SHP-1 suppresses cancer cell growth by promoting degradation of JAK kinases
Abstract
SHP-1 has been proposed to be a tumor suppressor gene for several cancers. The expression of SHP-1 protein is diminished or abolished in most leukemia and lymphoma cell lines and tissues, and in some non-hematopoietic cancer cell lines, such as estrogen receptor (ER) negative breast cancer cell lines and some colorectal cancer cell lines. However, we do not know whether the reduced SHP-1 expression is the cause of cancer diseases or the secondary effect of cancer developments. Here, we first demonstrate that SHP-1 has general tumor suppressing function in SHP-1 transfected cell lines. Transfected SHP-1 inhibits the growth of three lymphoma/leukemia cell lines (Ramos, H9, Jurkat) and one breast cancer cell line (HTB26). We also demonstrate a possible molecular mechanism for the tumor suppressing function of SHP-1: SHP-1 inhibits cell growth partly by negative regulation of activated JAK kinase. In addition, we find, for the first time, that SHP-1 down-regulates the level of TYK2 kinase in H9 cells and of JAK1 kinase in HTB26 cells, by accelerating their degradation. The SHP-1 accelerated degradation of JAK1 kinase in HTB26 cells was blocked with the treatment of MG132, a specific inhibitor for proteasome-mediated proteolysis. Our data suggest a new function of SHP-1 in the regulation of proteasome-mediated degradation pathway. © 2003 Wiley-Liss, Inc.
SHP-1, an SH2 domain-containing cytosolic protein tyrosine phosphatase, is a key regulator that controls the intracellular phosphotyrosine level. It is predominantly expressed in hematopoietic cells and epithelial cells [Neel and Tonks, 1997; Tamir et al., 2000; Zhang et al., 2000a]. SHP-1 contains two Src homology (SH2) domains, a neighboring catalytic domain, and a C-terminal tail. Its phosphatase activity is inhibited by the interaction between the N-terminal SH2 domain and the catalytic domain [Pei et al., 1993, 1994; Townley et al., 1993; Zhao et al., 1993; Yang et al., 2003]. SHP-1 acts as a negative regulator of intracellular signaling by three families of transmembrane receptors: growth factors with an intrinsic tyrosine kinase activity (e.g., c-kit, CSF-1, TrkA, and EGF) [Feng et al., 1993; Yi and Ihle, 1993; Tauchi et al., 1994; Vambutas et al., 1995; Chen et al., 1996], cytokine receptors (e.g., Epo-R, IFNα/β-R, IL-3R, and IL-2R [Yi et al., 1993, 1995; David et al., 1995; Klingmuller et al., 1995; Tauchi et al., 1995; Migone et al., 1998] and receptors involved in immune responses such as the T-cell receptor complex (TCR), CD5, and death receptor [Marengere et al., 1996; Pani et al., 1996; Plas et al., 1996; Perez-Villar et al., 1999; Daigle et al., 2002; Stefanova et al., 2003]. SHP-1 binds the immunoreceptor tyrosine-based inhibition motif (ITIM) of these receptors through its SH2 domains and dephosphorylates the down-stream proteins. The effect of SHP-1 is to terminate the signal of the activated receptor or to activate other terminating pathways such as apoptosis [Unkeless and Jin, 1997].
SHP-1 has been proposed to be a candidate tumor suppressor gene in lymphoma, leukemia, and other cancers because it functions as an antagonist to the growth-promoting and oncogenic potentials of tyrosine kinases [Plutzky et al., 1992, Wu et al., 2003]. Therefore, dysfunction of SHP-1 in lymphocytes is predicted to be associated with lymphoma, leukemia, and other related diseases. Extensive studies of SHP-1 protein and SHP-1 mRNA in cancer cell lines have revealed that the expression of SHP-1 protein is diminished or abolished in most leukemia and lymphoma cell lines and tissues, and in some non-hematopoietic cancer cell lines, such as estrogen receptor (ER) negative breast cancer cell lines and some colorectal cancer cell lines [Delibrias et al., 1997; Uesugi et al., 1999; Zhang et al., 2000b; Oka et al., 2001; Leon et al., 2002]. The decreased levels of SHP-1 protein and SHP-1 mRNA in these cell lines have been attributed to either methylation of the promoter region of the SHP-1 gene or a post-transcriptional block of SHP-1 protein synthesis [Zhang et al., 2000b; Oka et al., 2002]. In contrast, in some non-hematopoietic cell lines, such as ovarian cancer and ER positive breast cancer cell lines, SHP-1 protein is either normally expressed or over-expressed [Mok et al., 1995; Yip et al., 2000]. The data suggest that SHP-1 mainly plays negative roles in regulating signal transduction pathways in hematopoietic cells and in some non-hematopoietic cells.
