Microinjection of an antibody against the cysteine-protease involved in male chromatin remodeling blocks the development of sea urchin embryos at the initial cell cycle
Abstract
We reported recently that the inhibition of cysteine-proteases with E-64-d disturbs DNA replication and prevents mitosis of the early sea urchin embryo. Since E-64-d is a rather general inhibitor of thiol-proteases, to specifically target the cysteine-protease previously identified in our laboratory as the enzyme involved in male chromatin remodeling after fertilization, we injected antibodies against the N-terminal sequence of this protease that were able to inhibit the activity of this enzyme in vitro. We found that injection of these antibodies disrupts the initial zygotic cell cycle. As shown in this report in injected zygotes a severe inhibition of DNA replication was observed, the mitotic spindle was not correctly bipolarized the embryonic development was aborted at the initial cleavage division. Consequently, the injection of these antibodies mimics perfectly the effects previously described for E-64-d, indicating that the effects of this inhibitor rely mainly on the inhibition of the cysteine-protease involved in male chromatin remodeling after fertilization. These results further support the crucial role of this protease in early embryonic development. J. Cell. Biochem. 98: 335–342, 2006. © 2006 Wiley-Liss, Inc.
During male pronucleus formation in sea urchins, the complete set of sperm histones (SpH) is removed from sperm chromatin and replaced by maternally inherited CS histone variants [reviewed by Imschenetzky et al., 2003]. The loss of sperm histones was found to be a step-wise process; initially the H3-H4 tetramer disappears and finally at the time of the fusion of male and female pronucleus SpH1 and the SpH2A-SpH2B dimers are lost [Imschenetzky et al., 1991]. Consistent with this schedule of histone transitions, we have isolated hybrid nucleosome cores at an intermediate stage of decondensation, formed by phosphorylated SpH2A-SpH2B dimers and a subset of CS histone variants [Oliver et al., 2002]. We postulated previously that these SpH released from male chromatin are degraded by a nuclear cysteine-protease that was characterized in our laboratory. We had shown that this enzyme degrades SpH, leaving the CS histone variants unaffected [Imschenetzky et al., 1997]. We had reported that such selective degradation is modulated by post-translational modification of substrates. In this context, SpH1 and SpH2B are protected from being degraded at intermediate steps of male chromatin remodeling by phosphorylation and the female CS variants are protected from proteolysis by poly (ADP-ribosylation) [Morin et al., 1999a,b]. In agreement with previous reports showing that male chromatin remodeling is independent of protein synthesis after fertilization [Imschenetzky et al., 1991], this protease was found as an inactive precursor in the nucleus of unfertilized eggs that is activated and mobilized into male pronucleus after fertilization. The role of this protease in SpH degradation was further supported by the persistence of the complete set of SpH at the end of the initial cell cycle in zygotes treated with E-64-d, an inhibitor of cysteine-proteases. More recently we have reported that the inhibition of cysteine-proteases with E-64-d not only blocks SpH degradation after fertilization but also severely disturbs DNA replication, prevents mitosis, and blocks the initial cleavage division. In zygotes treated with E-64-d, the nuclear envelope breakdown that normally precedes metaphase was not observed, the correct centrosome polarization was abolished, and the normal organization of the mitotic furrow was altered [Concha et al., 2005b].
