Isolation and identification of 1α-hydroxy-3-epi-vitamin D3, a potent suppressor of parathyroid hormone secretion
Abstract
Since our original demonstration of the metabolism of 1α,25(OH)2D3 into 1α,25(OH)2-3-epi-D3 in human keratinocytes, there have been several reports indicating that epimerization of the 3 hydroxyl group of vitamin D compounds is a common metabolic process. Recent studies reported the metabolism of 25OHD3 and 24(R),25(OH)2D3 into their respective C-3 epimers, indicating that the presence of 1α hydroxyl group is not necessary for the 3-epimerization of vitamin D compounds. To determine whether the presence of a 25 hydroxyl group is required for 3-epimerization of vitamin D compounds, we investigated the metabolism of 1αOHD3, a non-25 hydroxylated vitamin D compound, in rat osteosarcoma cells (ROS 17/2.8). We noted metabolism of 1αOHD3 into a less polar metabolite which was unequivocally identified as 1αOH-3-epi-D3 using the techniques of HPLC, GC/MS, and 1H-NMR analysis. We also identified 1αOH-3-epi-D3 as a circulating metabolite in rats treated with pharmacological concentrations of 1αOHD3. Thus, these results indicated that the presence of a 25 hydroxyl group is not required for 3-epimerization of vitamin D compounds. Furthermore, the results from the same studies also provided evidence to indicate that 1αOH-3-epi-D3, like 1αOHD3, is hydroxylated at C-25. We then evaluated the biological activities of 1αOH-3-epi-D3. Treatment of normal rats every other day for 7 days with 2.5 nmol/kg of 1αOH-3-epi-D3 did not raise serum calcium, while the same dose of 1αOHD3 increased serum calcium by 3.39 ± 0.52 mg/dl. Interestingly, in the same rats which received 1αOH-3-epi-D3 we also noted a reduction in circulating PTH levels by 65 ± 7%. This ability of 1αOH-3-epi-D3 to suppress PTH levels in normal rats without altering serum calcium was further tested in rats with reduced renal function. The results indicated that the ED50 of 1αOH-3-epi-D3 for suppression of PTH was only slightly higher than that of 1α,25(OH)2D3, but that the threshold dose of the development of hypercalcemia (total serum Ca > 10.5 mg/dl) was nearly 80 times higher. These findings indicate that 1αOH-3-epi-D3 is a highly selective vitamin D analog with tremendous potential for treatment of secondary hyperparathyroidism in chronic renal failure patients. © 2005 Wiley-Liss, Inc.
Abbreviations used:
25OHD3, 25-hydroxyvitamin D3; 24(R),25(OH)2D3, 24(R),25 dihydroxyvitamin D3;1α,25(OH)2D3, 1α, 25-dihydroxyvitamin D3; 1α,24(R),25(OH)3D3, 1α,24(R),25-tryhydroxyvitamin D3; 1α,25(OH)2-24-oxo-D3, 1α,25-dihydroxy-24-oxovitamin D3; 1α,23,(S)25(OH)3-24-oxo-D3, 1α,23(S),25-trihydroxy-24-oxovitamin D3; 1α,23(OH)2-24,25,26,27-tetranor D3 or C-23 alcohol, 1α,23-dihydroxy-24,25,26,27-tetranorvitamin D3; 1,25(R)-dihydroxyvitamin D3-26,23 (S)-lactone, 1α,25(OH)2D3-lactone; 1α,25(OH)2-3-epi-D3, 1α,25-dihydroxy-3-epi-vitamin D3;1αOH-3-epi-D3, 1α-hydroxy-3-epi-vitamin D3; 1α,25(OH)2-16-ene-23-yne-D3, 1α,25-dihydroxy-16-ene-23-yne-vitamin D3; HPLC, high performance liquid chromatography; GC/MS, gas chromatography/mass spectrometry.
