High dose M-CSF partially rescues the Dap12−/− osteoclast phenotype†
Roberta Faccio and Wei Zou contributed equally to this project.
Abstract
Osteoclasts are macrophage derived cells and as such are subject to regulation by molecules impacting other members of the immune system. Dap12 is an adaptor protein expressed by NK cells and B and T lymphocytes. Dap12 also mediates maturation of myeloid cells and is expressed by osteoclasts which are dysfunctional in its absence. We find Dap12−/− osteoclast precursors fail to differentiate, in vitro, and the abnormality is partially rescued by high dose M-CSF. The relative paucity of osteoclast number, even in presence of high dose cytokine, is attended by dampened proliferation of precursor cells and their failure to normally migrate towards the osteoclast-recognized matrix protein, osteopontin. Furthermore, Dap12−/− osteoclasts generated in high dose M-CSF fail to normally organize their cytoskeleton. The incapacity of Dap12 null cells to undergo normal osteoclast differentiation is not due to blunted stimulation of major RANK ligand (RANKL) or M-CSF induced signaling pathways. On the other hand, when plated on osteopontin, Dap12−/− pre-osteoclasts do not activate the tyrosine kinase, Syk, which normally binds to the adaptor protein and transmits downstream signals. Attesting to the importance of the Dap12/Syk complex, Syk deficient macrophages do not undergo normal osteoclastogenesis. Furthermore, the same cells plated onto osteopontin, adhere poorly and fail to phosphorylate c-Src or Pyk2, two kinases central to organization of the osteoclast cytoskeleton. © 2003 Wiley-Liss, Inc.
Skeletal remodeling is an ever-occurring process in man which pivots on the activity and recruitment of the unique bone resorbing cell, the osteoclast [Chambers, 2000]. The osteoclast originates by hematopoietic precursors of the monocyte/macrophage family migrating to the bone environment, where, in the presence of the cytokines RANK ligand (RANKL) and M-CSF, they multinucleate and assume the unique osteoclast phenotype thus acquiring the capacity to degrade mineralized matrix [Teitelbaum, 2000; Boyle et al., 2003].
RANK signaling, activated by its ligand RANKL which is expressed on stromal cells and osteoblasts [Suda et al., 1999], is mediated by a series of protein kinases including c-Src, c-Jun N terminal kinase (JNK), p38, extracellular signal related kinase (ERK), phosphoinositol-3-kinase (PI-3K), and those activating NF-kB [Darnay et al., 1998; Galibert et al., 1998; Matsumoto et al., 2000; Lee et al., 2002]. M-CSF, which via its receptor, c-Fms, stimulates many of the same pathways, promotes proliferation of osteoclast precursors and survival of the mature resorptive cell [Tanaka et al., 1993; Woo et al., 2002]. Together, therefore, RANKL and M-CSF induce expression of genes, such as those encoding tartrate-resistant acid phosphatase (TRAP), cathepsin K (CATK), calcitonin receptor, and β3 integrin, which characterize the mature osteoclast and its committed precursors [Kudo et al., 2002; Faccio et al., 2003b].
Once the resorptive cell is in contact with bone, a series of matrix-derived signals, mediated largely through the αvβ3 integrin, prompt the osteoclast to reorganize its cytoskeleton and assume a unique polarized morphological and functional phenotype [McHugh et al., 2000; Faccio et al., 2003a]. Among the most dramatic of these polarized features is formation of the cell's ruffled membrane which is encompassed by a “sealing zone” or “actin ring” [Teti et al., 1991]. This circular structure, which serves to isolate the osteoclast resorptive microenvironment from the general extracellular space, is characterized by the presence of dense F-actin bundles associated with several cytoskeletal proteins and transmembrane receptors [Akisaka et al., 2001].
The fact that osteoclasts are derived from macrophages, cells which are fundamental to immune recognition, has led to a series of experiments which link the immune system to osteoclast recruitment and function. For example, T-lymphocycte-produced cytokines, including RANKL and TNFα, appear central to the enhanced osteoclastogenesis responsible for the bone loss attending menopause and the peri-articular bone erosions of rheumatoid arthritis [Cenci et al., 2000; Weitzmann et al., 2000; Romas et al., 2002]. In this context, the process of antigen presentation, itself, is also a fundamental event in pathological osteoclastogenesis [Jenkins et al., 2002].