At present, the regulation of degradation of key molecules participating in the control of cell cycle in eukaryotes has become a major topic. It is well established that the transitions from G1- to S-phase and from anaphase to telophase are regulated in crucial steps by the activity of two complexes: the Skp1-Cdc53/cullin-F-box complex (SCF) and the large multi-subunit anaphase promoting complex (APC/C). These complexes catalyze the ubiquitination of cell cycle regulatory proteins directing them to the degradation by the proteasome 26 S. The SCF complex regulates entry into S-phase by degradation of G1-associated cyclins and CKIs such as p27 and p21 [Deshaies, 1999; Spruck and Strohmaier, 2002]. The APC complex is involved in sister chromatid separation and exit from mitosis by degrading crucial cell cycle regulators, such as securine and the mitotic cyclins [reviewed by Wasch and Engelbert, 2005]. This complex also participates in the down-regulation of Cdt1 which occurs late in the cell cycle. APC/C-mediated proteolysis of Cdt1 and the inhibitory effect of geminin are required to prevent DNA re-replication in Xenopus extracts [Li and Blow, 2005]. Calpain-dependent proteolysis has also been implicated in cell cycle control, mostly in mammalians cells. It was reported that proteases belonging to the family of calpains were involved in cyclin D1 degradation [Choi et al., 1997], p53 stability [Glotzer et al., 1991; Zhang et al., 1997], the proteolysis of p107 a member of the retinoblastoma family [Jang and Choi, 1999], the degradation of p21, the proteolysis of p27 [Patel and Lane, 2000; Chen et al., 2001], and the chromosome alignment on the metaphase plate during mitosis [Honda et al., 2004]. In this context, it is important to precise which cystein-protease was inhibited by E-64-d and induced the embryonic cell cycle arrest that we had reported recently.
In this report we were able to obtain the immuno-inhibition in vivo of the cysteine-protease responsible for the SpH degradation associated to male chromatin remodeling by injecting antibodies against this enzyme that blocked its activity in in vitro assays. We demonstrate herein that in the zygotes injected with these antibodies DNA replication was severely inhibited, the mitotic furrow was not organized and early development was aborted at the initial cleavage division. Thus, the injection of these antibodies mimics perfectly the effects of E-64-d described previously [Concha et al., 2005b], indicating that the protease targeted by E-64-d is the same cysteine-protease that participates in SpH degradation after fertilization.
MATERIALS AND METHODS
Gametes and Embryos
Gametes were obtained from the sea urchin Tetrapygus niger that were collected from the Bay of Concepción, Chile. Insemination was performed and development took place in sea water at room temperature, under aeration. Developmental stages were followed by light microscopy as described previously [Imschenetzky et al., 1991]. For the experiments performed to analyze the effect of E-64-d, this compound dissolved in DMSO was added to the cultures 3 min after fertilization.
Protease Assay
Protease was isolated from nuclear extracts obtained from zygotes harvested 5 min after fertilization, purified by sucrose gradients followed by exclusion chromatography on Sephadex G-100 and used for in vitro assays as described previously [Imschenetzky et al., 1997]. Protease activity was determined by measuring the hydrolysis of (methyl-14C)SpH and (methyl-14C)SpH1. Units of activity were expressed as cpm released per minute per microgram of protein at 37°C (cpm/min/µg protein at 37°C).
Microinjection of Anti-Cysteine-Protease Antibodies
Antibodies against the synthetic KLH-peptide representing the N-terminal sequence of the SpH-protease were obtained and the IgG fraction of the serum was purified as described previously [Concha et al., 2005a]. Solutions of antibodies that had been dialyzed against buffer glycine 0.5 M pH 7.4 and concentrated to 1.4 mg/ml were used to inject the unfertilized eggs that were placed onto glass coverslips coated with 1% protamine. Control experiments were performed by injecting unfertilized eggs with the same protein concentration of IgG purified from pre-immune rabbit serum that had been dialyzed against buffer glycine 0.5 M pH 7.4. Eggs were fertilized over the coverslip and development was followed by light microscopy. Specimens damaged by the injection were discarded. At different times after insemination the zygotes were harvested and used for immuno-staining.
Immuno-Staining and Microscopy
Sea urchin embryos were harvested at different times after fertilization and processed as described previously [Concha et al., 2005a]. Anti-α-tubulin (1:500) primary antibodies (Sigma Chemical Company, St. Louis, MO) were used to label the mitotic spindle. Binding of primary anti-α-tubulin antibody was detected with FITC-conjugated goat anti-mouse IgG antibodies diluted 1:1,500 (Sigma). Fluorescent signals were observed by epifluorescence Nikon Eclipse E-600 microscope using a filter G-2F/C. To visualize the nucleus, the samples were stained with 1 µg/ml DAPI as previously described [Monardes et al., 2005].