A decade ago, we reported for the first time that the secosteroid hormone, 1α,25(OH)2D3, is metabolized to 1α,25(OH)2-3-epi-D3 in human keratinocytes [Reddy et al., 1994]. Since this original report there have been several studies which confirmed the metabolism of 1α,25(OH)2D3 through this new pathway in several tissues including bovine parathyroid cells [Reddy et al., 1997; Bischof et al., 1998; Brown et al., 1999a; Sekimoto et al., 1999; Siu-Caldera et al., 1999; Astecker et al., 2000; Masuda et al., 2000]. This new metabolic pathway is now well accepted in the literature as the “C-3 epimerization pathway” [Reddy et al., 2001]. Following the discovery of 1α,25(OH)2D3 metabolism into its 3-epimer, we started investigating the metabolism of a wide variety of synthetic vitamin D analogs into their respective 3-epimers. During these studies, we began identifying how changes in the structure of vitamin D compounds can affect the rate of 3-epimerization process. For example, one of the well studied vitamin D analogs 1α,25(OH)2-16-ene-23-yne-D3 is converted into its 3 epimer much more slowly than 1α,25(OH)2D3. However, when the stereochemistry of 20 methyl group of the same vitamin D analog is changed into an unnatural orientation, a dramatic increase in the rate of its C3-epimerization is noted [Reddy et al., 2000]. This observation led us to perform more studies to identify other structural changes in the vitamin D molecule that will alter the rate of 3-epimerization. Recent studies reported the metabolism of 25OHD3 and 24(R),25(OH)2D3 into their respective C-3 epimers [Kamao et al., 2001, 2004]. From these studies it became clear that the presence of 1α hydroxyl group is not necessary for the 3-epimerization of vitamin D compounds. In order to determine whether the presence of 25 hydroxyl group is a structural requirement for 3-epimerization of vitamin D compounds, we investigated the metabolism of 1αOHD3, a non-25 hydroxylated vitamin D compound, in rat osteosarcoma cells (ROS 17/2.8) and intact normal rats. We identified 1αOH-3-epi-D3 as a metabolite of 1αOHD3 in both in vitro and in vivo experimental conditions. Thus, we provided direct evidence to indicate that the presence of 25 hydroxyl group is not required for 3 epimerization of vitamin D compounds. Furthermore, we also found that 1αOH-3-epi-D3 is metabolized into 1α,25(OH)2-3-epi-D3 both in bone cells as well as in intact rats.
In our previous study we indicated that 1α,25(OH)2-3-epi-D3 is almost as potent as 1α,25(OH)2D3 in suppressing PTH secretion in cultured bovine parathyroid cells [Brown et al., 1999a]. As 1αOH-3-epi-D3 is the precursor for 1α,25(OH)2-3-epi-D3, we evaluated the possible therapeutic potential for 1αOH-3-epi-D3 in suppressing circulating PTH levels. We found that 1αOH-3-epi-D3 had very low calcemic activity in rats but yet was able to suppress PTH levels at non-calcemic doses, indicating the potential of 1αOH-3-epi-D3 for treatment of secondary hyperparathyroidism in patients with chronic kidney disease.
MATERIALS AND METHODS
Materials
ROS 17/2.8 cells were a kind gift from Dr. Sara Peleg (M.D. Anderson Cancer Center, Houston, TX). Streptomycin, penicillin, McCoy's, Dulbecco's modified Eagle's (DMEM), and Ham's F-12 media were obtained from Life Technologies (Gaithersburg, MD). Fetal calf serum (FCS) was purchased from Hyclone (Logan, UT). Tissue culture flasks and high performance liquid chromatography (HPLC) reagents were purchased from Baxter (McGaw Park, IL).
Vitamin D Compounds
25OHD3, 1αOHD3, 1αOH-3-epi-D3, 1α,25(OH)2D3, 1α,25(OH)2-3-epi-D3, 1α,24R,25(OH)3D3, and 1α,25(OH)2D3-lactone were synthesized at Hoffmann-La Roche (Nutley, NJ). 1α,25(OH)2-24-oxo-D3, 1α,23(S),25(OH)3-24-oxo-D3, and 1α,23(OH)2-24,25,26,27-tetranor D3 (C-23 alcohol) were biologically synthesized in the rat kidney perfusion system as previously described [Reddy and Tserng, 1989].
Cells and Cell Culture
Ros 17/2.8 cells were maintained in DMEM and Hams F-12 media (50/50, vol/vol) supplemented with 10% FCS and antibiotics [penicillin (100 IU/ml) and streptomycin (100 µg/ml)]. Cell culture medium was changed every 3–4 days. The cells were subcultured when approximately 80% confluent and were not subcultured beyond passage 5. For metabolism studies, 3 × 106 cells were seeded in T150 tissue culture bottles and grown to confluence. The incubations were carried out at 37°C in a humidified atmosphere under 5% CO2.
High Performance Liquid Chromatography (HPLC), Gas Chromatography/Mass Spectrometry (GC/MS), and 1H-Nuclear Magnetic Resonance (NMR) Analysis
HPLC analysis of the lipid extracts from the cells and media was performed with a Waters System Controller (Millennium 3.2) equipped with a photodiode array detector (Model PDA 996) to monitor the ultraviolet (UV) absorbing material at 265 nm. The vitamin D compounds were isolated and purified using both straight and reverse phase HPLC systems. Analysis by straight phase HPLC utilized a Zorbax-SIL column (9 × 250 mm) (Dupont, Wilmington, DE) eluted with two different solvent mixtures at a flow rate of 2 ml/min. The solvent mixtures used were as follows: 10% isopropanol in hexane (HPLC system no.1) or 6% isopropanol in hexane (HPLC system no.2). Analysis by reverse phase HPLC system utilized a Zorbax-ODS column (4.5 × 250 mm) (Dupont) eluted with 10% water in methanol at a flow rate of 1 ml/ min (HPLC system no.3).