The Janus kinase (JAK) family consists of four proteins in mammalian cells: JAK1, JAK2, JAK3, and TYK2. The JAK-STAT pathway transmits information received from extracellular polypeptide signals directly to target gene promoters in the nucleus. This is accomplished via transmembrane receptors, providing a mechanism for transcriptional regulation without second messengers [Aaronson and Horvath, 2002; Rane and Reddy, 2002]. SHP-1 has been shown to inhibit tyrosine phosphorylation of JAK kinases following their recruitment to receptor complexes [Yi and Ihle, 1993; Yi et al., 1993, 1995; Klingmuller et al., 1995; Bittorf et al., 1999]. Here, we report a general tumor suppressing function of SHP-1 by using three hematopoietic-related cancer cell lines and one breast cancer cell line as models. The tumor suppressing function of SHP-1 is achieved partly by its down-regulation of the level of phosphorylated JAK kinases. We also find that SHP-1 can not only inhibit the tyrosine phosphorylation of JAK kinases, but also decrease the expression level of JAK kinases by promoting their degradation. We present evidence for a novel function of SHP-1 in the negative regulation of JAK kinases and their degradation.
MATERIALS AND METHODS
Cell Culture and Induction of Differentiation
Romas, H9, Jurkat, HTB26, T47D cells were obtained from the American Type Culture Collection (Manassas, VA). Cells were passed routinely in RPMI 1640 medium supplemented with 10% fetal calf serum (FSC), 10 U/ml penicillin, 10 μg/ml streptomycin (for Jurkat cell) or with more additives 2 mM l-glutamine, 10 mM HEPES, 1 mM sodium pyruvate, 4.5 g/L glucose, 1.5 g/L bicarbonate (for Romas and H9 cells). HTB26 was maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS, 10 U/ml penicillin, and10 μg/ml streptomycin (Invitrogen, Carlsbad, CA). Lipofectamine™ 2000 (Invitrogen) was used to transiently transfect the plasmid DNAs of pCMV vector, SHP-1/pCMV and SHP-1M/pCMV into the targeted cells in 100 mm plates according to the protocol.
Cell Growth Curve and Cell Proliferation Curve
To measure cell growth, cells at a density of 2 × 104 cells/well were grown at 37°C in 24-well plates, in medium with or without 1.5% dimethyl sulfoxide (DMSO) (Sigma, St. Louis, MO). Cells were counted every 24 h after adding DMSO or 48 h after transient transfection. Cell proliferation rate was determined by the MTT assay using the CellTiter 96 Aqueous One Solution Cell Proliferation Assay protocol (Promega, Madison, WI). The amount of the formazan product resulting from bioreduction of MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] by cells, was monitored by recording the absorbance at 492 nm using a 96-well plate reader (Bio–Whittaker, Wakersville, MD).
Western Blotting
Cells were washed twice with cold PBS buffer, and lysed in Triton X-100 lysis buffer (10 mM Tris-HCl pH 7.4, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 20 mM Na4P2O7, 2 mM Na3VO4, 1% Triton X-100, 10% glycerol, 0.1% SDS, 0.5% deoxycholate, 1 mM PMSF, 1µg/ml leupeptin, and 5µl/ml aprotinin) for 30 min on ice. The extracts were sheared twice through a 20 gauge needle and centrifuged at 14,000 rpm for 15 min at 4°C. The protein concentration in supernatant was determined by Bradford assay using the Bio-Rad Protein Assay kit (Bio-Rad, Hercules, CA). Equal amounts of total protein were separated on 8–12% SDS–PAGE gels, then transferred on to nitrocellulose with Semi-Dry Transfer cell (Bio-Rad), and hybridized with primary antibody, and recognized with the secondary antibody and exposed to Kodak film.