Monitoring DNA Replication
To label the DNA replicated during the initial cleavage division a pulse of 10−3 M BrdU was added to cultures of embryos from 10 min uptil 40 min post insemination (p.i.). Samples were harvested at 120 min p.i. and processed by following the procedure described previously to remove the fertilization membrane [Concha et al., 2005b]. BrdU detection was performed as described previously [Oliver et al., 2003]. Monoclonal anti-BrdU antibodies (Boehringer, Manheim, Germany) diluted 1:10 in 0.5% (w/v) BSA in buffer A + Tween (150 mM NaCl, 50 mM Tris-HCl pH 7.4, 0.05% (v/v) Tween-20) were used as primary antibodies and FITC-linked sheep anti mouse IgG diluted 1:500 in 0.5% (w/v) BSA in PBS in buffer A + Tween as secondary antibody. Fluorescent signals were observed using a Nikon Eclipse E-600 microscope using a filter G-2F/C.
RESULTS
Injection of Anti-Protease Antibodies Blocks the Initial Cleavage Division
Initially we have determined the effect of the antibodies obtained against the N-terminal sequence of the cysteine-protease on the catalytic activity of this enzyme in vitro. The protease was obtained from nuclear extracts isolated from zygotes harvested 5 min post insemination, purified by sucrose gradients followed by exclusion chromatography on Sephadex G-100, and used for in vitro assays as previously described [Imschenetzky et al., 1997]. As shown in Figure 1, the antibodies inhibited the protease activity in a dose-dependent manner. Based on this observation, we decided that these antibodies may be used as a tool to inhibit the protease activity in vivo. Therefore, these antibodies were concentrated and injected into eggs before fertilization. As a control, eggs were injected with similar amounts of rabbit pre-immune IgG. The injected eggs were fertilized and the progress of the initial cleavage division was followed by light microscopy. In non-injected zygotes, as well as in zygotes injected with pre-immune IgG, the first cleavage divisions was observed at 90 min p.i. and the second occurred at 120 min p.i. indicating that the injection of pre-immune IgG does not affect the cleavage stages of development (Fig. 2A,C,E). As shown in Figure 2B,D, zygotes that were injected with the anti-protease antibodies were unable to perform the initial cleavage division at 90 min p.i. and remained undivided at 120 min p.i. In accordance with these results, zygotes incubated with the cysteine inhibitor E64-d (75 µM) also remained undivided at 120 min p.i (Fig. 2E,F). Control embryos and zygotes microinjected with pre-immune IgG reach normally the larval stage of development. In contrast, the zygotes injected with the antibodies and those treated with E-64-d remained arrested at the initial cleavage division and degenerated afterwards (results not shown).
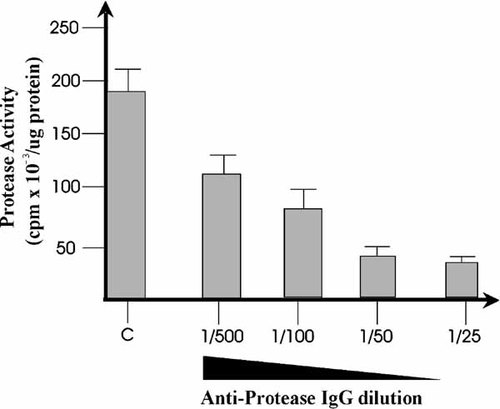
Cysteine-protease activity inhibition by anti-protease IgG. The protease was purified from nuclear extracts and then used for in vitro assays using 14C-SpH1 as substrate, as described in Materials and Methods. The proteolytic activity obtained in control assays (C) was compared with the assays containing increasing dilutions of anti-protease IgG. The anti-protease IgG dilutions used were the following: 1/500, 1/100, 1/50, and 1/25, respectively. Four independent assays were performed for each assay.