GC/MS analysis was performed using a Hewlett-Packard GC/MSD system, equipped with a series 5890 Series II chromatograph, a 5971 mass selective detector, and a 7673 GC autosampler (Agilent, Wilmington, DE). The vitamin D compounds were trimethylsilylated in 30 µl of a 1:1 mixture of acetonitrile and Power SIL-Prep (Alltech Associates, Deerfield, IL) and incubated at 70°C for 15 min. The trimethylsilyl ether derivatives were analyzed in quadruplicate. All analyses were done using a Hewlett-Packard 5-MS low-bleed capillary column (30 m × 0.25 mm × 0.25 µm film thickness, 5% phenyl methyl silicone), using UHP helium as a carrier gas at a flow rate of 0.8 ml/min. The oven temperature program was as follows: 150°C for 6 min, then increased at 10°C/min until reaching a final temperature of 300°C which was held for 10 min. Ionization was performed by electron impact with positive ion detection. Full scan spectra across the mass range of m/z 50–650 were acquired in each run and the published spectra were averaged and background subtracted. The 1H-NMR spectra were acquired on a Varian UNITYplus® 400-MHZ spectrometer. The samples were dissolved in deuterochloroform containing tetramethylsilane as an internal zero reference.
Metabolism Studies in ROS 17/2.8 Cells Using 1αOHD3 and 1αOH-3-epi-D3 as Substrates
The ROS 17/2.8 cells (3 × 106 cells/ml) were incubated with 1 and 10 µM concentrations of either 1αOHD3 or 1αOH-3-epi-D3 in 50 ml of medium containing 10% FCS. The incubations were stopped after 24 h with 10 ml of methanol and the lipids from both cells and media were extracted for HPLC analysis, using extraction procedure described earlier [Siu-Caldera et al., 1999]. Prior to lipid extraction, the cells and media were spiked with 5 µg of 25OHD3 which was used as an internal standard. The recovery of the internal standard was used to assess the extraction efficiencies of the various lipid-soluble vitamin D metabolites.
Control incubations without cells containing only media and the vitamin D compounds were performed. The control studies indicated that vitamin D compounds did not undergo any chemical change or breakdown either during the incubation period or during the extraction procedure (data not shown).
In Vivo Metabolism of 1αOHD3 in Rats
Sprague–Dawley rats (250 g; Taconic laboratories, Germantown, NY) were adapted to laboratory conditions for at least 5 days; the rats were housed two per cage with free access to food and water in a regulated environment, with a 12 h light-dark cycle. For the in vivo metabolism study using 1αOHD3 as substrate, we used four rats. Each rat was given a bolus dose of 500 µg of 1αOHD3, intravenously. 1αOHD3 was dissolved in ethanol and injected in vehicle consisting of 50% rat serum in normal saline solution. After 8 h, the blood was collected by catheterization of the aorta and immediately centrifuged. We obtained approximately 5 ml of serum from each rat. Lipids from the pooled serum were extracted and the vitamin D3 metabolites were analyzed by HPLC. A control experiment was performed in a rat treated with vehicle solution alone to assure that no metabolites were detected in serum before the rats were administered 1αOHD3 (data not shown).
In Vivo Activities of 1αOH-3-epi-D3 Versus 1αOHD3 in Normal Rats
Male Sprague–Dawley rats (250 g) were injected every other day with 1αOH-3-epi-D3 or 1αOHD3 at doses of 2.5 nmol/kg given intraperitoneally for one week (four injections). Blood samples were taken from the tail vein under ether anesthesia immediately before the first injection and at 24 h after each injection. Plasma was analyzed for total calcium and PTH.
Efficacy of 1αOH-3-epi-D3 versus 1α,25(OH)2D3 in Suppressing PTH in Uremic Rats
Female Sprague–Dawley rats (250 g) were subtotally (5/6) nephrectomized as described previously [Brown et al., 1999b; Ritter et al., 2002], and fed a diet containing 0.9% P and 0.6% Ca. Treatment with the vitamin D compounds began 1 month post-nephrectomy, when the rats had developed secondary hyperparathyroidism. A pretreatment blood sample was taken from each rat and analyzed for calcium, creatinine, and PTH. The rats were divided into two groups (n = 13) with comparable means and ranges of pretreatment creatinine and PTH levels. A dose escalation protocol was then initiated with one group receiving 1α,25(OH)2D3 starting at a dose of 0.01 nmol/kg and the other receiving 1αOH-3-epi-D3 starting at a dose of 0.04 nmol/kg. The rats were injected intraperitoneally every other day for 8 days and a blood sample was taken 24 h after the fourth injection. The every-other-day dosing was continued with a dose doubling every eighth day and a blood sampling 24 h after the four injection of each dose level. The dose escalation continued until the mean serum calcium levels rose above 10.5 mg/dl. PTH levels were determined in each blood sample. All animal protocols were approved by the Animal Studies Committee at Washington University School of Medicine.
Blood Chemistries
Plasma calcium was measured by atomic absorption spectrometry (model 503, Perkin-Elmer Corp., Norwalk, CT). PTH levels were determined using an ELISA for intact rat PTH (Scantibodies, Santee, CA).