These and other insights gained into the means by which osteoclast precursors differentiate and how the mature polykaryon resorbs bone have led to the identification of a number of new anti-osteoporosis therapeutic targets including CATK, c-Src, and the αvβ3 integrin, thus encouraging the exploration of other candidates [Wilder, 2002; Zaidi et al., 2003]. Among the most promising of such potential targets are intraosteoclastic signaling molecules which also function in the immune system [Paloneva et al., 2002; Kaifu et al., 2003].
Much of the information in hand regarding the molecular mechanisms of osteoclast formation and function is derivative of studies performed on human disease or genetically manipulated animals, particularly those with osteopetrosis [Marks, 1989; McLean and Olsen, 2001]. Presently, at least 24 genes or loci, the products of which positively or negatively regulate osteoclastogenesis and osteoclast function have been identified [Boyle et al., 2003; Teitelbaum and Ross, 2003]. Some such genes impact formation and/or survival of osteoclast precursors. Others mediate either the ability of these precursors to differentiate or the capacity of the mature osteoclast to resorb bone.
Dap12 is a transmembrane adapter molecule expressed in a variety of cells of the immune system [Tomasello et al., 1998; Lanier and Bakker, 2000]. In myeloid cells, Dap12 pairs with surface residing receptors including triggering receptor expressed on myeloid cells (TREMs) [Colonna, 2003]. The cytoplasmic domain of Dap12 contains the immunoreceptor tyrosine-based activation ITAM motif, which functions as a docking site for tyrosine kinases, including Syk [McVicar et al., 1998]. Interestingly, deletion of the Dap12 gene, in man, results in Nasu–Hakola disease (NHD), which includes skeletal abnormalities in its phenotype [Kondo et al., 2002; Kaifu et al., 2003]. Furthermore, Dap12 is expressed by osteoclasts and, as evidenced by the development of osteopetrosis in mice lacking the protein, is essential for normal osteoclast function [Kondo et al., 2002; Kaifu et al., 2003].
In this exercise, we turned to the molecular pathogenesis of Dap12 deficient osteopetrosis. We find that the failure of Dap12−/− myeloid cells to generate osteoclasts can be rescued substantially, but incompletely, by increasing ambient M-CSF. Regardless of cytokine concentration, however, the resorptive capacity and cytoskeleton of Dap12 deficient osteoclasts are deranged. Attesting to the importance of Dap12 associated signaling molecules in osteoclast function, bone marrow macrophages (BMMs) Syk lacking to differentiate into mature osteoclasts, and exhibit abnormal cytoskeletal function associated with dampened c-Src and Pyk2 phosphorylation.
MATERIALS AND METHODS
In Vitro Generation of OCs and Bone Resorption Assay
BMMs Dap12+/+ and Dap12−/− [Bakker et al., 2000], or Syk+/− and Syk−/− [Mocsai et al., 2002] kindly provided by Dr. C.A. Lowell (Department of Laboratory Medicine, University of California, San Francisco, CA), were isolated as previously described [Faccio et al., 2003b] and cultured for 3 days in α-MEM supplemented with 10% FCS and 1:10 CMG14–12 culture supernatant [Takeshita et al., 2000], which contained the equivalent of 100 ng/ml of recombinant M-CSF. A total of 5 × 104 cells were cultured in α-MEM containing 10% heat inactivated FBS with 100 ng/ml RANKL [McHugh et al., 2000] and increasing concentrations (from 10 to 100 ng/ml) of mouse recombinant M-CSF (R&D Systems, Inc., Minneapolis, MN) in 96-well tissue culture plates. Cells were fixed and stained for TRAP activity after 5 days in culture, using a commercial kit (Sigma 387-A, St. Louis, MO). Bone resorption was performed by culturing BMMs exposed to RANKL and M-CSF for 5 days onto dentine. Cells were fixed and stained with FITC–phalloidin to visualize F-actin to detect the actin ring organization or removed by brief treatment with 2 N NaOH. Resorption pits were visualized by hematoxylin staining (Sigma).
