Direct inhibition of interleukin-2 receptor α-mediated signaling pathway induces G1 arrest and apoptosis in human head-and-neck cancer cells
Abstract
Overexpression of the interleukin-2 receptor (IL-2R) α chain in tumor cells is associated with tumor progression and a poor patient prognosis. IL-2Rα is responsible for the high affinity binding of the receptor to IL-2, leading to activation of several proliferative and anti-apoptotic intracellular signaling pathways. We have previously shown that human squamous cell carcinoma of a head-and-neck line (PCI-13) genetically engineered to overexpress IL-2Rα exhibit increased transforming activity, proliferation, and drug resistance, compared to the vector control cells (J Cell Biochem 2003;89:824–836). In this study, we report that IL-2Rα+ cells express high levels of total and phosphorylated Jak3 protein and are more resistant to apoptosis induced by a Jak3 inhibitor than the control LacZ cells. Furthermore, we used daclizumab, a monoclonal antibody specific to IL-2Rα, and determined the effects of IL-2Rα inhibition on cell cycle and apoptosis as well as the involvement of potential cell cycle and apoptosis regulatory proteins. We found that daclizumab induces G1 arrest, associated with down-regulation of cyclin A protein, preferentially in IL-2Rα+ cells, but not in LacZ cells. In addition, daclizumab activates apoptotic death program via Bcl-2 down-regulation preferentially in IL-2Rα+ cells. Finally, daclizumab also sensitizes IL-2Rα+ cells to other apoptotic stimuli, although the effect is moderate. These results indicate that daclizumab inhibits the proliferative potential of IL-2Rα+ cells via inhibition of cell cycle progression and induction of apoptosis. © 2005 Wiley-Liss, Inc.
Interleukin-2 (IL-2) is the primary cytokine of activated T-lymphocytes and can drive clonal expansion and effector cell maturation [Robb et al., 1984; Thornton et al., 2004]. IL-2 stimulation can also lead to the growth of natural killer (NK) cells [Whiteside and Herberman, 1995] and human tumor epithelial cells [Reichert et al., 2000] possibility through regulating the cyclin-dependent kinase inhibitors p21 and p27 [Reichert et al., 2000]. IL-2 medicates its biologic effects by binding to the IL-2 receptor (IL-2R) complex. IL-2R is composed of three chains: α, β, and γ [Sharon et al., 1986; Takemoto, 1989]. The α chain (CD25+, Tac, p55) is involved in IL-2 and IL-15 signaling, while IL-2Rβ and γ chains are involved in several cytokine-signaling pathways including IL-4, IL-7, IL-9, IL-15, and IL-21 [Noguchi et al., 1993; Russell et al., 1993; Anderson et al., 1995; Kimura et al., 1995].
Heterodimerization of IL-2Rβ and γ leads to an intermediate binding affinity (Kd = 10−9M) and is sufficient for downstream signaling, but heterotrimerization of all three subunits leads an overall to high affinity to the ligand (Kd = 10−11M) [Taniguchi and Minami, 1993]. Although IL-2Rβγ complex is fully competent to signal, it appears that only the IL-2Rαβγ complex is the physiologically relevant form of IL-2R, because mice lacking IL-2Rα are phenotypically the same as IL-2-deficient mice [Schorle et al., 1991; Sadlack et al., 1995]. The stoichometry of these receptors has recently come to light. Vamosi et al. [2004] have shown all three subunits are co-expressed in lipid rafts of T cells even in the absence of IL-2. Additionally, IL-2Rα and IL-15Rα form homodimers/oligomers and may provide a switching mechanism for the IL-2Rβγ heterodimer complex depending upon either IL-2 or IL-15 ligand stimulation [Vamosi et al., 2004].
Although there is much literature on the functionality of IL-2R in hematologic cell systems, the role that IL-2R plays in tumorogenic cells is not well characterized. Several studies have found overexpression of IL-2Rα in lung, ovarian, cervical, leukemic, and lymphomic cancers [Kaczmarski and Mufti, 1991; Barton et al., 1993; McDoniels-Silvers et al., 2002; Rocha-Zavaleta et al., 2004]. The levels of IL-2Rα protein expression have been used as a prognostic indicator, and high levels of IL-2Rα protein correlate with poor survival rates in cancer patients [Kaczmarski and Mufti, 1991; Tsai et al., 2001; Ohno et al., 2002; Rosso et al., 2002].
We have previously shown that a squamous cell carcinoma of the head-and-neck cell line (PCI-13) stably transfected with IL-2Rα cDNA (IL-2Rα+) has increase transforming activity and accelerated proliferation rates and is resistant to apoptosis-inducing drugs, compared to the vector control cells (LacZ) [Kuhn et al., 2003]. However, whether IL-2Rα is directly responsible for these unique cellular behaviors remains unknown. In the current study, we wanted to further elucidate the role of IL-2Rα in PCI-13 cells overexpressing IL-2Rα cDNA by using daclizumab, a humanized monoclonal antibody that specifically inhibits the binding of IL-2Rα to the IL-2Rβγ complex [Vincenti et al., 1998]. Treatment of IL-2Rα+ and control LacZ cells with daclizumab causes growth inhibition selectively in IL-2Rα+ cells, associated with a G0–G1 arrest and decreased cyclin A protein expression. Daclizumab treatment also induces apoptosis in IL-2Rα+ cells, without affecting the LacZ cells, possibly due to the decrease of Bcl-2 protein levels. Treatment with daclizumab also sensitizes IL-2Rα+ cells to the apoptosis inducing drugs ALLN, VP-16, and taxol, although the effect is moderate. Our data have confirmed that daclizumab inhibits the growth of head-and-neck squamous carcinoma cells, and revealed possible involved new molecular mechanisms.