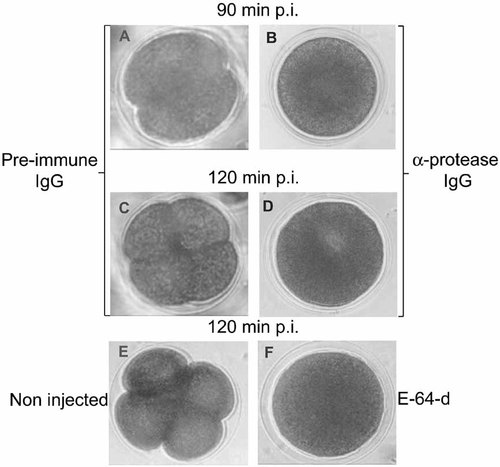
Injection of anti-protease IgG antibodies blocks the initial cleavage divisions. Anti-protease IgG antibodies were injected into unfertilized eggs as described in Materials and Methods. Control experiments were performed by injecting unfertilized eggs with similar amounts of pre-immune IgG. After fertilization the zygotes were observed by light microscopy to follow the initial cleavage divisions. Zygotes harvested at 90 min p.i., injected with pre-immune IgG (A) or with anti-protease IgG (B). Zygotes harvested at 120 min p.i., injected with pre-immune IgG (C) or with anti-protease IgG (D). Non-injected zygotes at 120 min p.i. cultured in sea water (E) and in the presence of 75 µM of E-64-d (F).
To investigate the effect of the protease inhibition on the nuclear dynamics following fertilization, the zygotes were harvested at different times after fertilization and stained with DAPI to visualize the nucleus of the zygotes. As shown, the fusion of male and female pronucleus in the zygotes injected with the pre-immune IgG (30 min p.i.) occurs in schedule with those injected with the anti-cysteine-protease IgG antibodies and also with those treated with E-64-d (Fig. 3 plates A, B, and C), although the extent of male pronucleus decondensation in zygotes injected with the anti-protease antibodies and in those treated with E-64-d was lower compared to those injected with the pre-immune IgG. In the zygotes injected with the pre-immune IgG the first nuclear division was observed at 90 min p.i., while in the zygotes injected with the anti-protease IgG, as well as in those treated with E-64-d, the zygotic nucleus remains undivided at this time (Fig. 3 plates D, E, and F).
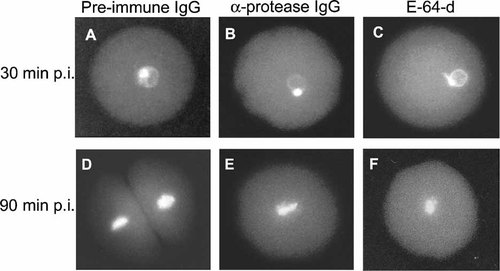
Injection of anti-protease IgG antibodies impedes the initial nuclear division. Unfertilized eggs were injected with similar amounts of pre-immune IgG or alternatively with anti-protease IgG antibodies as described in Materials and Methods. Eggs were fertilized, harvested at 30 min p.i. and 90 min p.i., and stained with DAPI dye. Zygotes harvested at 30 min p.i. injected with pre-immune IgG (A) or with anti-protease IgG (B). Non-injected zygotes cultured in sea water containing 75 µM of E-64-d (C). Zygotes harvested at 90 min p.i. injected with pre-immune IgG (D) or with anti-protease IgG (E). Non-injected zygotes cultured in sea water containing of 75 µM of E-64-d (F).
Taken together these results indicate that the injection of anti-protease IgG blocks the initial nuclear division and the first cell division. These effects were identical to those resulting from the treatment of zygotes with E-64-d.
Injection of Anti-Protease Antibodies Inhibits the Initial S-phase and Alters the Organization of the Mitotic Spindle
To determine the impact of the injection of anti-protease antibodies on S-phase progression we monitored BrdU incorporation into DNA during the two initial embryonic cell cycles. The embryos were harvested at a stage of four blastomers that was attained at 120 min p.i. and processed for BrdU detection as described in Materials and Methods. As shown, the incorporation of BrdU into DNA was clearly visible in the four nuclei of non-injected embryos and those injected with the pre-immune-IgG (Fig. 4 plates A, B). In contrast, BrdU incorporation into DNA was undetectable in the zygotes injected with the anti-protease IgG antibodies, as well as in those treated with E-64-d (Fig. 4 plates C and D). These results demonstrate that injection of the anti-protease antibodies blocks DNA replication during the initial embryonic development. Moreover, the effect of the injection of the anti-protease antibodies mimics perfectly the inhibition of the S-phase that results from the E-64-d treatment.
