Increases in cytosolic calcium, but not fluid flow, affect aggrecan mRNA levels in articular chondrocytes†
Portions of this work were presented at the 2001 Biomedical Engineering Society meeting.
Abstract
Fluctuations in intracellular free calcium concentration ([Ca2+]i) is thought to be one mechanism by which cells transduce mechanical signals into biological responses. Primary cultures of bovine articular chondrocytes (BAC) respond to oscillating fluid flow with a transient rise in [Ca2+]i. However, specific down-stream effects of [Ca2+]i on gene expression and phenotype in BAC remain to be defined. The present work was designed to determine whether [Ca2+]i mobilization regulates aggrecan mRNA levels. [Ca2+]i was transiently elevated by exposing BAC to the [Ca2+]-specific ionophore, ionomycin. The results show that ionomycin increases [Ca2+]i in a dose-dependent fashion. Semi-quantitative real time (RT)-PCR was used to study the effects of increased [Ca2+]i on steady state levels of aggrecan mRNA. Four hours after a brief exposure to 1.5 μM ionomycin, BAC displayed a nearly four-fold decrease in aggrecan mRNA levels compared to control cells. This effect of ionomycin on aggrecan mRNA was no longer evident 6 or 10 h later. Despite previous observations that oscillating fluid flow elicits increased [Ca2+]i in BAC, it did not affect aggrecan mRNA levels. Taken together, these data suggest that ionomycin-induced [Ca2+]i fluctuations regulate aggrecan mRNA levels, but that flow induced [Ca2+]i fluctuations do not. © 2003 Wiley-Liss, Inc.
Chondrocytes, the cells responsible for the regulation of cartilage matrix metabolism, are highly sensitive to their mechanical environment and physical signals are required to maintain the cartilage matrix in a balanced state of assembly and turnover. Joint immobilization, for example, results in decreased collagen and proteoglycan synthesis, as well as increased metalloproteinase production [Tammi et al., 1983]. The resulting loss of extracellular matrix (ECM) macromolecules from cartilage leads to alterations in the biomechanical properties of the tissue [Jones et al., 1999]. Conversely, increased exercise is associated with increased rates of proteoglycan synthesis and deposition into the cartilage matrix [Caterson and Lowther, 1978; Tammi et al., 1983]. While the importance of physical loading in cartilage homeostasis is well established, specific mechanical signals to which chondrocytes respond have not been completely defined. Normal joint loading induces several mechanical events at the tissue and cellular levels. These signals include deformation of the ECM and cells, fluid flow, and osmotic pressure gradients [Guilak et al., 1999].
The cartilage ECM is composed mostly of water, type II collagen, and the sulfated proteoglycan aggrecan [Sandy et al., 1997]. Several aggrecan molecules bind to a single molecule of hyaluronic acid, forming large proteoglycan networks. With most forms of physical activity, joints are compressed in a cyclic fashion. As a joint is loaded or compressed, water is forced out of the pores formed by the proteoglycan and collagen network. As the joint is unloaded, the direction of fluid flow is reversed. Therefore, cyclic compression, as seen with the gait cycle, for example, exposes chondrocytes to an oscillating (back and forth) fluid flow profile [Jacobs et al., 1998].
Bovine articular chondrocytes (BAC) respond to both steady [Yellowley et al., 1997] and oscillating [Edlich et al., 2001] flow profiles with increased [Ca2+]i. Pharmacological studies demonstrated that steady flow promotes influx of Ca2+ through G-protein linked and stretch activated ion channels in the plasma membrane and mobilization of inositol 1,4,5-trisphosphate (IP3)—sensitive intracellular stores [Yellowley et al., 1999]. The mechanisms contributing to the effects of oscillating flow on [Ca2+]i remain to be defined. In addition, specific down-stream effects of flow-induced [Ca2+]i mobilization on chondrocyte gene expression are largely undefined.