MATERIALS AND METHODS
Reagents
Dulbecco's modified Eagle medium (DMEM), penicillin, and streptomycin were purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS), propidium iodide, ribonuclease RNase A, ALLN (or LLnL), etoposide (VP-16), taxol, Hoechst 33258, dimethyl sulfoxide (DMSO), and other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO). Monoclonal antibody to Bcl-2 (Ab-1) and polyclonal antibody to Bcl-X (Ab-1) were purchased from Oncogene Research Products (Boston, MA). Monoclonal antibodies to cyclin A (BF-683), cyclin D1 (R-124), and polyclonal antibodies to actin (C-11), Jak3 (C-21), and pJak3 (Tyr 980) as well as anti-goat, anti-rabbit, and anti-mouse IgG-horseradish peroxidase were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal antibodies to Cdk2 (D-12) were purchased from BD Biosciences Pharmingen (San Diego, CA). Fluorogenic peptide substrate Ac-DEVD-AMC (for caspase-3/-7 activities) and the specific Jak3 inhibitor WHI-P131 were obtained from Calbiochem (San Diego, CA). Daclizumab was a generous gift from Hoffman-La Roche (Nutley, NJ).
Cell Cultures
Human squamous cell carcinoma of the head-and-neck cell line, PCI-13 (Pittsburgh Cancer Institute-13) [Heo et al., 1989], stably transfected with the LacZ vector alone or IL-2Rα cDNA as previously described [Lin et al., 1993], was used in the current study. These transfected PCI-13 cell lines were cultured in DMEM, supplemented with heat inactivated 10% (v/v) FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were maintained in 5% CO2 in air atmosphere at 37°C, and passed by trypsinization (0.05% Trypsin, 0.53 mM EDTA; GIBCO, Carlsbad, CA).
Apoptotic Morphology Changes
Apoptotic morphology was assessed using phase-contrast microscopy as described previously [Smith et al., 2002; Kuhn et al., 2003].
Cell Extract Preparation and Western Blotting
Whole cell extracts were prepared as described previously [An et al., 1998]. Analysis of pJak3, cyclin A, cyclin D1, cdk-2, actin, Bcl-2, and Bcl-XL protein expression was performed using monoclonal or polyclonal antibodies according to previously reported protocols [An et al., 1998]. The relative density (RD) of protein expression was quantitated using Total Lab (Nonlinear USA, Inc., Durham, NC) and was normalized to loading control (actin).
Cell Cycle Analysis
At each time point cells were collected, washed twice with PBS, and fixed in 70% ethanol for 2 h at 4°C. The cells were then centrifuged, re-suspended in 1 ml of sample buffer containing propidium iodide [1× PBS, 50 μg propidium iodide, 1 mg/ml glucose, and 100 U/ml ribonuclease A] and incubated at room temperature for 30 min in the dark. Propidium iodide staining, indicative of G0/G1, S, and G2/M phase distribution of the cell population, was visualized with FACScan (Becton Dickinson Immunocytometry, CA) and cell cycle distribution was determined by ModFit LT cell cycle analysis software (Verity Software, Topsham, ME). The cell cycle distribution is presented as the percentage of cells containing G0/G1, S, and G2/M DNA content.
Fluorogenic Assays
To measure cell-free caspase-3 activity, whole cell extracts (30 μg) from untreated or treated PCI-13 cells were incubated with 20 μM of the fluorogenic substrate for caspase-3/-7 (Ac-DEVD-AMC) for 30 min at 37°C in 100 μl of assay buffer (50 mM Tris, pH 8.0). Measurement of the hydrolyzed AMC groups was performed on a VersaFluor™ Fluorometer (Bio-Rad, Hercules, CA) as described previously [Kuhn et al., 2003].
Nuclear Staining
After each drug treatment, both detached and attached PCI-13 cells were collected as described previously [An et al., 1998], washed twice in PBS, fixed in 70% ethanol for 2 h, and stained in 50 μM Hoechst 33258 for 30 min in the dark. Morphology was visualized at 10× on a fluorescent microscope (Zeiss; Thornwood, NY). Digital images taken with Axiovision 1.4.