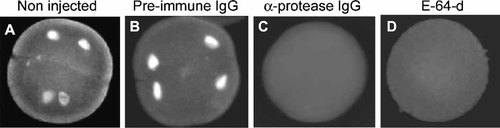
Injection of anti-protease IgG antibodies abrogates S-phase of the initial cell cycle. Unfertilized eggs were injected with pre-immune IgG or with anti-protease IgG antibodies. BrdU (0.1 mg/ml) was added after fertilization and the zygotes were harvested at 120 min p.i. fixed and processed for detection of BrdU incorporation into DNA with FITC labeled anti-BrdU antibodies, as described in Materials and Methods. Non-injected zygotes (A), zygotes injected with pre-immune IgG (B), zygotes injected with anti-protease IgG (C). Non-injected zygotes incubated in 75 µM E-64-d (D).
To visualize the mitotic spindle, zygotes were harvested 60 min p.i. and stained with anti-α-tubuline antibody as described in Materials and Methods. A bipolarized mitotic spindle was visualized in non-injected zygotes and in those injected with pre-immune IgG (Fig. 5 plates A and B). Strikingly the organization of the mitotic spindle was severely damaged by the injection of the anti-protease antibodies (Fig. 5 plate C). Therefore, our results demonstrate that the activity of this protease is required for proper organization of the mitotic spindle during the first embryonic cell cycle. These results are identical to the effect on mitosis of E-64-d previously described, where it was found that the polarization of the mitotic spindle during the first cell cycle was abolished by this treatment [Concha et al., 2005b].
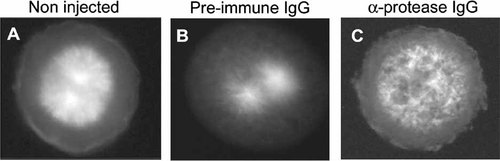
Injection of anti-protease IgG antibodies disturbs the formation of the mitotic spindle during the initial cell cycle. Unfertilized eggs were injected with pre-immune IgG or with anti-protease IgG antibodies. Eggs were fertilized and zygotes were harvested at 60 min p.i. fixed and processed for anti-α-tubuline immuno-staining to visualize the mitotic spindle as described in Materials and Methods. Non-injected zygotes (A), zygotes injected with pre-immune IgG (B), zygotes injected with anti-protease IgG (C).
DISCUSSION
We show in this report that the immuno-inhibition in vivo of the cysteine-protease involved in male chromatin remodeling induces the arrest of the S-phase and disorganizes the mitotic spindle at the initial cell cycle of sea urchin embryos. These effects are followed by the blocks of the initial nuclear division and of the formation of first cleavage furrow thereby aborting the embryonic development at the first cleavage stage. These deleterious effects on the initial embryonic cell cycle, produced by the injection of these antibodies, mimic the results obtained when E-64-d, an inhibitor of cysteine-proteases, is added to the zygotes after fertilization [Concha et al., 2005b]. Taken together our results indicate that the effects on the cell cycle, described previously for E-64-d, rely mainly on the inhibition of the activity of the SpH cysteine-protease.
Different proteolytic pathways had been implicated previously in fertilization and in the control of embryonic cell cycle. The ubiquitin-mediated proteasome pathway has been involved in the correct termination of S-phase and subsequent initiation of M-phase of the initial embryonic cell cycle [Kawahara et al., 2000]. However, the effects of the injection of the anti-protease antibodies on the initial cell cycle, described in this report, seem to be unrelated with the ubiquitin-dependent proteasome pathway. As it is well established, E-64-d, the inhibitor that mimics the results obtained by injecting the eggs with the antibody described in this report, has no effect on proteasome activity [Tamai et al., 1986]. In addition, we have reported previously that first S-phase was not inhibited by treatment of the zygotes with MG132, an inhibitor of proteasomes [Concha et al., 2005b].