The present studies were undertaken to investigate the hypothesis that flow-induced [Ca2+]i mobilization affects aggrecan mRNA. Several lines of evidence suggest that aggrecan gene expression is regulated by mechanical signals. For example, the aggrecan promoter region contains three shear stress response elements (SSRE) [Valhmu et al., 1998a]. Moreover, discrete mechanical events including compression [Smith et al., 1996; Valhmu et al., 1998b], stretch [Xu et al., 2000], steady fluid flow [Hung et al., 2000], and osmolality [Palmer et al., 2001] differentially regulate the aggrecan gene in vitro. Our results show that increased [Ca2+]i down-regulates steady state levels of aggrecan mRNA. Conversely, oscillating fluid flow, which was shown previously to induce increased [Ca2+]i in BAC, does not affect aggrecan mRNA. These data suggest that [Ca2+]i regulates aggrecan gene expression in BAC but oscillating fluid flow does not.
METHODS
Isolation and Culture of BAC
BAC were harvested and cultured as described previously [Yellowley et al., 1997]. Briefly, articular cartilage was removed from bovine hock joints, chopped into small pieces, and digested for 2 h at 37°C in a solution containing 0.15 mg/ml DNAase, 2 mg/ml collagenase, and 0.1 mg/ml hyaluronidase. Cells were plated in 75 cm2 tissue culture flasks and cultured in RPMI 1640 medium (Gibco BRL, Grand Island, NY) containing 20% fetal bovine serum (FBS; Hyclone Laboratories, Logan UT) and 2% penicillin/streptomycin (Gibco BRL, Grand Island, NY). After the cells reached confluence (7–10 days), they were subcultured onto glass slides (75 × 38 mm) at 7 × 104 cells/cm2 for fluid flow experiments or onto coverslips at 3 × 104 cells/cm2 for measurements of [Ca2+]i. Both culture surfaces were coated with type II collagen (0.5 mg/ml). We have previously demonstrated that cells isolated and cultured under these conditions retain phenotypic characteristics of chondrocytes in vivo [Yellowley et al., 1997]. All experiments were conducted on the third day following subculture in RPMI 1640 medium containing 2% FBS.
Quantification of [Ca2+]i
[Ca2+]i was quantified using the fluorescent [Ca2+] indicator fura-2 (Molecular Probes, Eugene, OR) as described previously [Yellowley et al., 1997; Jacobs et al., 1998]. Calibration ratios were determined with [Ca2+] standards supplied by Molecular Probes. Cells were loaded with 1 μM fura-2-am for 30 min in phenol red-free medium. The coverslip was then mounted in an open perfusion chamber and placed on the stage of a microscope equipped for fluorescent imaging (Nikon Diaphot 300, Nikon, Melville, NY). Ionomycin was added and removed by carefully pipetting directly into and from the chamber. Cells were alternately illuminated at 340 and 380 nm and emitted light was detected by an ICCD camera at a rate of one image every 2 s. Data acquisition and analysis software was used to capture and calculate fluorescent signal intensity which was converted to [Ca2+]i values (Metafluor, Universal Imaging, West Chester, PA). Each experiment consisted of a 1 min baseline recording, followed by a 3 min exposure to ionomycin (0.5–1.5 μM), a wash step, and a 5 min recovery interval. Each cell in the field of view was manually outlined utilizing Metafluor software and a time course of [Ca2+]i was determined for each cell independently. All data were transferred to a Microsoft Excel spread sheet for further analysis.
Transients in [Ca2+]i were examined using rainflow analysis as described previously [Jacobs et al., 2000]. For each outlined cell, the peak [Ca2+]i was determined for both baseline and ionomycin periods. Cells exhibiting a 20% increase in [Ca2+]i compared to baseline values were considered responsive to ionomycin.