Immunostaining of Apoptotic Cells In Situ
Immunostaining of apoptotic cells was performed by addition of the FITC-VAD-FMK marker and visualized on an Axiovert 25 microscope (Zeiss). Briefly, LacZ and IL-2Rα+ cells were grown to ∼80% confluency in 60 mm dishes, and then treated with 25 μg/ml daclizumab for 24 h. In some experiment, the pretreated cells were further treated with another apoptosis stimulus for 24 h. Detection of caspase activity was determined according to the manufacturer's protocol with a few modifications. Briefly, total cell population was collected and incubated with a 10 μM FITC-VAD-FMK for 20 min in the dark. Cells were then centrifuged at 300g for 3 min, washed 3× in PBS, and then resuspended in 50 μl PBS. Cell suspension was then transferred to glass slides in the presence of Vector Shield mounting medium with DAPI. Images were captured using AxioVision 4.1 and adjusted using Adobe Photoshop 6.0 software. Apoptotic cells were quantified by counting the number of apoptotic cells over the total number of cells in the same field.
RESULTS
Direct Inhibition of IL-2Rα Induces G1 Arrest and Cyclin A Down-Regulation Preferentially in IL-2Rα+ Cells
Previously we reported that IL-2Rα+ cells had increased proliferation rates, associated with high expression of several cell cycle proteins [Kuhn et al., 2003]. To determine whether IL-2Rα overexpression is responsible for these events, we used daclizumab, the monoclonal antibody specific to IL-2Rα [Vincenti et al., 1998]. LacZ and IL-2Rα+ cells were plated at 100,000 cells/well, and subsequently treated with 25 μg/ml of daclizumab for 24, 48, or 72 h (Fig. 1A). We found that daclizumab inhibited ∼70% of cell proliferation in IL-2Rα+ cells after 72 h, while LacZ cells showed only 9% inhibition of cell growth (Fig. 1A). In a similar experiment, both LacZ and IL-2Rα+ cell lines were treated with 25 μg/ml of daclizumab for 24, 48, and 72 h, followed by flow cytometry analysis. Daclizumab treatment arrested most of IL-2Rα+ cells in G0–G1 phase of the cell cycle (Fig. 1B). G0–G1 populations of IL-2Rα+ cells increased in a time-dependent manner, with the highest increase (by 57%) observed after 72 h (Fig. 1B). However, much less growth arrest (by up to 20%) was observed in LacZ cells treated with daclizumab (Fig. 1B). To confirm the differential effects of daclizumab were not due to its stability, both LacZ and IL-2Rα+ cells were treated with daclizumab at 25 μg/ml once per day for 3 days. Again, much higher levels of G0–G1 arrest were observed in IL-2Rα+ than in LacZ cells (57% vs. 19%; 3× columns in Fig. 1B). These data confirm the anti-proliferative effects of daclizumab on IL-2Rα+ cells shown in Figure 1A and suggest that the observed effect of daclizumab is due to specific inhibition of IL-2Rα.
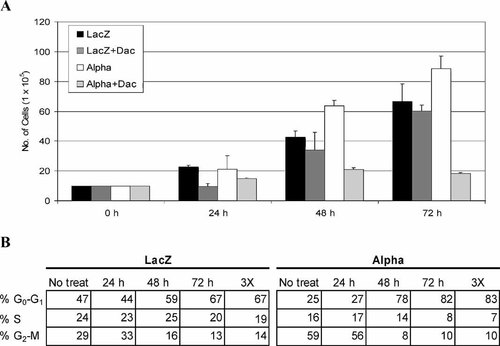
Inhibition of IL-2Rα signaling decreases cell proliferation and induces G0–G1 cell cycle arrest in IL-2Rα+ cells. A: LacZ and α cells were plated in 6-well plates (1 × 105 cell/well) and treated with 25 μg/ml daclizumab. At indicated time points, cells were collected via trypsinization and total cell population was calculated with a hemacytometer. Results shown are from three independent experiments (±SD). B: Cells were either treated once at 0 h or treated once a day for 3 days as indicated (3×). Cell cycle analysis was then performed using propidium iodide to assess DNA content. Cell cycle distribution is presented as the percentage of cells containing G0/G1, S, and G2/M DNA content.
We speculate that selective growth-inhibitory effect of daclizumab is due to inhibition of some cell cycle regulatory proteins in IL-2Rα+, but not LacZ, cells. To test this idea, the expression of several cell cycle proteins was measured in both IL-2Rα+ and LacZ cells after treatment with 5, 10, or 25 μg/ml of daclizumab for 24 h (Fig. 2A). We found that daclizumab treatment caused a dose-dependent decrease on cyclin A protein expression in IL-2Rα+ cells, by 15, 32, and 52% at 5, 10, and 25 μg/ml, respectively (Fig. 2A,B). In contrast, no change in cyclin D1 protein expression was found in IL-2Rα+ cells treated with daclizumab at any of the doses (Fig. 2A). Cdk2 expression decreased very little in IL-2Rα+ cells with 10 and 25 μg/ml daclizumab treatment (Fig. 2A). Interestingly, minor decreases in cyclin A expression were found in LacZ cells treated with daclizumab at 5 and 10 μg/ml, but at the maximal dose (25 μg/ml) a 25% increase in cyclin A expression was observed (Fig. 2A,B). Similar to IL-2Rα+ cells, there was little or no change in cyclin D1 expression in treated LacZ cells (Fig. 2A). Cdk2 expression remained relatively unchanged in LacZ cells treated with daclizumab at 5 and 10 μg/ml, but increased with 25 μg/ml daclizumab treatment (Fig. 2A).