The potential role of calpains after fertilization in sea urchins is yet unknown, but in Xenopus eggs it was reported that a calpain is involved in the proteolysis of the c-mos proto-oncogene product on fertilization [Watanabe et al., 1989]. Alternatively, calpains were shown to be expressed during mouse spermatogenesis and localized in the acrosomal cap. Based on the finding that calpains inhibition reduced the acrosomal reaction, its potential function in the events preceding fertilization was suggested [Ben-Aharon et al., 2005]. Although E-64-d also inhibits proteases that belong to the family of calpains, the potential effect of the antibodies used in this report on proteases that belong to the calpain family should be discarded, based on two lines of evidence. The first is that the antibodies used in this report were obtained against the N-terminal sequence of the cysteine-protease, which has no sequence similarity with calpains known thus far. The second is that the treatment of sea urchin zygotes with PD 150606, an inhibitor of calpains, does not affect the normal organization of the mitotic furrow [Concha et al., 2005b]. In addition to calpains, a cysteine-protease, the CD separase, was reported to regulate mitosis-associated events in other systems. This protease is involved in chromatid separation at the metaphase to anaphase transition [reviewed by Uhlmann, 2003]. Although this type of protease has not been described in embryonic systems, the predictable effects of the inhibition of the CD separase on the initial cell cycle does not correlate with the effects of the injection of the anti-protease antibodies described here. As reported CD separase inhibition specifically blocks the metaphase/anaphase transition, but has no effect on S-phase initiation or progress. Furthermore, there is no homology at the amino acid level between the sequence of the CD separases and the N-terminal peptide of the cysteine-protease that was used to obtain the antibodies used in this report. Hence, the potential inhibition of a protease CD-separase-like by the antibodies used in this report seems unlikely.
Interestingly, the N-terminal sequence of the SpH protease exhibits a remarkable amino acid sequence homology with proteases that belong to the cathepsine family of proteases and most particularly with cathepsine L. Based on this homology we had previously analyzed the effect of cathepsine L inhibitor on sea urchin zygotes obtaining a disruption of the mitotic spindle that is consistent with that resulting from the treatment with E-64-d [Concha et al., 2005b], or the injection of the anti-protease antibodies described here. Based on these lines of evidence, we postulate that the cysteine-protease that was immuno-inhibited in vivo in this report belongs to the cathepsine L family of proteases. Although most of the current information regarding cathepsine L describes this protease linked to lysosomes or to the extra cellular milieu, the protease targeted by the antibodies used in this report resides in the nuclei of zygotes and at mitosis localizes on the mitotic spindle [Concha et al., 2005a]. In this context, the idea that this protease is a nuclear cathepsine L variant involved in male chromatin remodeling and subsequently in cell cycle control after fertilization may be considered controversial. However, the potential role of proteases in cell cycle control that are members of the cathepsine family was suggested previously for other systems. A strong repression of cell proliferation induced by myeloid and erythroid nuclear termination stage-specific protein (MENT), an endogenous nuclear inhibitor of cathepsines K, L, and V, was reported [Irving et al., 2002]. More recently a nuclear variant of cathepsine L was identified and postulated to be involved in cell cycle control. This cathepsine L variant, described for a non-embryonic system, catalyses the limited proteolysis of the CDP/cux transcription factor at the G1/S transition of the cell cycle, modulating its activity as a transcription factor relevant for the expression of several genes involved in cell cycle control, such as DNA polymerase α, dihidrofolate reductase (DHFR), dihydroorotase (CAD), and cyclin A [reviewed by Goulet and Nepveu, 2004]. Thus far, it is not known if the cathepsine L-like isoform targeted in this report by the injection of antibodies may be related to the cathepsine L nuclear isoform identified in other cells. It is also unknown if the mechanisms related to cell division that were altered by the inhibition of this cysteine-protease may be correlated to the potential role of the cathepsine L nuclear isoform that participates in CDP/Cux proteolytic processing. Unfortunately these pathways are far from being totally understood yet.
In summary, our results further reinforce the crucial role that SpH cysteine-protease has on the events that follow fertilization. Interestingly, potential new roles for this enzyme on cell cycle control appear as an emergent issue that we feel merits further investigation in the future.
Acknowledgements
We thank Dr. Soraya Gutierrez for helpful comments and the critical reading of this manuscript.