Antibodies
Anti-human SHP-1 rabbit antibody was generated against full-length SHP-1 as previously described [You and Zhao, 1997]. The following antibodies were purchased: polyclonal anti-actin antibody (Sigma), polyclonal anti-JAK1, JAK2 antibodies (BioSource, Camarillo, CA), polyclonal anti-TYK2, anti-JAK3 anti-phospho-JAK1(pTyr1022/1023), anti-phospos-JAK2(pTyr1007/1008), and anti-phospho-TYK2(pTyr1054/1055) antibodies (Santa Cruz, Biotechnology, Santa Cruz, CA).
Screening the HTB-26 Stable Cell Lines and the H9 Mixed Stable Cell Line
For HTB26 cells, cells transiently transfected with SHP-1/CMV or SHP-1M/CMV vectors were diluted at 1:5–1:10, cultured in medium with different concentration of G418 for 4 weeks. The cells were then diluted to one cell per well in 96-well plates and grown in the presence of G418 till reaching sufficient density and screened for the expression of SHP-1 or SHP-1M proteins. For H9 cells, cells transiently transfected with SHP-1/CMV or SHP-1M/CMV vectors were cultured in medium with different concentration of G418 for 6 weeks and screened for the expression of SHP-1 or SHP-1M proteins.
Study of JAK1 Degradation in HTB26 Cells
SHP-1 and SHP-1M stably transfected HTB-26 cells (5 × 106) that had been pretreated with 20 μg/ml cycloheximide were cultured in plates and harvested after incubation in culture medium for 0, 1, 2 and 4 h. JAK1 kinase expression was analyzed using the method described above. For the inhibition of proteasome-mediated degradation, 50 μM MG-132 was added into SHP-1 and SHP-1M stably transfected HTB26 cells, respectively, when cell number grown to 5 × 106. The cells were harvested 4 h later for JAK1 kinase expression analysis.
RESULTS
SHP-1 Suppresses the Growth of Lymphoma/Leukemia Cancer Cells
The level of SHP-1 protein is dramatically reduced or abolished in most hematopoietic-related cancer cell lines and tissues [Wu et al., 2003]. We do not know whether the reduced SHP-1 expression is the cause of cancer diseases or the secondary effect of cancer developments. However, as SHP-1 negatively regulates hematopoietic cell development, decreased or abolished SHP-1 protein level is predicted to result in the abnormal proliferation of hematopoietic cells and therefore to promote cancer cell growth and development. If SHP-1 deficiency contributes to the abnormal proliferation of these cells, then overexpression of SHP-1 may suppress their growth. To test this hypothesis, three SHP-1 deficient cell lines were selected: Romas cell from Burkitt's lymphoma, H9 cell from T cell lymphoma, and Jurkat cell from T-cell leukemia. SHP-1 protein expression is undetectable in H9 cells (Fig. 1A), and is dramatically reduced in both Romas and Jurkat cells [Delibrias et al., 1997; Oka et al., 2001; Leon et al., 2002].