RESULTS
Metabolism of 1αOHD3 in ROS 17/2.8 Cells
We first examined the metabolism of 1αOHD3 in ROS 17/2.8 cells, which express the C-3 epimerization pathway but not the C-24 oxidation pathway. Figure 1 shows the HPLC profiles of the lipid extracts obtained when ROS 17/2.8 cells were incubated for 24 h with either 1 µM (panel A) or 10 µM (panel B) concentrations of 1αOHD3. It can be seen that 1αOHD3 (1 µM) was metabolized into a more polar metabolite (peak 2) and a less polar metabolite (peak A) in ROS 17/2.8 cells. Both of the metabolites possess the characteristic UV chromophore of vitamin D (λmax 265, λmin 228) (Fig. 1, panel A-inset). The polar metabolite (peak 2) co-migrated with the synthetic standard of 1α,25(OH)2D3 on both the straight and reverse phase HPLC systems and exhibited a mass spectrum identical synthetic standard 1α,25(OH)2D3 (data not shown). The conversion of 1αOHD3 into 1α,25(OH)2D3 shows evidence for the hydroxylation of 1αOHD3 at C-25 position. The less polar metabolite (peak A) co-migrated with the synthetic standard of 1αOH-3-epi-D3 on both straight and reverse phase HPLC systems. Hence this metabolite was tentatively identified as 1αOH-3-epi-D3, the C-3 epimer of 1αOHD3. In order to produce an adequate amount of the metabolite A for more definitive structure identification, the ROS 17/2.8 cells were incubated with a higher concentration of 1αOHD3 (10 µM) for 72 h. Metabolite A was purified using both straight and reverse phase HPLC systems as described in Materials and Methods, and the purified metabolite A was subjected to GC/MS and 1H-NMR analysis for structure identification. It can be seen from Figure 1, panel B that at a higher concentration (10 µM), 1αOHD3 was metabolized not only into 1αOH-3-epi-D3 (peak A) and 1α,25(OH)2D3 (peak 2), but also into metabolite 1 and metabolite U. Metabolite 1 was identified as 1α,25(OH)2-3-epi-D3 by GC/MS analysis and its co-elution with the synthetic standard on both straight and reverse phase HPLC systems [data not shown as it was reported earlier [Reddy et al., 2001]]. This finding indicates that 1α,25(OH)2-3-epi-D3 may be produced either from hydroxylation of 1αOH-3-epi-D3 at C-25 position or C-3 epimerization of 1α,25(OH)2D3. The identity of the minor metabolite U was not established.
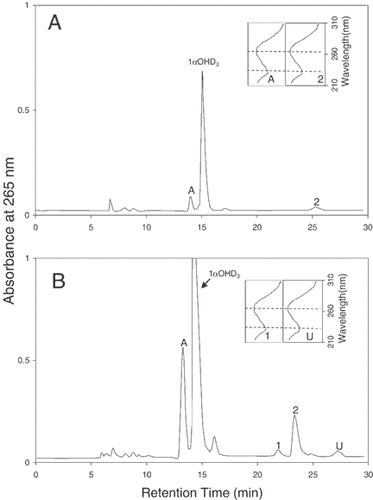
HPLC profiles of 1αOHD3 and its metabolites produced in ROS 17/2.8 cells incubated with 1 µM (panel A) or (10) µM (panel B) concentrations of the substrate for 24 h. HPLC was performed using a Zorbax-SIL (9 × 250 mm) column eluted with 10% isopropanol in hexane at a flow rate of 2 ml/min. Peaks A, 1, and 2 were identified as 1αOH-3-epi-D3, 1α,25(OH)2-3-epi-D3, and 1α,25(OH)2D3 respectively. The identity of peak U, a minor metabolite of 1αOHD3 was not established. The UV spectra of the metabolite peaks (A, 1, 2, and U) are shown in the inset.
Identification of Metabolite A as 1αOH-3-epi-D3 by GC/MS and 1H-NMR
Figure 2 shows the identical mass spectral characteristics for the trimethylsilylated 1αOH-3-epi-D3 synthetic standard (lower panel) and for trimethylsilylated metabolite A (upper panel). The trimethylsilylated synthetic 1αOH-3-epi-D3 and the trimethylsilylated metabolite A exhibited a molecular ion at m/z 544. The fragments at m/z 454 and 364 are formed by sequential elimination of one and two trimethylsilanol moieties from m/z 544. The loss of 131 Da from the A ring (m/z 413) and the detection of ion fragment at m/z 217, arising from A-ring cleavage, denote the presence of hydroxylation at C-1 and C-3 on the A-ring. In addition, to the identical mass spectra, the retention time (rt) on GC/MS confirmed the identity of the 1αOH-3-epi-D3 metabolite (rt. 14.06 min), which is identical to that of the synthetic standard (rt. 14.06 min). As the two other stereoisomers of 1αOHD3 namely 1βOHD3 and 1βOH-3-epi-D3 were not available, 1H-NMR was necessary for absolute stereochemical characterization of the metabolite. Metabolite A was produced in sufficient quantities for 1H-NMR analysis by incubating ROS 17/2.8 cells with 10 µM 1αOHD3 in 20 culture bottles as described earlier. We obtained 160 µg of metabolite A for 1H-NMR analysis. The 1H-NMR spectrum of 1αOH-3-epi-D3 (metabolite A) and its parent compound 1αOHD3, were compared (Fig. 3). The spectra were the same except for differences in the chemical shifts between ring A protons. The largest chemical shift difference was observed for H-3, 4.23 ppm and 4.06 ppm for the parent compound (panel A) and metabolite (panel B), respectively. This observation is highly indicative of epimerization occurring at C-3. Comparison of the 1H-NMR spectrum of metabolite A (panel B) with that of the authentic 1αOH-3-epi-D3 (panel C) shows that the spectra are superimposable, thereby confirming that metabolite A and authentic 1αOH-3-epi-D3 have identical structures. This result leads to the final conclusion that 1αOHD3 is metabolized into its C-3 epimer, 1αOH-3-epi-D3 in bone cells.