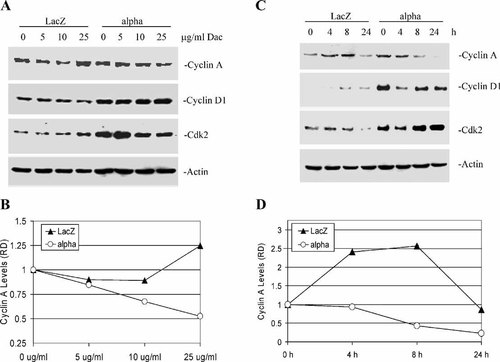
Daclizumab decreases cyclin A protein expression. A: Dose response. LacZ and IL-2Rα+ cells were treated with 5, 10, or 25 μg/ml daclizumab for 24 h. B: The relative density (RD) of cyclin A protein expression was quantitated and normalized to actin from panel A. C: Kinetic analysis. LacZ and IL-2Rα+ cells treated for 4, 8, and 24 h with 25 μg/ml daclizumab. D: Densitometry of cyclin A protein expression from kinetic experiment (C).
Next, we examined the kinetics of daclizumab's effect on both IL-2Rα+ and LacZ cell lines (Fig. 2C). There was a time-dependent decrease in cyclin A in IL-2Rα+ cells treated with the specific IL-2Rα antibody (Fig. 2C). Greater than 80% of cyclin A protein expression was abolished after 24 h treatment of daclizmab (Fig. 2D). Both cyclin D1 and cdk2 proteins decreased transiently after 4 h treatment in IL-2Rα+ cells, but began to return to basal levels after 8 h (Fig. 2C). Again, we observed different responses from LacZ cells. Daclizumab treatment seemed to stimulate cyclin A expression up to 8 h, but returned to basal levels after 24 h (Fig. 2C,D). Cyclin D1 expression began to increase after 8 h, lasting up to 24 h in LacZ cells treated with daclizumab, however the levels of cdk2 were almost completely abolished after 24 h incubation (Fig. 2C). These data indicate that the selective proliferation inhibition (Fig. 1A) and G0–G1 arrest induction (Fig. 1B) by daclizumab in IL-2Rα+ cells are a consequence of the decreased expression of cyclin A protein selectively in these cells.
IL-2Rα Inhibition Induces Apototic Cell Death and Bcl-2 Protein Down-Regulation Preferentially in IL-2Rα+ Cells
We and others have documented that overexpression of IL-2Rα is associated with resistance to apoptosis induction [Phillips et al., 2000; Guo et al., 2002; Kuhn et al., 2003]. Since daclizumab is a specific antibody of IL-2Rα and its binding to IL-2Rα causes inactivation of the receptor-mediated signal transduction pathways [Vincenti et al., 1998; Goebel et al., 2000], we examined whether daclizumab could induce apoptosis selectively in cells overexpressing IL-2Rα. We first treated both IL-2Rα+ and LacZ cells with 25 μg/ml daclizumab, followed by assaying the caspase-3 activity levels. Daclizumab treatment for 4 and 8 h induced caspase-3 activation (by twofold) only in IL-2Rα+ cells, but not LacZ cells (Fig. 3A). A 24 h treatment with daclizumab caused a much higher increase in caspase-3 activity in IL-2Rα+ cells than in LacZ cells (3.5-fold vs. 0.5-fold; Fig. 3A).
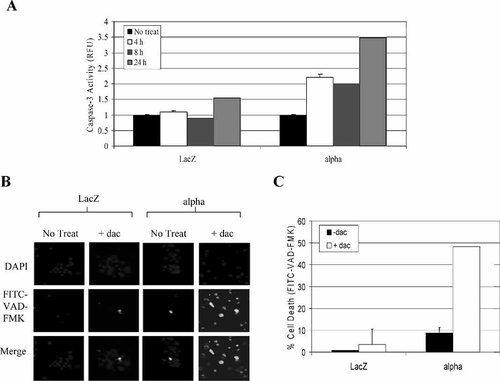
Daclizumab treatment alone induces activation of caspase-3/-7 in IL-2Rα cells. A: LacZ and IL-2Rα+ cells were treated with 25 μg/ml daclizumab for 4, 8, and 24 h. Cell extract was then incubated with fluorogenic peptide substrate specific for caspase-3/-7 activity. Measurement of the cleaved AMC groups was performed on a multilabel plate reader (Perkin-Elmer) and normalized to the untreated cells (±SD). B: LacZ and IL-2Rα+ cells were treated with 25 μg/ml daclizumab for 24 h. Following the treatment, the cells were then incubated with a FITC-conjugated marker that binds to activated caspases. Cell suspension was then transferred to glass slides in the presence of Vector Shield mounting medium with DAPI. Images were captured using AxioVision 4.1 and adjusted using Adobe Photoshop 6.0 software. C: Quantification of apoptotic cells (in B) was calculated by counting the number of apoptotic cells over the total number of cells in the same field. Data are mean of duplicate experiments ±SD.