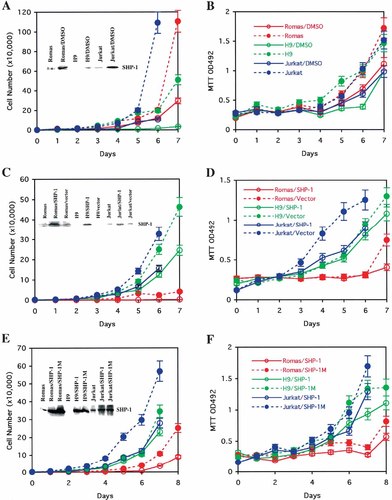
Induction of SHP-1 expression can suppress the growth of three lymphomatic originated cancer cells. The cell growth curves are shown in A, C, E and the cell proliferation rate as determined by MTT assay, monitored with the absorbance at 492 nm are shown in B, D, F for Romas (red), H9 (green), and Jurkat (blue) cells. The results with lower SHP-1 expression are shown in dashed line with solid circle, and those with higher SHP-1 expression are shown in solid line with open circle. A and B: Comparison of cell growth and cell proliferation rate of Romas, H9, and Jurkat cells (dashed line) with those in the presence of 1.5% of dimethyl sulfoxide (DMSO) (solid line). DMSO induces SHP-1 expression, as shown in the insert of A. C and D: Comparison of cell growth and cell proliferation rate of Romas, H9, and Jurkat cells transfected with SHP-1 gene (solid line) with those transfected with vector only (dashed line). E and F: Comparison of cell growth and cell proliferation rate of Romas, H9, and Jurkat cells transfected with the SHP-1 gene (solid line) with those transfected with the inactive SHP-1M (C455S mutant) gene (dashed line). The figure legends are shown as inserts in the right-panel figures, and the SHP-1 protein expression under the corresponding experimental conditions are shown as inserts in left-panel figures. The curves represent the average of at least three independent experiments.
DMSO, a reagent that is widely used to suppress cell growth, is known to induce SHP-1 expression and suppress the growth of K562 cells [Bruecher-Encke et al., 2001]. We first examined the effects of 1.5% DMSO on the growth of the above three cancer cell lines. Cell growth curves and cell prolieration rates for Romas (red), H9 (green), and Jurkat (blue) were determined in the presence (solid lines) and in the absence (dashed lines) of DMSO (insert of Fig. 1A,B). In the presence of DMSO, cell growth was reduced 2–3 fold for Romas cells, 10–20 fold for H9 cells, and 3–10 fold for Jurkat cells. In each cell type, the cell proliferation rate was also reduced 2–3 fold in the presence of DMSO. The suppressing effect of DMSO on cell growth was correlated with SHP-1 expression. Addition of DMSO to the growth medium induced SHP-1 expression by 3–5 fold in all three cell lines (insert of Fig. 1A). Therefore, the reduced cell growth with the DMSO treatment correlates with induced SHP-1 expression.
To examine directly whether or not elevating SHP-1 protein levels in cancer cells is sufficient to reduce their growth, cell growth rate and cell proliferation rate were compared for Romas (red), H9 (green), and Jurkat (blue) cells transiently transfected with the SHP-1 gene (solid line) or with the pCMV vector (dashed line), respectively (Fig. 1C,D). Cell growth of the SHP-1 transfected cells was suppressed by 2–3 fold in both H9 and Jurkat cells and by 10 fold in Romas cells, relative to pCMV-transfected cells. The cell proliferation rate was also reduced by 1–3 fold in all SHP-1-transfected cells (Fig. 1C,D). In addition, the suppression of cancer cell growth by SHP-1 protein depends on its phosphatase activity. If cells are transiently transfected with a phosphatase-deficient mutant of SHP-1 (SHP-1M, carrying the C455S substitution), growth is not suppressed (Fig. 1E,F). The growth rate of SHP-1M transfected cells (dashed line) was two to four times faster than that of SHP-1 transfected cells. The cell proliferation rate also was two to four times faster in SHP-1M transfected cells than in SHP-1 transfected cells. Thus, three different experiments indicate that increased levels of SHP-1 protein expression, achieved via either DMSO treatment or transiently tranfected SHP-1 gene, can suppress the growth of Romas, H9, and Jurkat cells. This suppressing function also depends on the phosphatase activity of SHP-1.
SHP-1 Suppresses the Activation of JAK1 and Reduces the Expression of TYK2 in H9 Cells
SHP-1 has been reported to directly dephosphorylate the JAK kinases and to negatively regulate cell proliferation [Yi and Ihle, 1993; Klingmuller et al., 1995]. Because the JAK kinases control cell proliferation, perhaps SHP-1 controls cell proliferation by negatively controlling the amount of activated JAK kinases. To test this hypothesis, we examined the effects of SHP-1 expression on the activation of all JAK kinases (JAK1, JAK2, JAK3, and TYK2 kinases) in H9 cells. For this purpose, plasmid DNA containing both the G418 resistance gene and the gene encoding SHP-1 or SHP-1M was transfected into H9 cells. The mixed SHP-1 and SHP-1M transfected H9 cells were selected by growing the SHP-1 tranfected H9 cells and the SHP-1M transfected H9 cells in the presence of G418 for 6 weeks, respectively, and were used for the following analysis.
