, cox-2 is a feed-forward enzyme, which is induced by its own product, PGE2 [Pilbeam et al., 1995. In µg, this feed-forward regulation becomes uncoupled, with PGE2 levels significantly increased in both the 1 g (P < 0.05) and the µg (P < 0.001) osteoblasts when compared to gr controls. The underlying mechanism for this uncoupling is currently under study using modeled µg and understanding the loss of feed-forward induction of cox-2 at the cellular level may help us understand the loss of bone in astronauts.
Messenger RNA and protein content of FGF-2, an important transcription and autocrine growth factor in bone [Stachowiak et al., 1994; Keresztes and Boonstra, 1999, was depressed in the µg samples. There are at least three isoforms of fgf-2, both the PKA and PKC signaling pathways act synergistically to stimulate synthesis of each of these forms [Stachowiak et al., 1994. PKA results in more fgf-2 being associated with the intracellular (transcription factor) pathway, while PKC causes accumulation in the cytoplasm and secretion [Stachowiak et al., 1994. We found that after 24 h in microgravity, the cells had a significant fivefold decrease in both FGF-2 protein and message when compared to gr samples. This corresponds well with the gene chip data of Hammond et al. who reported a sixfold decrease in mRNA signal for fgf-2 message and its receptor in rat kidney cells grown on the Space shuttle for 6 days [Hammond et al., 2000. Since fgf-2 is known to regulate bcl2 in small lung cancer cells, we analyzed message for bcl2 and found that its expression was also lowered in osteoblasts 24 h after activation in µg. The role of the cytoskeleton in growth and its relationship to cell cycle and the anabolic MAPK signal transduction pathway is being actively investigated in this laboratory. We have recently reported that gravity strain can stimulate osteoblast cytoskeleton and growth through a MAPK mechanism, most likely a PKA pathway [Hatton et al., 2003. The PKA pathway might also be effected in microgravity since it is known to mediate induction of fgf-2 [Stachowiak et al., 1994 and promote growth in osteoblasts [Barry et al., 1999; Fitzgerald et al., 2000.
In coordination with the changes in induction of gene expression, the cells demonstrated a significant difference in shape (roundish/elliptic), nuclear structure, and texture (less/more bright particles, less/more densely packed, less/more contrasted) when exposed to µg. Mathematical analysis of morphology demonstrated a significant differentiation of single cells grown under varying gravity conditions. To apply an a-posteriori classification scheme to discriminate microgravity conditions the number of specimens has to be increased. The cause of the elongation of nuclei and elongation of cytoskeleton in microgravity is not known, although Rijken et al. noted cell shape changes previously [de Groot et al., 1991. It is possible that it is a result of a microgravity-induced inhibition of anabolic stimulation. The only example we found of similar nuclear elongation was caused by limiting cell access to mitogens [Wang and Walsh, 1996.
Studies by Wang and Walsh [Foote et al., 1996 demonstrated that when myocytes undergo growth factor withdrawal, a portion of the cells form elongated nuclei, a shape much like those seen in our spaceflight samples in µg. The classification results additionally show that 1 g in-flight cells differ only little from gr although the shape is slightly changed. Table IV shows that only texture features with relatively low F-values allow a 78.7% correct classification. Differences are possibly due to the 19 h storage of the quiescent osteoblasts in µg in the Type I containers prior to serum activation and being placed in a 1 g environment. In addition, Wang and Walsh 1996 reported that myocytes had a spindle-shaped elongation of cells, which accompanied the elongation of the nucleus. Our studies here as well as our previous studies in space flight [Fitzgerald and Hughes-Fulford, 1996 suggest that there is an elongation of the nucleus in cells grown in microgravity. These findings are supported by the observations of Guignandon et al. 1995 who also observed that intermittent effect of gravity causes flight-induced shape changes that included focal contact plaque reorganization. When cells were blocked in G2/M with nocodazole the flight-induced decrease in adhesion was ameliorated, suggesting that microtubules play a role in gravity-induced changes of the nuclear structure. Results from several investigators suggest that the actin cytoskeleton is a necessary component of the cell cycle, especially needed for transition from G1-S phase and for anchorage dependent DNA synthesis [Carvalho et al., 1998; Pavalko et al., 1998. Restrictions to the cytoskeleton formation inhibit growth [Bohmer et al., 1996; Brighton et al., 1996; Hughes-Fulford and Lewis, 1996; Rosenberg, 2003 and can induce differentiation [Ingber et al., 1995; Hii et al., 1998; Dike et al., 1999. A review of osteoblast signaling also suggest that key pathways may be linked to stress and related gravity forces [Hughes-Fulford, 2004. The obvious changes in nuclear morphology in microgravity conditions may be a key component in regulation of growth and differentiation and may further link cytoskeletal anomalies in space-flown cells to nuclear function suggesting that the cytoskeleton is an important component of osteoblast physiology in microgravity and on earth.
Beside the visual recognizability and mathematically measurable shape changes of the nuclei, the deviations of nuclear structure suggest that cell functions are changed under microgravity. Analogous to nuclear texture changes during cell cycle (nearly no texture in synthesis periods, increased texture in periods of standstill like G0/G1), these cells show a certain cell cycle quiescence, suggesting a lag in the anabolic signaling processes. Most likely cell cycle progression slows in the microgravity environment since the size of most of the microgravity cells does not show a large G2 (doubling) size. The fixed cells are only 0.4-µm thick so the diameter is a good measure of DNA in cells that are anchored (not rounded in preparation for mitosis or coming out of mitosis).
In this study, sera activation failed to fully stimulate synthesis of cox-2, cpla2, c-myc, TGFβ, OC, PCNA, bcl2, and fgf-2 message as well as FGF-2 protein in µg, suggesting that gravity is needed for some early anabolic signals. Conversely, it is of interest to note that cox-2, OC, and fgf-2 are induced by mechanical stress [Hatton et al., 2003 and therefore their downregulation in the absence of stress during spaceflight suggests an earthbound mechanism for these anabolic responses. It is likely that cellular studies of the osteoblast in microgravity will provide new mechanistic models and pharmaceutical targets for disuse osteoporosis in astronauts and the elderly. Moreover, since all terrestrial organisms have evolved in a 1 g environment, these studies add to our understanding of the effect of earth's gravity on fundamental biological laws underlying life itself.
Acknowledgements
We thank Janice Voorsluys and Theresa Marsh for their thoughtful comments and review of this manuscript. The authors thank the following members of the Laboratory of Cell Growth who assisted in preparation of this manuscript; Sina Sayyah, Emily Sear, Yvonne Ha, and Janice Voorsluys. This research was supported in part by NASA grants NAG-2-1086, NAG-2-1286, NCC-2-136 and the Department of Veterans Affairs Research Service Merit Review in support of MHF.