To further assess the apoptosis-inducing effects of daclizumab, IL-2Rα+, and LacZ cells were treated with 25 μg/ml daclizumab for 72 h. An in situ marker specific for activated caspases was then added to the cells (Fig. 3B). We found that the untreated IL-2Rα+ cells showed an average of 9% apoptosis (basal line), possibly due to their high cell cycling profile. However, when IL-2Rα+ cells were treated with daclizumab, there was a significant increase in caspase-associated apoptosis (by 48%; Fig. 3B,C). In contrast, LacZ cells treated with daclizumab had little increase in caspase activity (∼10%; Fig. 3C).
To further confirm the apoptosis-inducing ability of daclizumab, both LacZ and IL-2Rα+ cell lines were treated with 25 μg/ml daclizumab for 48 h, followed by separation of the attached and detached cell populations. Both attached and detached cell populations were then used for detection of apoptotic nuclear changes (columns 1, 2 in Fig. 4). We found that after 48 h treatment with daclizumab, only IL-2Rα+ cells became detached. All the detached IL-2Rα+ cells exhibited typical apoptotic nuclear condensation and fragmentation (Fig. 4). The cellular detachment may be triggered by apoptosis, since even the remaining attached IL-2Rα+ cells showed apoptotic nuclear morphology (Fig. 4). No detachment was found in the treated LacZ cells; consistently, these cells contained normal, round nuclei (Fig. 4). When a DNA fragmentation assay was performed using samples prepared from LacZ and IL-2Rα+ cells treated with or without 25 μg/ml daclizumab for 48 h, DNA fragmentation was observed only in IL-2Rα+ cells treated with daclizumab (data not shown). These results suggest that IL-2Rα inhibition induces apoptosis preferentially in IL-2Rα+ cells.
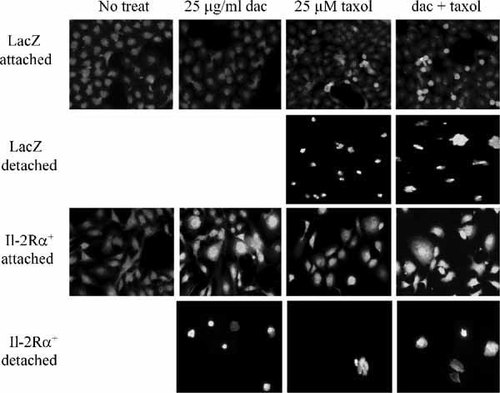
Daclizumab treatment induces apoptosis and sensitizes the effects of taxol in IL-2Rα+ cells. LacZ and IL-2Rα+ cells were pretreated with, or without, 25 μg/ml daclizumab for 24 h, followed by addition of 25 μM taxol for 24 h. Both detached and attached cell populations were stained with 50 μM Hoechst 33258 and apoptotic nuclei were visualized with fluorescent microscopy (10×).
We then determine whether daclizumab treatment could cause inhibition of some anti-apoptotic proteins selectively in IL-2Rα+ cells. We examined levels of both Bcl-2 and Bcl-XL proteins in IL-2Rα+ and LacZ cells after daclizumab treatment (Fig. 5). Bcl-2 protein expression in IL-2Rα+ cells decreased in a daclizumab dose-dependent manner, with a complete inhibition observed at 25 μg/ml (Fig. 5A,B). Bcl-XL protein levels were not affected at any of the doses of daclizumab in IL-2Rα+ cells (Fig. 5A). LacZ cells have no detectable Bcl-2 protein expression (Fig. 5A). We have previously reported the presence of an unknown Bcl-2-related 48 kDa protein (p48) observed in the LacZ, but not in the IL-2Rα+ cells [Kuhn et al., 2003]. Although the function of this protein is yet underdetermined, we observed a dose-dependent decrease in its expression in LacZ cells treated with daclizumab (Fig. 5A,B).
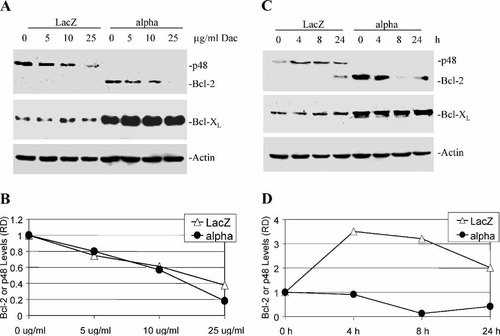
Daclizumab decreases Bcl-2 protein expression. A: Dose response. LacZ and IL-2Rα+ cells were treated with 5, 10, or 25 μg/ml daclizumab for 24 h. The unknown Bcl-2-related p48 protein is indicated. B: Relative density (RD) analysis of the dose-dependent decrease of Bcl-2 and p48 shown represented by panel A. C: Kinetic analysis. LacZ and IL-2Rα+ cells treated for 4, 8, and 24 h with 25 μg/ml daclizumab. D: Densitometry of Bcl-2 and p48 protein expression from kinetic experiment in panel C.