The mixed SHP-1 transfected H9 cells showed slower growth rate as compared with the mixed SHP-1M transfected H9 cells (Fig. 2A). Growth of the SHP-1 transfected cells was slowed down by more than 20 fold as compared with that of the SHP-1M transfected cells. Cell proliferation rate for the SHP-1 transfected cells was also reduced by 2–3 fold as compared with that of the SHP-1M transfected cells. Overall, the results were consistent with those obtained from the transiently transfected H9 cells (Fig. 1).
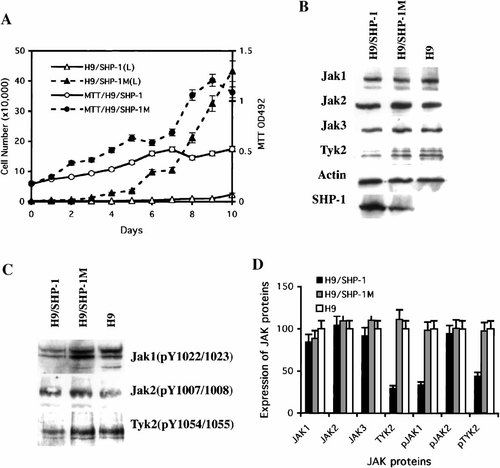
SHP-1 suppresses the growth of H9 cells by promoting the degradation of TYK2 kinase. A: The cell growth curve (diamond, Y axis on left) and cell proliferation rate (circle, Y axis on right) for the mixed SHP-1 (solid line) and SHP-1M (dashed line) transfected H9 cells. B: The amount of total JAK1, JAK2, JAK3, and TYK2 kinases in the mixed SHP-1 and SHP-1 transfected H9 cells and in parental H9 cells, as determined by immunoblotting with actin as a control. The expression of SHP-1 protein was also examined. C: The amount of active JAK1, JAK2, and TYK2 kinases in mixed SHP-1 and SHP-1M transfected H9 cells and parental H9 cells, as determined using the monoclonal antibody for bi-phosphorylated JAK proteins. D: The amount of the JAK kinases and of the phosphorylated JAK kinases in the mixed SHP-1 and SHP-1M transfected H9 cells, as compared with that in the parental H9 cells.
The level of phosphorylated (activated) JAK proteins was determined for H9 cells and two mixed transfected H9 cells using the anti-phospho-JAK antibodies (Fig. 2C). Because there is no anti-phos-JAK3 antibody available, the amount of activated JAK3 kinase was examined using anti-phosphotyrosine antibody against JAK3 that had been immunoprecipitated using anti-JAK3 antibody. No activated JAK3 kinase was observed in any of the three H9 cell lines (data not shown). The amount of activated JAK2 kinase was the same among the three H9 cells (Fig. 2C,D). However, the levels of activated JAK1 and activated TYK2 kinases were reduced by 50% in SHP-1 transfected H9 cells relative to the parental H9 cells and the SHP-1M transfected H9 cells. Because activated JAK kinases can phosphorylate STAT proteins and promote cell proliferation, the reduction in activated JAK1 and TYK2 in SHP-1 transfected H9 cells is predicted to reduce their growth and proliferation. Therefore, our data suggest that the transfected SHP-1 suppresses the growth of the SHP-1 deficient H9 cell line via negative regulation of activated JAK1 and TYK2 kinases.