Oscillating Fluid Flow
Cells were exposed for 3 min to oscillating fluid flow (±20 dynes/cm2; 1 Hz) in a parallel plate flow chamber [Frangos et al., 1985] with a rectangular fluid volume of 56 mm × 24 mm × 0.28 mm. Control slides were placed in the parallel plate flow chamber but oscillating fluid flow was not applied. The rate of flow was monitored with an ultrasonic flow meter (Transonic Systems, Inc., Ithaca, NY). Immediately following application of fluid flow, the glass slides were transferred to 100 mm tissue culture dishes containing RPMI 1640 plus 2% FBS and returned to the incubator for 1–4 h before isolation of total RNA.
Determination of Relative Aggrecan mRNA Levels
After the indicated recovery intervals, cells were lysed and homogenized with the QIAshredder system (Qiagen, Inc., Valencia, CA). Total RNA was isolated using the RNeasy Mini Kit (Qiagen, Inc.) and quantified by spectrophotometry. Relative changes in aggrecan mRNA levels were determined using semi-quantitative real time reverse transcription PCR (RT-rt-PCR). An aggrecan probe tagged at the 5′end with a fluorescent reporter dye, VIC and at the 3′ end with a quencher, 6-carboxytetramethylrhodamine (Applied Biosystems, Foster City, CA), hybridizes to a central region of the gene. During primer extension, the 5′–3′ nuclease activity of TAQ polymerase degrades the probe. Thus, the reporter is separated from the quencher, and PCR amplification is detected as fluorescence. (Prism 7,700 Sequence Detection System, Applied Biosystems, Foster City, CA). Aggrecan primers and probe were based on bovine sequence (Accession u766150 J05028 Y00319 X16486 M25616). 18S rRNA was amplified using a kit (Perkin–Elmer-Applied Biosystems, Foster City,CA). For each sample, the aggrecan gene and 18S rRNA were amplified in the same tube. Aggrecan/18S data were collected and for each time point, ratios obtained from cells exposed to ionomycin or to oscillating fluid flow were expressed relative to ratios obtained from control cells.
Statistics
To investigate the dose effect of ionomycin on [Ca2+]i, 59, 145, 82, and 165 cells were exposed to 0.5, 0.75, 1.0, and 1.5 μM ionomycin, respectively. Statistical analysis was conducted using Statistica Software (StatSoft, Tulsa, OK). Proportions were compared using a χ2 test. Multiple comparisons were conducted using analysis of variance followed by an LSD post-hoc test. In experiments designed to determine the effects of ionomycin on aggrecan gene expression, a total of 5–8 slides from three separate cell isolations were used for each group. In experiments designed to determine the effects of oscillating fluid flow on aggrecan gene expression, a total of five slides from two separate cell isolations were used for each group. For gene expression studied, a two tailed t-test was used to make statistical comparisons between control and experimental conditions at each time point. Values were considered statistically different at P < 0.05.
RESULTS
Initial experiments were conducted to determine a dose and time course of ionomycin exposure which, like oscillating fluid flow [Edlich et al., 2001], transiently increased [Ca2+]i. Chondrocytes were exposed briefly to the Ca2+ ionophore, ionomycin as described in “Methods.” Figure 1 shows that nearly all cells exposed to ionomycin (1.5 μM) displayed a significant increase in [Ca2+]i. Moreover, after the drug was removed, [Ca2+]i levels rapidly returned to baseline. Figure 2 (top) shows the dose-dependent effect of ionomycin on peak [Ca2+]i (P < 0.00001). Figure 2 (bottom) shows that most doses of ionomycin tested (0.75–1.5 μM) elicited [Ca2+]i responses in nearly 100% of cells. The lowest dose tested elicited a response in 62.71% of cells (P < 0.0001).