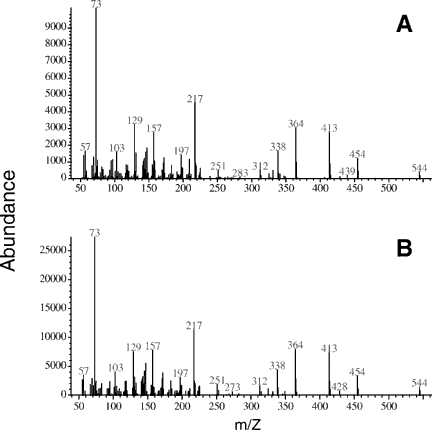
Mass spectra of trimethylsilyl derivatives of metabolite A produced in ROS 17/2.8 cells and the synthetic standard of 1αOH-3-epi-D3. Upper panel: metabolite A, retention time:14.06 min; lower panel: synthetic standard of 1αOH-3-epi-D3, retention time: 14.06 min.
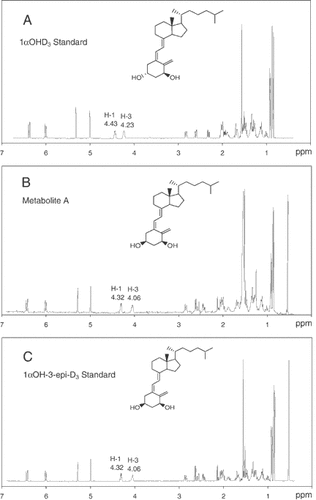
1H-NMR spectra of the synthetic standard of 1αOHD3 (panel A), metabolite A (panel B), and the synthetic standard of 1αOH-3-epi-D3 (panel C).
Metabolism of 1αOH-3-epi-D3 in ROS 17/2.8 Cells
In the previous experiment, we have shown that at higher concentrations of 1αOHD3 (10 µM) we could detect the metabolism of 1αOHD3 not only into 1αOH-3-epi-D3 and 1α,25(OH)2D3 but also 1α,25(OH)2-3-epi-D3 in ROS 17/2.8 cells (Fig. 1). In order to determine whether 1αOH-3-epi-D3 can undergo C-25 hydroxylation to form 1α,25(OH)2-3-epi-D3 in ROS 17/2.8 cells, we next examined the metabolism of 1αOH-3-epi-D3. Figure 4 shows the HPLC profiles of the lipid extracts obtained when ROS 17/2.8 cells were incubated for 24 h with 1 or 10 µM concentrations of 1αOH-3-epi-D3 (panels A and B). As seen in Figure 4, 1αOH-3-epi-D3 at both substrate concentrations (1 and 10 µM) was metabolized into a polar metabolite (peak 1) which was identified as 1α,25(OH)2-3-epi-D3. These findings together with the results of the previous experiment show evidence for C-25 hydroxylation of both 1α OHD3 and 1αOH-3-epi-D3 in vitro.
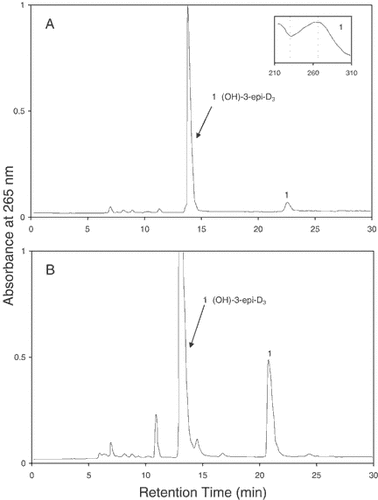
HPLC profiles of 1αOH-3-epi-D3 and its metabolites produced in ROS 17/2.8 cells incubated with 1 µM (panel A) or 10 µM (panel B) concentrations of the substrate for 24 h. The HPLC analysis was performed under the same chromatographic conditions as described in the legend in Figure 1. Peak 1 was identified as 1α,25(OH)2-3-epi-D3and its UV spectrum is shown in the inset.