The negative regulation of the phosphorylated JAKs in SHP-1 transfected H9 cells could be achieved by at least two possible pathways, one is direct dephosphorylation of the phorphorylated JAKs, and the other is reduced expression of JAKs. As SHP-1 is a protein tyrosine phosphatase, it could directly inactivate the phosphorylated JAKs by dephosphorylation. In support of this hypthosis, SHP-1 is reported to associate with and dephosphorylate JAK2 [Klingmuller et al., 1995; Jiao et al., 1996] and also to down-regulate IL-2 induced tyrosine phosphorylation of JAK1 and JAK3 [Migone et al., 1998]. However, there are no reports addressing possible regulation of JAK protein expression by SHP-1. To explore this possibility, the levels of the four JAK kinases were examined in three H9 cell lines using standard Western analysis (Fig. 2B,D). The levels of JAK1, JAK2, and JAK3 kinases was very similar among the three H9 cell lines. However, the protein expression level of TYK2 kinase was reduced by one to two times in SHP-1 transfected H9 cells, relative to H9 cells and SHP-1M transfected H9 cells. Therefore, our data suggest that SHP-1 can inhibit the growth of H9 cells by at least two pathways, one is by direct dephosphorylation of JAK1 kinase, and the other is by down-regulation of the expression of TYK2 kinase. To our knowledge, this is the first evidence that SHP-1 down-regulates the expression of a JAK kinase.
SHP-1 Suppresses the Growth of a SHP-1-Deficient Breast Cancer Cell Line
We have demonstrated (above) that SHP-1 can suppress the cell growth of three lymphoma/leukemia cell lines and that SHP-1 can down-regulate the expression of TYK2 kinase in SHP-1 transfected H9 cells. To determine whether SHP-1 can suppress the growth of non-hematopoietic related cancer cell lines, we have examined the effect of transiently transfecting SHP-1 or SHP-1M in breast cancer cell lines in which SHP-1 protein is either over-expressed (T47D) [Yip et al., 2000] or absent (HTB26) (the insert of Fig. 3A). The expression of SHP-1 is up-regulated in ovarian cancer and in ER-positive breast cancer cell lines, but decreased or abolished in most prostate cancer cell lines and some ER-negative breast cancer cell lines. For the cell line in which SHP-1 is over-expressed SHP-1 (T47D), transient tranfection of the SHP-1 gene did not slow its growth rate or reduce its cell proliferation rate (data not shown). However, for the cell line in which SHP-1 expression is undetectable (HTB26), the growth rate of the SHP-1-transfected HTB26 cells (red solid line) was reduced 2–3 fold relative to that of the SHP-1M transfected HTB26 cells (red dashed line) (Fig. 3A). Cell proliferation rate was also reduced by 50% (Fig. 3B). These data suggest that SHP-1 can suppress the growth of SHP-1-deficient breast cancer cell lines.
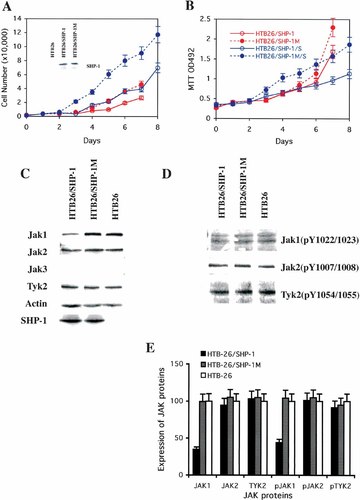
The transfected SHP-1 gene can suppress growth of a SHP-1 deficient breast cancer cell line by promoting the degradation of JAK1 kinase. Comparison of cell growth (A) and cell proliferation rate (B) of HTB26 (green) cells transiently transfected with the SHP-1 gene (solid line with open circle) and the inactive SHP-1M (C455S) gene (dashed line with solid circle) and of HTB26 cell stably transfected with the SHP-1 and SHP-1M (C455S) genes (blue). Figure legends are also shown as an insert in B. The SHP-1 protein expression in corresponding cells is shown as an insert in A. The curves represent the average of at least three independent experiments. C: The amounts of total JAK1, JAK2, JAK3, and TYK2 in SHP-1 and SHP-1M stably transfected HTB26 cell lines and in original HTB26 cell lines. The amount of actin was examined as a control. The expression of SHP-1 protein was also examined. D: The amount of active JAK1, JAK2, and TYK2 in those three cell lines, as determined with the monoclonal antibodies for activated JAK proteins. E: The amount of the JAK kinases and of the phosphorylated JAK kinases in the stable SHP-1 and SHP-1M transfected HTB26 cells, as compared with that in the original HTB26 cells.