Proliferation Assay
BMMs from Dap12+/+ and Dap12−/− mice were cultured in the presence of increasing concentrations of M-CSF with or without RANKL (100 ng/ml). After 2 days, viable cell number was calculated using the MTT (3-[4,5 dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; Sigma) method or Cell Proliferation ELISA system (Amersham Pharmacia Biotech, Piscataway, NJ). Briefly, 10 μl MTT (5 mg/ml) were added in each well, containing 100 μl culture media, and incubated at 37°C for 4 h. The reaction was terminated with 150 μl isopropanol/0.04 N HCl and MTT absorbance determined at the optical density of 570 nm. Six-wells were used for each variable and each experiment was repeated twice. For the ELISA assay, BrdU was added to each well to a final concentration of 10 μM. Cells were incubated at 37°C for additional 2 h and BrdU incorporation was detected following manufacturer's instruction.
Immunoblot
BMMs from Dap12+/+ and Dap12−/− mice were cultured for 3 days in the presence of 100 ng/ml RANKL and 10 or 100 ng/ml purified M-CSF, starved for 2 h and stimulated for the indicated times with 100 ng/ml RANKL or for c-Fms signaling, 10 and 100 ng/ml purified M-CSF. Pre-osteoclasts were lifted with trypsin/EDTA, re-suspended in serum-free medium and plated on 5 μg/ml OPN-coated dishes for the indicated times. Cells were washed twice with ice-cold PBS and lysed in the buffer containing 10 mM Tris (pH 7.4), 150 mM NaCl, 1 mM EDTA, 0.2% sodium deoxycolate, 1% NP 40, 1 mM NaF, 2 mM Na3VO4, and 1× protease inhibitor cocktail (Sigma). Forty micrograms of cell lysates were boiled in the presence of SDS sample buffer for 5 min and subjected to electrophoresis on 8% SDS–PAGE. Polyclonal anti-ERK1/2 or anti-phospho-ERK1/2 MAPK polyclonal antibodies (Cell Signaling Technology, Inc., Beverly, MA) were used for ERK-MAPK immunoblot. IκBα polyclonal antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and phospho-IκBα, phospho p38, and p-JNK antibodies were purchased from Cell Signaling Technology. Anti-c-Fos and -Syk were obtained from Santa Cruz Biotechnology, Inc. Phospho-Src antibody was purchased from Cell Signaling and phospho-Pyk2 from Biosource International (Camarillo, CA).
RNA Preparation and Reverse-Transcription Polymerase Chain Reaction (RT-PCR) Analyses
Total RNA from cultured cells was isolated by the guanidine/phenol method. For RT-PCR analysis, cDNAs were synthesized from 1 μg of total RNA using reverse transcriptase and oligo dT primers in a volume of 20 μl, and the reaction mixture was finally adjusted to 100 μl with TE buffer for PCR analysis. PCR was performed with 1 μl of cDNA reaction mixture by using Platinum Pfx polymerase (Invitrogen, Carlsbad, CA) and appropriate primers in a volume of 50 μl. The following primers for CATK, 5′-GGAAGAAGACTCACCAGAAGC-3′ and 3′-GCTATATAGCCGCCTCCACAG-5′; for matrix metalloproteinase-9 (MMP-9), 5′-CCTGTGTGTTCCCGTTCATCT-3′ and 3′-CGCTGGAATGATCTAAGCCCA-5′; for calcitonin receptor, 5′-CATTCCTGTTACTTGGTTGGC-3′ and 3′-AGCAATCGACAAGGAGTGAC-5′; and for GAPDH, 5′-ACTTTGTCAAGCTCATTTCC-3′ and 3′-TGCAGCGAACTTTATTGATG-5′ were used.
The samples were transferred to a programmable thermal cycler (Hybaid US, Franklin, MA) that had been preheated to 95°C and incubated for 21–40 PCR cycles. Each cycle consisted of a denaturation step at 95°C for 1 min, an annealing step at 60°C for 1 min, and an extension step at 72°C for 1 min. Ten microliters aliquots of PCR products were separated by electrophoresis on a 1.5% agarose gel.