In a kinetics experiment, we found that Bcl-2 protein levels in IL-2Rα+ cells decreased as early as 4 h after treatment with 25 μg/ml daclizumab, remained suppressed up to 8 h, and began to recover after 24 h (Fig. 5C,D). Again, there was no discernable change in Bcl-XL protein expression in the treated IL-2Rα+ cells (Fig. 5C). In LacZ cells treated with 25 μg/ml daclizumab, expression of Bcl-2 was not detected up to 8 h, but a long exposure film shows the appearance of Bcl-2 after 24 h (Fig. 5C). Expression of the Bcl-2-related p48 was high at 4, 8 and 24 h (Fig. 5C,D). Bcl-XL protein expression in LacZ cells remained unchanged throughout all time points (Fig. 5C). Our results indicate that the selective apoptosis induction by daclizumab in IL-2Rα+ cells is associated with the decreased expression of Bcl-2 protein in these cells.
IL-2Rα+ Cells Express High Levels of Total and Phosphorylated Jak3 Protein and Are Resistant to Apoptosis Induced by a Jak3 Inhibitor
It has been shown that Jak3 kinase is activated upon IL-2 ligand interaction with its receptor [Johnston et al., 1994]. We investigated the potential role of Jak3 kinase in IL-2Rα-overexpressing PCI-13 cells (Fig. 6). We first measured levels of total Jak3 protein (Fig. 6A). We found approximately threefold higher expression of Jak3 protein in IL-2Rα+ cells compared to the vector control cells (Fig. 6A). We then determined levels of phosphorylated Jak3 protein since it is associated with activation of Jak3 [Johnston et al., 1994]. Western blot assay using a polyclonal antibody specific to Tyr-980 pJak3 shows that IL-2Rα+ cells express a higher level of phosphorylated/activated Jak3 than LacZ cells (by 2.6-fold; Fig. 6A). Interestingly, the same antibody also detected a protein band of 42 kDa (p42, denoted by **; Fig. 6A) that was present in much higher levels (by 4.4-fold) in IL-2α+ cells than LacZ cells. Whether the p42 is a modified form of pJak3 or a substrate of pJak3 needs further investigation. Therefore, IL-2Rα over-expression results in increased levels of total Jak3 and phosphorylated/activated Jak3 protein.
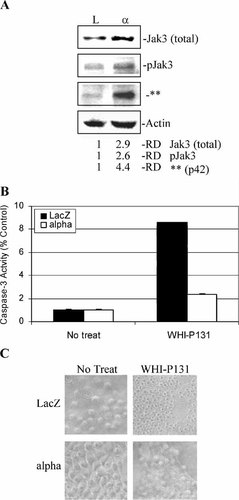
IL-2Rα+ cells expressing high levels of total Jak3 and phosphorylated Jak3 protein are resistant to apoptosis induced by a Jak3 inhibitor. A: Western blot analysis of total Jak3 and phosphorylated Jak3 protein expression in growing LacZ and IL-2Rα+ cells. ** Denotes an ∼42 kDa band (p42) that may be modified Jak3 or a putative target. The RD of protein expression was quantitated and normalized actin. LacZ and IL-2Rα+ cells were treated with 100 μM WHI-P-131 for 24 h, followed by measuring cell-free caspase-3 activity (B) and apoptotic morphology (C). B: Cell-free caspase-3 activity assay. Data shown is the mean of three independent experiments. C: Apoptotic morphology was assessed at 40× resolution.
If Jak3 kinase is required for tumor cell survival, we would expect that IL-2Rα+ cells are more resistant to apoptosis induction by a Jak3 inhibitor than LacZ cells. To test this hypothesis, both LacZ and IL-2Rα+ cell lines were treated for 24 h with WHI-P131, a pharmacologic inhibitor that specifically inhibits activity of Jak3, but not Jak1 and Jak2 [Sudbeck et al., 1999], followed by measurement of caspase-3 activity. Indeed, much higher levels of caspase-3 activity were detected in LacZ cells than in IL-2Rα+ cells (8.5-fold vs. 2.3-fold; Fig. 6B). Consistently, phase-contrast microscopy showed that LacZ cells rounded up and exhibited blebbing of the plasma membrane, a characteristic of apoptosis [Kerr et al., 1972], while IL-2Rα+ cells had very little apoptotic morphological changes (Fig. 6C). Therefore, high levels of pJak3 are associated with resistance to apoptosis by WHI-P131 in IL-2Rα-overexpressing cells.