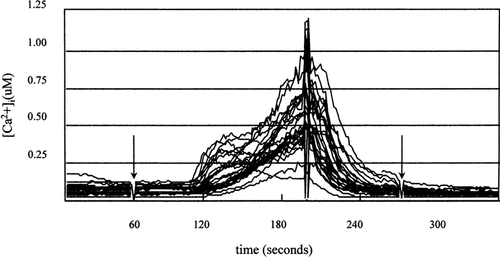
Representative [Ca2+]i traces of chondrocytes exposed to 1.5 μM ionomycin. Each line represents an individual cell response. [Ca2+]i was monitored for a 1 min base line period, 1.5 μM ionomycin was added (left arrow), and ionomycin was washed out with fresh medium. The artifact at 200 s is due to exchange of medium during the washing step. The right arrow indicates completion of the washing step.
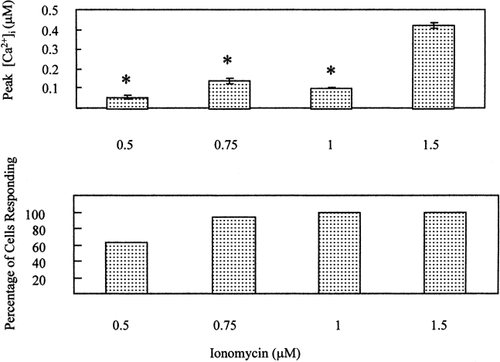
Top: Effect of ionomycin dose on peak [Ca2+]i in BAC. [Ca2+]i was measured in cells exposed for 3 min to a range of ionomycin concentrations. Data are mean and SEM of peak [Ca2+]i of 59, 145, 82, and 165 cells exposed to 0.5, 0.75, 1.0, and 1.5 μM ionomycin, respectively. Statistics were conducted by ANOVA followed by an LSD post-hoc test. *P < 0.000001 compared to peak [Ca2+]i of cells exposed to 1.5 μM ionomycin. Bottom: Effect of ionomycin concentration on the percentage of cells responding with increased [Ca2+]i. Data are the percentage of cells responding to 0.5, 0.75, 1.0, and 1.5 μM ionomycin in 2, 5, 2, and 5 slides, respectively. Proportions were compared using a χ2 test for trend (P < 0.0001).
In order to determine whether [Ca2+]i modulates aggrecan mRNA levels, chondrocytes were exposed to ionomycin (1.5 μM), as in Figure 1. Published data indicate that aggrecan gene expression is modulated as early as 2–4 h following exposure to a mechanical signal [Smith et al., 1996; Hung et al., 2000]. Therefore, after ionomycin was washed out, the cells were allowed to recover for 4, 6, or 10 h. At the end of the experiment, total RNA was harvested as described in “Methods.” Figure 3 shows that ionomycin leads to a transient decrease in aggrecan mRNA levels as determined by RT-rt-PCR. Four hours after exposure to ionomycin, relative aggrecan mRNA levels in control and ionomycin-exposed cells were 1.00 ± 0.10 and 0.29 ± 0.05, respectively (P < 0.001; Fig. 3). Conversely, exposure to 0.75 μM ionomycin did not significantly affect aggrecan mRNA abundance at any of the times tested (data not shown).
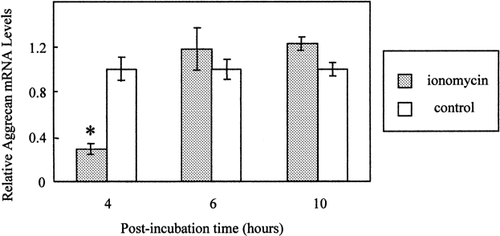
Effect of ionomcyin on aggrecan mRNA levels. BAC were exposed for 3 min or 1.5 μM ionomycin and then allowed to recover at 37°C for 4, 6, or 10 h. Data are mean and SEM of aggrecan/18S mRNA levels from 5 to 8 slides. * P < 0.001 versus. aggrecan expression in 4 h control cells.