SHP-1 Reduces the Expression of JAK1 in HTB26 Cells
To examine whether or not SHP-1 can down-regulate JAKs in other cell lines, we generated SHP-1 and SHP-1M stably transfected HTB26 cell lines using standard methods. Both cell growth rates and cell proliferation rates were reduced in SHP-1 transfected HTB26 cells (blue solid line) relative to SHP-1M transfected HTB26 cells (blue dashed line). These results are consistent with the results in transiently transfected HTB26 cells (Fig. 3A,B).
The levels of four JAK kinases and the levels of three activated JAK kinases were compared among HTB26 cells and the SHP-1 and SHP-1M stably transfected HTB26 cells (Fig. 3C,D). SHP-1 did not affect the expression level or the phosphorylation level of JAK2 and TYK2 kinases under the conditions tested. Both the amounts of JAK2 and TYK2 kinases and the amounts of active JAK2 and TYK2 kinases were the same among three HTB26 cells. As expected, JAK3 kinase is expressed only in hematopoietic cells [Rane and Reddy, 2002], no expression of JAK3 kinase was observed in HTB26 cells. However, the JAK1 kinase was reduced by 60–70% in SHP-1 transfected HTB26 cells relative to SHP-1M transfected HTB26 cells and the parental HTB26 cells (Fig. 3C,E). The level of active JAK1 kinase was also reduced by 50–70% in SHP-1 transfected HTB26 cells relative to the SHP-1M and parental HTB26 cells (Fig. 3D,E). In other words, both JAK1 kinase and active JAK1 kinase were reduced when SHP-1 was expressed via stable transfection. Therefore, as observed in SHP-1 transfected H9 cells, SHP-1 can regulate the level of phosphorylated JAK1 kinase by decreasing its expression in SHP-1 transfected HTB26 cells.
SHP-1 Accelerates the Degradation of JAK1 in HTB26 Cells and of TYK2 in H9 Cells
The data indicate that SHP-1 decreases JAK1 kinase level in HTB26 cells and TYK2 kinase level in H9 cells. This regulation could be achieved either at the level of protein synthesis or at the level of protein stability. To examine whether the stability of JAK kinases differs between SHP-1 transfected cells and SHP-1M transfected cells, cycloheximide, a protein synthesis inhibitor, was used to stop protein synthesis. The amount of JAK1 kinase in both cell lines was then examined in the following hours by Western analysis. As shown in Figure 4A and B, JAK1 kinase was reduced at a faster rate in SHP-1 transfected HTB26 cells than in SHP-1M transfected cells. The half life of JAK1 kinase was about 1 h shorter in SHP-1 transfected HTB-26 cells than in SHP-1M transfected HTB-26 cells. Therefore, SHP-1 indeed accelerates the degradation of JAK1 in HTB26 cells.
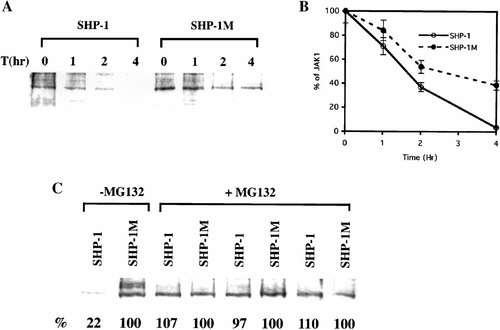
SHP-1 can accelerate the proteasome-regulated degradation of JAK1 kinase in HTB26 cells. A: The rate of degradation of total JAK1 proteins in SHP-1 and SHP-1M stably transfected HTB26 cell lines. The cells were pretreated with cycloheximide, grown in the culture medium for the specific time. The cell lysate was loaded for Western analysis. B: Quantitative representation of the degradation of total JAK1 proteins in SHP-1 and SHP-1M stably transfected HTB26 cells as shown in A. The intensity of the total JAK1 protein was scanned and normalized against the intensity at 0 h. The curve represents the average of at least three independent experiments. C: The effects of MG132, an inhibitor of proteasome-mediated proteolysis, on the degradation of JAK1 kinase in SHP-1 and SHP-1M stably transfected HTB26 cells. Percentage of JAK1 kinase in SHP-1 transfected HTB26 cells relative to that in SHP-1M transfected HTB26 cells was labeled at the bottom of each lane.