Adhesion and Migration Assay
Dap12+/+ or Dap12−/− pre-osteoclasts were lifted with Trypsin–EDTA. Adhesion assay was performed on coverslips coated with 5 μg/ml human osteopontin. A total of 5 × 104 pre-osteoclasts diluted in α-MEM + 0.5% BSA, with or without 100 ng/ml M-CSF, were added to each well. Following 1 h of incubation at 37°C, cells were washed and TRAP stained. Migration assay was performed using transwell filters, 8 μm pore size (Costar, Cambridge, MA), wherein the lower side of the membrane was coated with the same concentration of osteopontin for 2 h at room temperature. In some experiments, 100 ng/ml M-CSF was added in the lower compartment of the transwell as chemoattractant. Cells attached to the top surface of the membrane were removed with cotton swabs. Cells that had migrated to the lower side were viewed at 300× magnification, and the number of cells per field determined. Results represent the averages from 15 fields ± SE of a representative experiment.
RESULTS
M-CSF Partially Rescues Dap12−/− Osteoclasts
Attesting to failed osteoclast function in vivo, Dap12 deficient mice develop progressive osteopetrosis [Kaifu et al., 2003]. Furthermore, Dap12−/− osteoclast differentiation and function are attenuated in vitro. Because we have shown that a similar osteoclast phenotype, namely that of the β3 integrin-deleted mouse, is rescued by high dose M-CSF [Faccio et al., 2003b], we asked if the cytokine has salutary effects on cells lacking Dap12. When cultured in the presence of RANKL, low dose M-CSF (10 ng/ml) induces TRAP expression by Dap12−/− BMMs and many cells become binucleated (Fig. 1). In contrast, increasing the concentration of the cytokine to 100 ng/ml yields many large, TRAP-expressing mutant polykaryons. On the other hand, although Dap12−/− osteoclasts generated in high dose M-CSF approximate their wild type counterpart in size, their morphology is not completely normalized nor are they as numerous.
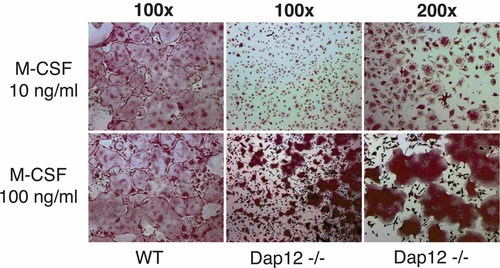
M-CSF partially rescues osteoclastogenesis of Dap12−/− cells. Wild type (WT) and Dap12−/− osteoclasts were generated from bone marrow macrophages (BMMs) cultured for 5 day with RANK ligand (RANKL) (100 ng/ml) and low (10 ng/ml) or high (100 ng/ml) dose M-CSF. Images represent tartrate-resistant acid phosphatase (TRAP) stained cultures of osteoclasts at 100× or 200× magnification. Within 5 days WT cells form numerous large multinucleated cells independent of M-CSF concentration. Although less pronounced, many Dap12−/− cells become large, multinucleated, and TRAP positive only when cultured in the presence of high dose M-CSF.
To determine if the relative paucity of Dap12−/− osteoclasts reflects blunted precursor division, we measured the proliferative rate of early and committed osteoclast progenitors, namely BMMs and mononuclear TRAP expressing pre-osteoclasts, respectively, in the presence of increasing doses of M-CSF (Fig. 2). Consistent with the failure of Dap12−/− cells to generate normal numbers of osteoclasts even in the presence of high dose M-CSF, the proliferative response of Dap12−/− precursors to M-CSF is less particularly as the concentration of the cytokine increases.
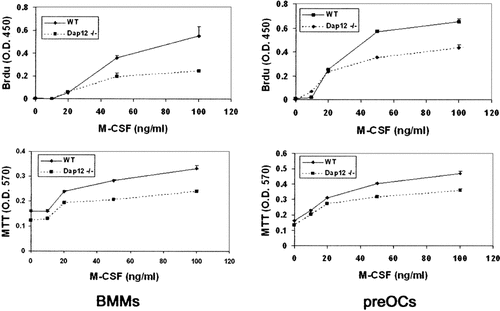
Defective proliferation of Dap12−/− cells. WT and Dap12−/− BMMs or pre-osteoclasts were cultured for 3 days in the presence of increasing concentrations of M-CSF after which proliferation was evaluated by BrdU incorporation or MTT assay. The proliferative response of Dap12−/− cells is diminished particularly as the concentration of cytokine increases.