Although oscillating fluid flow increases [Ca2+]i in chondrocytes [Edlich et al., 2001] and the present data show that [Ca2+]i modulates aggrecan mRNA, a direct link between oscillating flow and aggrecan has not been established. In order to address this issue, chondrocytes were exposed for 3 min to oscillating fluid flow (20 dynes/cm2; 1 Hz, approximate walking frequency), conditions previously shown to elicit increased [Ca2+]i in BAC [Edlich et al., 2001], and allowed to recover for 1–4 h as described in “Methods.” Figure 4 shows that 3 min of oscillating fluid flow does not affect aggrecan mRNA levels in BAC under the conditions tested. 2–4 h of oscillating fluid flow also did not affect aggrecan mRNA levels (data not shown).
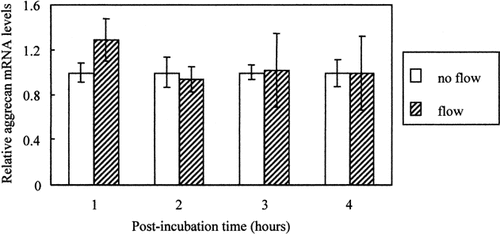
Effect of oscillating fluid flow on aggrecan mRNA levels. BAC were exposed for 3 min to oscillating fluid flow (20 dynes/cm2; 1 Hz) and then allowed to recover at 37°C for 1–4 h. Data are mean and SEM of aggrecan/18S mRNA levels from 4 to 5 slides.
DISCUSSION
Chondrocytes maintain the cartilage ECM in a balanced state of synthesis and turnover by producing both matrix molecules and the proteases that degrade them. Because mechanical signals are known to modulate articular cartilage metabolism, numerous in vitro studies have been designed to determine the effects of various mechanical events on ECM genes in chondrocytes. Stretch [Millward–Sadler et al., 2000; Xu et al., 2000] and compression [Smith et al., 1996; Valhmu et al., 1998b] both upregulate aggrecan gene expression significantly. Conversely, steady flow results in decreased aggrecan promoter activity [Hung et al., 2000].
Recently, oscillating fluid flow has been identified as a potent mechanical stimulus for chondrocytes, as well as bone cells in vitro [Jacobs et al., 1998; Edlich et al., 2001; You et al., 2001]. Because most forms of physical activity are cyclical in nature, chondrocytes are likely to be exposed to time-dependent variations in their mechanical environment. For example, as the cartilage matrix is intermittently compressed during the gait cycle, water moves back and forth within the pores formed by the proteoglycan and collagen network. Thus, in addition to loading-induced oscillations in matrix compression and osmotic pressure, cells within cartilage are exposed to a reversing, or oscillating, fluid flow profile.
Although the importance of physical loading to normal cartilage metabolism is well established, the mechanisms by which cells convert mechanical signals into biochemical responses are largely undefined. Recently, changes in [Ca2+]i have been proposed as one mechano-transduction mechanism. For example, release of Ca2+ from internal stores is required for the effects of oscillating fluid flow on osteopontin mRNA levels in bone cells [You et al., 2001]. Data from our laboratory indicate that chondrocytes respond to oscillating fluid flow, at a rate which induces the same shear stress as that examined in the current study, with increased [Ca2+]i [Edlich et al., 2001]. In order to determine whether flow-induced [Ca2+]i might modulate chondrocyte phenotype, we exposed cells to ionomycin, a Ca2+ ionophore. Our results indicate that increased [Ca2+]i reduces aggrecan mRNA levels. Oscillating fluid flow, on the other hand, does not affect aggrecan mRNA levels in BAC.