To determine whether the SHP-1-dependent accelerated degradation of JAK1 kinase occurs via the proteasome-mediated pathway, we have examined the effect of MG132 on JAK1 kinase levels in SHP-1 and SHP-1M transfected HTB26 cell lines. MG132 is a specific inhibitor of proteasome-mediated proteolysis. In the absence of MG132, the amount of JAK1 kinase in SHP-1 transfected HTB26 cells was only 22% of that in SHP-1M transfected HTB26 cells. However, in the presence of MG132, the amount of JAK1 kinase was the same between SHP-1 and SHP-1M transfected HTB26 cells (Fig. 4C). Thus, MG132 prevents the SHP-1 accelerated degradation of JAK1 kinase, suggesting that the degradation of JAK1 is regulated by proteasome-mediated pathway.
DISCUSSION
We have demonstrated that SHP-1 can suppress the growth of three hematopoietic related lymphoma/leukemia cell lines and one non-hematopoetic related cancer cell line. SHP-1 protein expression is decreased in Romas and Jurkat cells and undetectable in H9 and HTB26 cells. These data are consistent with the report that increased SHP-1 expression can suppress the growth of K562, a leukemia cell line [Bruecher-Encke et al., 2001], and of PC-3, a prostate cancer cell line [Zapata et al., 2002]. In addition, SHP-1 protein expression is decreased in more than 90% of hematopoietic related cancer cell lines and tissues, and in some non-hematopoietic related cancer cell lines [Wu et al., 2003]. We do not know the pathological function of decreased SHP-1 expression in cancer development. However, our data demonstrate that for those cell lines with lower or abolished SHP-1 expression, the restoration of SHP-1 expression can suppress cancer cell growth. This result suggests SHP-1 may have applications in gene therapy treatment for cancer patients.
The molecular mechanism for the tumor suppressing function of SHP-1 remains unclear. Several reports have shown that SHP-1 can inhibit the tyrosine phosphorylation of JAK kinase following their recruitment to receptor complexes that is facilitated by their binding to the receptors via their SH2 domain. SHP-1 has also been shown to bind and dephosphorylate JAK kinases directly [David et al., 1995; Klingmuller et al., 1995; Jiao et al., 1996; Migone et al., 1998]. We have demonstrated that the level of phosphorylated (active) JAK1 and TYK2 kinases was decreased in SHP-1 transfected H9 cells, and that the level of phosphorylated JAK1 kinase was also decreased in SHP-1 transfected HTB26 cells, consistent with previous reports. As active JAKs can phosphorylate STAT proteins and promote cell proliferation, the reduced levels of phosphorylated JAK1 and TYK2 kinases in SHP-1 transfected H9 and HTB26 cells would be expected to reduce their growth and proliferation.
To our surprise, we made the discovery that not only could SHP-1 inhibit the level of phosphorylated JAK kinases, but it could also reduce the total amount of JAK kinase proteins. We observed reduced protein level of different JAKs in different cancer cell lines, such as reduced TYK2 kinase in SHP-1 transfected H9 cells and reduced JAK1 kinase in SHP-1 transfected HTB26 cells. The reduced expression of JAKs would result in reduced active JAK kinases in SHP-1 transfected H9 cells and HTB26 cells. Therefore, our data suggest that SHP-1 can negatively regulate the activated JAK kinases by at least two pathways: one is to negatively regulate the level of phosphorylated JAKs as reported before. The other is to negatively regulate the expression level of JAKs. We have further demonstrated that the reduced JAK kinase expression was achieved by the accelerated degradation of JAK kinases in the SHP-1 transfected cells. The degradation is controlled by proteasome-mediated pathway. The SHP-1 accelerated degradation of JAK kinases can be inhibited with MG132, an inhibitor of the proteasome-mediated proteolysis.
Acknowledgements
We thank Dr. Xin-hua Feng for kind advice and suggestion. We also thank members in the Zhou laboratory for helpful discussion.