IL-2Rα Inhibition Sensitizes IL-2Rα Cells to Other Apoptotic Stimuli
It has been shown that the head-and-neck cancer PCI-13 cells overexpressing IL-2Rα have a decreased sensitivity to chemotherapeutic agents, while vector control cells remain susceptible [Kuhn et al., 2003]. To assess whether the monoclonal antibody to IL-2Rα would sensitize the overexpressing cells to other apoptosis stimuli, IL-2Rα+ and LacZ cells were pretreated with or without daclizumab for 24 h, followed by treatment with the proteasome inhibitor ALLN (LLnL) or a chemotherapeutic drug VP-16 or taxol for additional 24 h (Fig. 7). Induction of caspase-3 activity in IL-2Rα+ cells without pretreatment of daclizumab ranged from 2.0- (LLnL) and 2.6-fold (VP-16) to 1.3-fold (taxol) (Fig. 7B). IL-2Rα+ cells pretreated with daclizumab alone had almost a threefold increase in caspase-3 activity (Fig. 7B), and addition of LLnL, VP-16, and taxol further increased caspase-3 activity by two, five, and fourfold, respectively (Fig. 7B). Treatment with LLnL, VP-16, and taxol in LacZ cells increased caspase-3 activity by 8-, 23-, and 14-fold, respectively (Fig. 7Y). When LacZ cells were pretreated with daclizumab for 24 h, followed by introduction with these apoptosis-inducing drugs, the levels of caspase-3 activity were slightly lower than drug treatment alone (Fig. 7A).
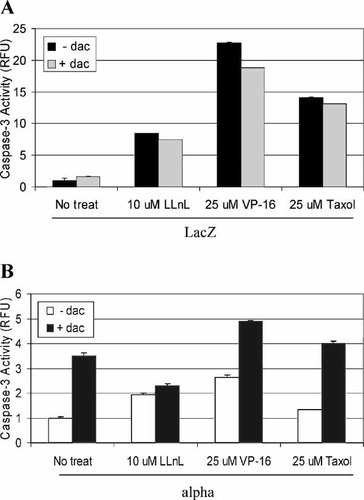
Inhibition of IL-2Rα signal transduction leads to increased sensitivity to chemotherapeutic drugs. LacZ and IL-2Rα+ cells were preincubated with 5 μg/ml daclizumab for 24 h, followed by treatment with 10 μM LLnL, 25 μM VP-16, or 25 μM taxol for 24 h in LacZ (A) and IL-2Rα+ cells (B). Caspase-3 activity was determined by incubating with fluorogenic substrate for 4 h and measuring free AMCs. Standard deviations are shown with error bars from a mean of at least three independent experiments.
To further determine the sensitizing effects of daclizumab in IL-2Rα+ cells, nuclear condensation and fragmentation assay was performed. Little detachment and nuclear fragmentation were observed in IL-2Rα+ cells treated with taxol without daclizumab (Fig. 4, column 3). IL-2Rα+ cells pretreated with daclizumab followed by taxol treatment induced more detachment and nuclear morphology change in the attached cell population (Fig. 4, column 4 vs. 3). Although taxol treatment of LacZ cells increased populations of detachment and apoptotic nuclei, pretreatment with daclizumab did not increase the effect of taxol (Fig. 4, column 4 vs. 3). Therefore, daclizumab pretreatment sensitizes IL-2Rα+, but not LacZ, cells.
DISCUSSION
To further elucidate the role of that IL-2Rα+ overexpression has in tumor growth and survival, we obtained a humanized monoclonal antibody that binds specifically to IL-2Rα (daclizumab; Zenepax). Daclizumab is currently approved for treatment in kidney transplant patients to abrogate IL-2-mediated activation of lymphocytes, a critical pathway in the cellular immune response involved in allograft rejection [Vincenti et al., 1998]. Daclizumab treatment is therefore predicted to lead to inhibition of all the downstream proliferative and anti-apoptotic signaling pathways mediated by IL-2Rα.
Proliferation of IL-2Rα+ cells was effectively inhibited by daclizumab, while the LacZ cells had a significantly reduced sensitivity to IL-2Rα inhibition (Fig. 1A). The cell cycle distribution pattern differs dramatically between the untreated vector and IL-2Rα+ cells (Fig. 1B). Almost one-half of the LacZ cell population resides in the G0–G1 phase, which resembles the customary pattern observed in cycling cells. IL-2Rα+ cell distribution shows that 59% of the cells are in the G2–M phase, similar to what we have observed previously [Kuhn et al., 2003]. Treatment with daclizumab for 72 h effectively inhibited cell cycle progression in the IL-2Rα+ cells with a 57% increase of the cells in G0–G1 compared to LacZ, which only increased ∼20% (Fig. 1B). This observation is confirmed by the examination of several cell cycle proteins. Notable decrease in cyclin A expression is found in IL-2Rα+ cells treated with daclizumab in both a dose- and time-dependent manner (Fig. 2). Therefore, daclizumab selectively and effectively inhibits cell cycle progression in IL-2Rα+ cells associated with inhibition of cyclin A protein expression.
The role IL-2Rα plays in cell survival of solid tumorigenic cells is not well understood. If the cell surface receptor is responsible for the anti-apoptotic properties, then inhibition of IL-2Rα should lead to apoptosis. We also predicted that daclizumab should have no effect on LacZ cells. Although LacZ cells express endogenous IL-2Rα on their membrane, they have vastly different cell cycle and anti-apoptotic protein expression phenotype compared to IL-2Rα cells. Daclizumab did not induce caspase-3 activation in LacZ cells. Conversely, when IL-2Rα cells were treated with daclizumab, we observed a 3.5-fold increase in caspase-3 activity (Fig. 3A) and increase overall caspase activity by almost 50% (Fig. 3B,C). Furthermore, daclizumab treatment induced detachment and apoptotic nuclear changes in IL-2Rα+, but not LacZ cells (Fig. 4).