In vivo Metabolism of 1αOHD3 in Rats
We next examined whether 1αOH-3-epi-D3 exists as an in vivo metabolite in rats given pharmacological substrate concentrations of 1αOHD3. Figure 5, panel B shows the HPLC profile of unmetabolized 1αOHD3 (peak S) and six of its metabolites detected in 20 ml of serum obtained from four rats treated with 1αOHD3. The elution positions of various authentic standards of vitamin D3 metabolites are shown in panel A we observed a less polar peak along with several polar peaks. The less polar metabolite (peak A) eluting before the substrate (Peak S) was identified as 1αOH-3-epi-D3, which had been observed in our in vitro studies with ROS 17/2.8 cells. The polar metabolites were identified as the following metabolites: 1α,25(OH)2-3-epi-D3 (peak 3), 1α,25(OH)2D3 (peak 4), 1α,25(OH)2-24-oxo-D3 (peak 5), 1α,24(R),25(OH)3D3 (peak 6), and 1α,25(OH)2D3-lactone (peak 7). The initial identity of each of the metabolites was achieved by UV absorption spectrophotometry [the metabolites possessed the characteristic vitamin D cis-triene chromophore (Fig. 5, panel B-inset)] and co-elution with the authentic standards on various HPLC systems described in Materials and Methods. The peaks J1 and J2 were considered to be contaminants based on their UV spectra (the contaminants did not exhibit the UV spectral characteristics which are typical to a vitamin D cis/triene chromophore). The final identification of all the metabolites derived through the side chain oxidation pathways with the exception of 1α,25(OH)2-24-oxo-D3 was obtained through GC/MS [data not shown as it was reported earlier [Sekimoto et al., 1999]]. The novel less polar metabolite in the serum of rats (peak A) was identified as 1αOH-3-epi-D3 through its co-elution with synthetic 1αOH-3-epi-D3 on both straight and reverse phase HPLC systems and by GC/MS as described earlier. These findings demonstrate that 1αOHD3 is metabolized through the C-3 epimerization pathway in vivo.
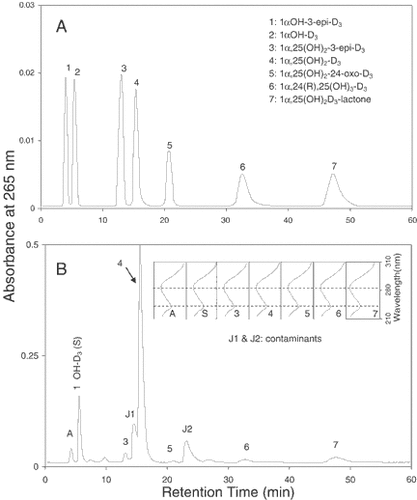
HPLC profiles of various metabolites of 1αOHD3 and 1αOH-3-epi-D3 in rat serum. Panel A: synthetic standards of various vitamin D3 metabolites. Panel B: vitamin D3 metabolites in the lipid extract of 20 ml of serum obtained from 4 rats. Each rat received 500 µg of 1αOHD3 intravenously, 8 h prior to sacrifice. The HPLC analysis of the lipid extract was performed under the same chromatographic conditions as described in the legend to Figure 1. The UV spectra of the metabolite peaks (A,S, 1, 2,3,6 and 8) are shown in the inset.
In Vivo Activities of 1αOHD3 Versus 1αOH-3-epi-D3
The calcemic activity of 1αOH-3-epi-D3 was compared to that of 1αOHD3. Rats were injected every other day for 7 days (four injections) with 2.5 nmol/kg of either 1α,OH-3-epi-D3 or 1αOHD3. Figure 6 shows the changes in serum calcium measured 24 h after each injection of the 2.5 nmol/kg dose. 1αOHD3 produced a steady increase in serum calcium during the treatment period, with an average increase of 3.39 ± 0.52 mg/dl. In contrast 1αOH-3-epi-D3 elicited only a transient, but not significant, rise in serum calcium at day 3, and the final calcium was not different from pretreatment levels (−0.05 ± 0.38 mg/dl). Despite the lack of change in serum calcium of rats receiving 1αOH-3-epi-D3, it was significant to note that the serum PTH levels in the same rats decreased by 64.9 ± 7.2 % by the end of the 1 week treatment.
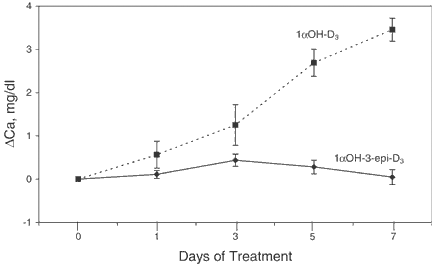
Calcemic activities of 1αOHD3 (solid circles) and 1αOH-3-epi-D3 (open circles). Normal rats were injected every other day with 2.5 nmol/kg of the vitamin D compounds, and plasma calcium was measured 24 h after each injection. Data are expressed as mean ± SD (n = 3).