Dap12−/− Osteoclasts Fail to Normally Organize Their Cytoskeleton or Resorb Bone
Since Dap12−/− osteoclasts generated in high dose M-CSF differ morphologically from their wild type counterparts we asked if they also differ functionally. To this end, we generated Dap12−/− osteoclasts on dentine slices, in high dose M-CSF, and stained them with FITC–phalloidin. Documenting that the abnormal shape of these polykaryons is reflective of their cytoskeletal organization, confocal microscopy reveals they are incapable of actin ring formation as the cytoskeletal protein forms numerous clusters distributed peripherally in the mutant cells (Fig. 3A). In keeping with their dysfunctional cytoskeleton, Dap12−/− osteoclasts, established in parallel cultures, are incapable of degrading mineralized matrix as evidenced by a complete absence of dentin resorptive lacunae (Fig. 3B). Therefore, high dose M-CSF promotes formation of multinucleated Dap12−/− osteoclasts but, unlike its impact on β3 integrin deleted cells [Faccio et al., 2003a], is incapable of normalizing their cytoskeleton.
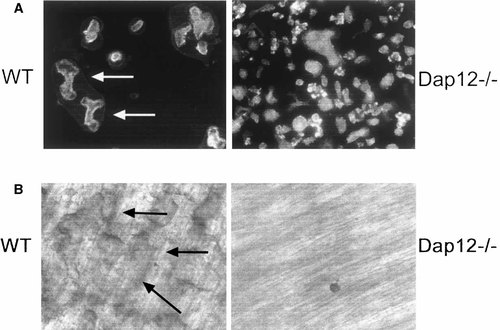
Dap12−/− osteoclasts fail to organize their cytoskeleton or resorb mineralized matrix. WT and Dap12−/− osteoclasts were generated on dentine slices in the presence of RANKL and high dose M-CSF. After 5 days, the cells were fixed and stained with FITC–phalloidin to visualize actin organization (A) or removed to identify bone resorption pits (B). While WT osteoclasts form numerous well defined actin rings (A, arrows) and resorptive pits (B, arrows), Dap12−/− osteoclasts exhibit a disorganized cytoskeleton (A) and fail to resorb bone (B).
RANK and c-Fms Signal Normally in Dap12−/− Cells
The fact that M-CSF only partially rescues the phenotype of Dap12−/− osteoclasts raised the possibility that the cytokine, even in abundance, is incapable of inducing terminal differentiation of the cells. To determine if such is the case, we cultured BMMs, with time, in RANKL and low or high dose M-CSF and measured three markers of osteoclast differentiation by RT-PCR. As seen in Figure 4, CATK, MMP-9, and calcitonin receptor mRNA levels are diminished in day two and four Dap12−/− osteoclastogenic cultures maintained in low M-CSF, but the same markers are completely normalized in high concentration of the cytokine. These data are consistent with the posture that Dap12−/− osteoclast precursors fail to fully differentiate in RANKL plus low dose M-CSF because they do not normally transmit intracellular signals induced by these cytokines. To test this hypothesis, we first assessed intracellular signaling pathways induced by RANKL and found that three such events, namely activation of p38 and AKT, as well as phosphorylation and degradation of IκBα, are indistinguishable from normal in Dap12−/− BMMs, indicating that Dap12 is not required for RANK signaling (Fig. 5A). Furthermore, despite the inability of low concentrations of the cytokine to induce osteoclast formation, Dap12−/− pre-osteoclasts normally activate the M-CSF responsive signaling molecules, ERK and c-Fos, regardless of concentration of the cytokine (Fig. 5B,C). Therefore, and again in contrast to β3 integrin deficient osteoclasts [Faccio et al., 2003b], the ability of M-CSF to partially rescue the Dap12 phenotype is not dependent on ERK and c-Fos activation.