The exact nature of the Bcl-2-related 48 kDa protein (p48) observed by Western blot (Fig. 5A,C) is unknown at this time. Our preliminary data suggests that it is a Bcl-2-related protein that is detectable by multiple antibodies against Bcl-2 (data not shown). Additionally, p48 is not affected by phosphatase treatment leading us to believe that it is not a phosphorylated form of Bcl-2, nor is it a protein complex because additional heating, β-mercaptoethanol, and SDS had no effect [Kuhn et al., 2003]. Further characterization of this protein is needed to determine its importance, if any, in the apoptotic cellular machinery.
The mechanism of drug resistance in tumors overexpressing IL-2Rα is not entirely understood. Previously we reported that IL-2Rα+ cells are resistant to a tripeptidyl proteasome inhibitor (LLnL) and two chemotherapeutic drugs (VP-16 and taxol). We hypothesized that the downstream signaling pathways, such as the Jaks and signal transducer and activators of transcription (STATs), stimulated transcription of proliferative and anti-apoptotic genes (e.g., cyclin D and Bcl-XL), leading to their drug resistance [Kuhn et al., 2003]. We found that pretreatment with daclizumab sensitized, in a moderate degree, IL-2Rα+ cells but not LacZ cells (Figs. 4 and 7). One possible explanation for the modulate effect is the G0/G1 cell cycle arrest observed in IL-2Rα+ cells after daclizumab treatment (Fig. 1). Several previous reports have shown that arresting cells in G0/G1 confers resistance to apoptotic stimuli [Kuroki et al., 1996; Ketley et al., 1997, 2000]. Most chemotherapeutic drugs target actively cycling cells. For instance, VP-16 targets topoisomerase II, which is responsible for cleavage and rejoining of double-stranded DNA, allowing the separation of intertwined DNA strands essential for DNA replication and transcription [Isaacs et al., 1998], and taxol binds to tubulin, stabilizing their polymerization, leading to inhibition of chromosome separation in anaphase [Gligorov and Lotz, 2004]. Thus, resting cells do not provide an effective target.
Another possibility why daclizumab does not provide an additive or synergistic effect to these cell death-inducing drugs (LLnL, VP-16, and taxol) is probably due to the inability to decrease the anti-apoptotic protein Bcl-XL. The amount of Bcl-XL protein expression in IL-2Rα+ cells is considerably more than LacZ cells (Fig. 5). While we observed dose- and time-dependent decreases in Bcl-2 protein expression after 25 μg/ml treatment with daclizumab in IL-2Rα+ cells, there was no change to Bcl-XL expression (Fig. 5). This reduced expression of Bcl-2 may be responsible for the daclizumab-induced apoptosis observed (Figs. 3 and 4). Further studies into the role of Bcl-XL in the anti-apoptotic properties in cells overexpressing IL-2Rα are needed.
Previously we have demonstrated that overexpression of IL-2Rα in a squamous cell carcinoma of the head-and-neck cell line (PCI-13) is associated with increased drug resistance, proliferation, and transforming activity [Kuhn et al., 2003]. Jak3 kinase has been shown to be activated upon IL-2 ligand interaction with its receptor [Kuhn et al., 2003]. However, treatment with the Jak3 inhibitor WHI-P131 had a moderate effect on apoptosis induction in IL-2Rα+ cells compared to the vector control treated cells, 2.3-fold and 8.5-fold, respectively (Fig. 6). This indicates that anti-apoptotic signaling pathway mediated by Jak3 in IL-2Rα+ cells is not solely responsible for the aggressive phenotype observed in this cell line. Other survival kinase pathways are activated after IL-2R stimulation, such as the Ras/Raf/Mek/Erk- and PI3K/Akt-mediated anti-apoptotic survival pathways [Miyazaki et al., 1995].
In summary, we have shown that daclizumab inhibits proliferation, associated with decreased cyclin A protein expression and subsequent G0–G1 arrest in IL-2Rα+ cells, but not the vector control cells. Daclizumab is also capable of inducing apoptosis in IL-2Rα+ cells, which is likely due to a decrease in Bcl-2 protein. Pretreatment with daclizumab does lead to increased sensitization of IL-2Rα overexpressing cells to the apoptosis-inducing drugs LLnL, VP-16, and taxol. However, this effect is moderate probably due to the G0–G1 cell cycle arrest and unchanged Bcl-XL expression. The research presented here confirms the proliferation-inhibitory properties of daclizumab and reveals some involved molecular mechanisms.
Acknowledgements
We thank Hoffman-La Roche (Nutley, NJ) for providing Daclizumab as a generous gift, Dr. Teresa Whiteside at the University of Pittsburgh (Pittsburgh, PA) for the PCI-13 cells, and the Flow Cytometry Core at Karmanos Cancer Institute, Wayne State University, School of Medicine (Detroit, MI).