Efficacy of 1αOH-3-epi-D3 Versus 1α,25(OH)2D3 in Suppressing PTH in Uremic Rats
The suppression of PTH by 1αOH-3-epi-D3 in the absence of changes in calcium in normal rats suggested that 1αOH-3-epi-D3 may be useful for the treatment of secondary hyperparathyroidism associated with chronic renal failure. We tested the efficacy of 1αOH-3-epi-D3 in a rat model of renal insufficiency and secondary hyperparathyroidism. The rats underwent a partial (5/6) nephrectomy and were fed a diet containing 0.9% P and 0.6% Ca for one month to promote secondary hyperparathyroidism. The rats then were treated with escalating doses of 1α,25(OH)2D3 or 1αOH-3-epi-D3, and the plasma calcium and PTH were determined at the end of each 1-week dose increment. Figure 7 (upper panel) shows the elevations in plasma Ca with increasing doses of the two compounds. Hypercalcemia, defined as plasma calcium >10.5 mg/dl, was achieved with doses of 0.08 nmol/kg of 1α,25(OH)2D3 and about 8 nmol/kg of 1αOH-3-epi-D3. In contrast to the 100-fold difference in calcemic activities, the two compounds were similar in their potencies to reduce plasma PTH levels (Fig. 7, lower panel). Significant suppression was observed with 1α,25(OH)2D3 only at doses of 0.02 nmol/kg and higher. However, even the lowest dose of 1αOH-3-epi-D3 (0.04 nmol/kg) produced significant suppression of about 60% that was maintained over 5 additional dose doublings. The more rapid drop in PTH in the 1α,25(OH)2D3-treated rats is due to suppression by the greater increment in plasma calcium.
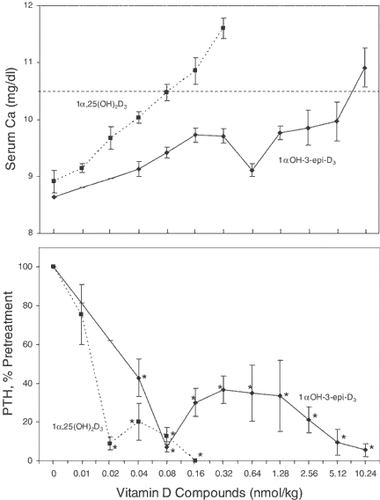
Effects of 1α,25(OH)2D3 and 1αOH-3-epi-D3 on plasma calcium (upper panel) and plasma PTH (lower panel) in uremic rats. Partially nephrectomized rats with established secondary hyperparathyroidism were treated with 1α,25(OH)2D3 or 1α(OH)-3-epi-D3 in a dose escalation protocol as described in Methods. At the end of each dosing period, plasma PTH and calcium were measured. Plasma total calcium (upper panel) is expressed as the increase from pretreatment values (in mg/dl) and given as mean ± SD (n = 12). PTH values (lower panel) are expressed as percent of pretreatment value and given as mean ± SEM (n = 12). *P < 0.05 versus pretreatment PTH level.
DISCUSSION
In the first part of this paper we reported that bone cells (ROS 17/2.8) directly metabolize 1αOHD3 into 1αOH-3-epi-D3. The identity of 1αOH-3-epi-D3 produced in ROS 17/2.8 cells was established using the techniques of UV absorption spectrophotometry, co-elution with the synthetic standard on both straight and reverse phase HPLC systems, GC/MS and 1H-NMR analysis. We also observed that 1αOHD3 is metabolized in bone cells not only into 1αOH-3-epi-D3, but also into 1α,25(OH)2D3 and 1α,25(OH)2-3-epi-D3. This finding suggests that 1α,25(OH)2-3-epi-D3 from 1αOHD3 may be formed through two pathways: (a) C-25 hydroxylation of 1αOHD3 into 1α,25(OH)2D3 followed by the C-3 epimerization pathway or (b) C-3 epimerization of 1αOHD3 into 1αOH-3-epi-D3 followed by C-25 hydroxylation. Thus, in both these pathways, two steps are involved in the conversion of 1αOHD3 into 1α,25(OH)2-3-epi-D3. Furthermore, we also identified 1αOH-3-epi-D3 and 1α,25(OH)2-3-epi-D3 as circulating metabolites in rats treated with pharmacological amounts of 1αOHD3. These results indicate that unlike the C-24 oxidation pathway, in which a hydroxyl group at C-25 position is essential for C-24 hydroxylation, the first step in the C-24 oxidation pathway [Reddy and Tserng, 1989], the hydroxyl group at C-25 position is not a pre-requisite for C-3 epimerization.