The apparent discrepancy between the effects of oscillating fluid flow and increased [Ca2+]i on aggrecan mRNA levels might be explained in at least two ways. First, the dose of ionomycin (1.5 μM) that modulates aggrecan causes an increase in [Ca2+]i that is markedly higher than that elicited by oscillating or steady fluid flow. Peak [Ca2+]i values were approximately 0.125 μM, 0.175 μM, 0.14 μM, and 0.40 μM in cells exposed to oscillating fluid flow [Edlich et al., 2001], steady fluid flow [Yellowley et al., 1997], 0.75 μM ionomycin, and 1.5 μM ionomycin, respectively. Thus, [Ca2+]i might regulate aggrecan mRNA in a dose-dependent fashion and physiological levels of fluid flow do not lead to an increase in [Ca2+]i sufficient to affect aggrecan mRNA levels. It is possible that fluid flow levels, not normally experienced in vivo, increase [Ca2+]i to levels sufficient to affect aggrecan mRNA levels. However, the physiological relevance of such a finding would be unclear. Another possibility is that ionomycin and oscillating fluid flow might mobilize [Ca2+]i from different sources. Ionomycin inserts into various cellular membranes and acts as Ca2+/H+ exchanger. Thus, both extracellular and intracellular sources of [Ca2+] contribute to the ionomycin-induced rise in [Ca2+]i. For oscillating fluid flow, the contributions of specific plasma membrane [Ca2+] channels, G proteins, and IP3-gated intracellular Ca2+ stores remain to be defined. A large body of literature strongly suggests that different temporal and spatial patterns of [Ca2+]i mobilization have distinct effects on cells [reviewed in Bootman et al., 2001]. Thus, mobilization of different [Ca2+] stores is a possible explanation for the differential effects of ionomycin-induced and flow-induced changes in [Ca2+]i on aggrecan mRNA levels.
Together with published data, the present results suggest that chondrocytes may be equipped to differentiate between specific mechanical signals. While oscillating fluid flow, for intervals ranging from 3 min–4 h, does not appear to affect aggrecan mRNA levels, published data suggest that other mechanical signals do modulate expression of this gene. For example, a 2 h exposure to steady fluid flow (16 dynes/cm2) leads to decreased aggrecan promotor activity by a calcium-independent mechanism [Hung et al., 2000]. Conversely, 1 h of static compressive loading (0.1 mPa) leads to induction of aggrecan mRNA expression [Valhmu et al., 1998b]. Similar to oscillating fluid flow, the effects of static compression on aggrecan mRNA were transient; aggrecan mRNA expression returned to baseline values after 24 h of loading [Valhmu et al., 1998b]. At a higher magnitude (10 mPa), 4 h of dynamic compression upregulates aggrecan mRNA levels, while static loading has no effect [Smith et al., 1996]. Conversely, application of a constant hyperosmotic stress suppresses aggrecan gene expression, while dynamic osmotic loading has no effect [Palmer et al., 2001]. In addition, in the presence of interleukin-1β, cyclic tensile strain (6%, .05 Hz) induces increased aggrecan mRNA expression [Xu et al., 2000]. Thus, chondrocytes appear to differentiate between various mechanical signals.
Specific down-stream affects of various mechanical signals may be due to activation of different signal transduction pathways. In addition to [Ca2+]i mobilization [Yellowley et al., 1997; Yellowley et al., 1999], steady flow affects nitric oxide [Das et al., 1997], G proteins [Das et al., 1997; Yellowley et al., 1999], and mitogen activated protein (MAP) kinase [Hung et al., 2000] in chondrocytes. Moreover, steady fluid flow affects aggrecan promotor activity through a mechanism that requires MAP kinase activity, but not [Ca2+]i mobilization [Hung et al., 2000]. While the role of [Ca2+]i in compression-induced aggrecan gene expression has not been directly investigated, activation of phosholipase C and cAMP were both required for the observed affect of compressive loading [Valhmu et al., 1998b]. Except for [Ca2+]i mobilization, signaling mechanisms activated in chondrocytes by oscillating fluid flow have not been identified. Thus, oscillating and steady fluid flow might regulate multiple pathways in chondrocytes that have different and competing effects on the aggrecan gene. Together with published data, our results suggest that various mechanical signals may affect cellular phenotype through multiple signal transduction pathways.
Acknowledgements
We thank Deborah Grove, PhD, for designing primers and completing the RT-rt-PCR protocols.