In the second part of this study we evaluated the biological activities of 1αOH-3-epi-D3. We have previously shown that 1α,25(OH)2-3-epi-D3 has nearly the same activity as 1α,25(OH)2D3 in suppressing the PTH secretion in cultures of bovine parathyroid cells despite its lower affinity for the vitamin D receptor [Brown et al., 1999a]. Based on the results of this in vitro study, we performed the present in vivo study. In a small group of normal rats, we first observed a dramatic suppression of PTH by 1αOH-3-epi-D3 in the absence of any increase in serum calcium. This observation was then confirmed in more detail in rats with kidney failure and established secondary hyperparathyroidism using a dose-escalation protocol. 1αOH-3-epi-D3 was only slightly less active in suppressing PTH than 1α,25(OH)2D3. However, the dose of 1αOH-3-epi-D3 required to achieve hypercalcemia (total plasma Ca>10.5 mg/dl) was about 100 times higher than that of 1α,25(OH)2D3. This high degree of selectivity for 1αOH-3-epi-D3 in suppressing PTH in vivo suggests a tremendous potential for 1αOH-3-epi-D3 in the treatment of secondary hyperparathyroidism. In recent years, the natural vitamin D hormone, 1α,25(OH)2D3, has been largely supplanted by new vitamin D analogs in the treatment of secondary hyperparathyroidism and the associated renal osteodystrophy in patients with chronic kidney diseases. Studies in the kidney failure rat model showed that the less calcemic analogs, 22-oxa-1α,25(OH)2D3 and 19-nor-1α,25(OH)2D2, have been estimated to have 6- and 3-fold wider therapeutic windows than 1α,25(OH)2D3 [Slatopolsky et al., 1995; Brown, 2000; Hirata et al., 2002]. Our present findings indicate that the therapeutic window for 1αOH-3-epi-D3 may be as much as 50-fold wider than that of 1α,25(OH)2D3. Confirmatory studies, performed with the same fixed dose protocol used for the other analogs, are necessary for a true comparison. Nonetheless, 1αOH-3-epi-D3 appears to have great potential for treatment of secondary hyperparathyroidism.
The mechanisms responsible for the differential effects of 1αOH-3-epi-D3 compared to 1α,25(OH)2D3 are not clear. It is conceivable that 1αOH-3-epi-D3 like other vitamin D prodrugs such as 1αOHD2 and 1αOHD3 has to undergo activation via hydroxylation of the side chain at carbon 25 before it can exert its biological activities in target tissues such as the parathyroid glands. We have recently observed that 1αOHD2 and 1αOHD3 can suppress PTH synthesis by cultured parathyroid cells (Brown, A.J., submitted for publication), suggesting that these “prodrugs” are activated by hydroxylation within parathyroid cells. The high selectivity of 1αOH-3-epi-D3 in the parathyroid glands could be due to either local (autocrine) activation in parathyroid cells or to interaction with a unique receptor, these possibilities are under investigation. The very low calcemic activity of 1αOH-3-epi-D3 can be attributed primarily to a greatly reduced potency in stimulating intestinal calcium transport. Its calcemic effects are very much less than those of 1αOHD3, illustrating the tremendous influence of the stereochemistry of the hydroxyl group at carbon 3 on biological activity. Again, the mechanisms for this are unclear. The relative rates of hydroxylation/activation of 1αOH-3-epi-D3 and 1αOHD3 are not known. The presumed activation products, 1α,25(OH)2-3-epi-D3 and 1α,25(OH)2D3 differ only about 10-fold in their affinities for the vitamin D receptor [Brown et al., 1999a], and the same receptor is thought to mediate vitamin D actions in both the intestine and parathyroid glands. Thus, other factors such as differential rates of catabolism, cellular uptake or intracellular trafficking of the compounds may be responsible for the selectivity of 1αOH-3-epi-D3 [Brown, 2000]. Recently, Plum et al. [2004] reported that several vitamin D analogs lacking most of the side chain retained VDR binding activity and were potent suppressors of PTH in vivo, but they had greatly reduced calcemic activities. Although the mechanism for the selectivity of the side chain truncated analogs is not known, these findings illustrate the possibility of developing analogs that target the parathyroid glands.
In conclusion, we report for the first time that bone cells can directly metabolize 1αOHD3 into 1αOH-3-epi-D3. Also, we report that 1αOH-3-epi-D3 is an in vivo metabolite of 1αOHD3 under pharmacological substrate concentrations. The conversion of 1αOHD3 into 1αOH-3-epi-D3 indicates that a hydroxyl group at C-25 position is not a pre-requisite for C-3 epimerization. Furthermore, our results also indicate that 1αOH-3-epi-D3 like 1αOHD3, is hydroxylated at C-25 position to form 1α,25(OH)2-3-epi-D3. Assessment of in vivo biological activities in both normal and kidney failure rats demonstrated that 1αOH-3-epi-D3 has much lower calcemic activity than 1αOHD3, and that 1αOH-3-epi-D3 can suppress PTH effectively in the absence of hypercalcemia. Thus, 1αOH-3-epi-D3 appears to have a great potential application for treatment of secondary hyperparathyroidism.
Acknowledgements
We thank Ms. H. Sekimoto and Dr. Mei-Ling Siu-Caldera for their expert technical assistance in generating sufficient quantities of 1αOH-3-epi-D3 for structure identification and Mr. Matthew Robinson for his help in the preparation of this manuscript.