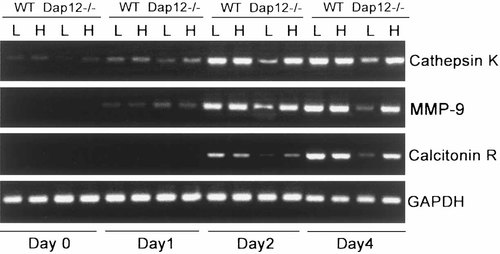
High dose M-CSF induces expression of osteoclast specific mRNAs by Dap12−/− cells. WT and Dap12−/− BMMs were cultured for 4 days with RANKL and 10 ng/ml (L) or 100 ng/ml (H) M-CSF. The indicated osteoclastogenic markers were analyzed by PCR. Untreated BMMs (day 0) do not express osteoclast markers. After 2 days in low or high M-CSF, WT cells express cathepsin K (CATK), matrix metalloproteinase-9 (MMP-9), and calcitonin receptor. At each day, expression of these markers by Dap12−/− cells is diminished in low M-CSF but normalized in high M-CSF.
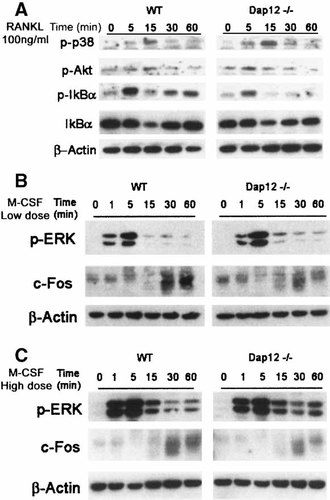
RANKL and M-CSF signaling is normal in Dap12−/− pre-osteoclasts. BMMs stimulated with RANKL (A) and pre-osteoclasts exposed to low (B) or high dose M-CSF (C) for up to 1 h, were subjected to immunoblot to detect activation of the indicated proteins. Phosphorylation of p-38, AKT, and IκBα in response to RANKL, and IκBα degradation and re-synthesis occurs equally in WT and Dap12−/− BMMs. Phosphorylation of ERK and activation of c-Fos in pre-osteoclasts stimulated with low or high dose M-CSF is similar in WT or Dap12−/− pre-osteoclasts. β-Actin serves as loading control.
High Dose M-CSF Is Required for Spreading and Migration
Cytoskeletal organization of osteoclasts is dependent upon recognition of extracellular matrix. Thus, we assessed the capacity of Dap12−/− pre-osteoclasts, in the presence or absence of M-CSF, to adhere and migrate to osteopontin, an extracellular matrix protein recognized by the osteoclast integrin, αvβ3. Both wild type and Dap12−/− pre-osteoclasts attach to the matrix protein within 30 min (Fig. 6A). On the other hand, whereas wild type cells spread on osteopontin within this time frame, those lacking Dap12 do not. Importantly, the spreading defect of the mutant osteoclast precursors is rescued by high dose M-CSF. Similarly, migration to OPN is decreased two-fold in cells lacking DAP12 (Fig. 6B). Adding high dose M-CSF as chemoattractant to the well approximately doubles the migratory capacity of both wild type and Dap12 deficient pre-osteoclasts but fails to rescue the phenotype of the mutant cells. Thus, consistent with the partial rescue of Dap12−/− osteoclastogenesis by high dose M-CSF, the cytokine corrects some but not all of the mutant cell's capacity to recognize extracellular matrix.
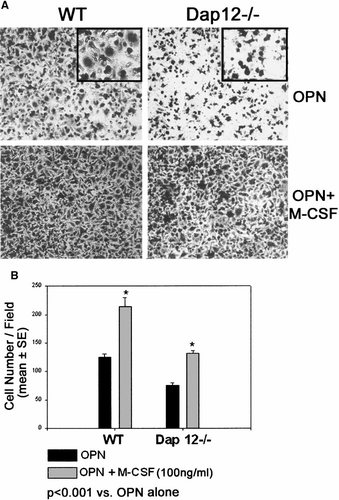
Spreading on and migration to osteopontin by Dap12−/− pre-osteoclasts is abnormal. A: WT and Dap12−/− preosteoclasts were plated on OPN coated coverslips. While WT cells adhere and spread onto OPN within 30 min, Dap12−/− pre-osteoclasts attach to the protein but do not spread (insert). Adding M-CSF increases adhesion of both cell types and rescues the spreading defect of Dap12−/− cells. B: Pre-osteoclast migration was assessed using transwells in which the lower membrane was coated with OPN, in the absence or presence of high dose M-CSF as chemoattractant. While M-CSF increases the directed migration of both cell types, the motility of Dap12−/− cells remains less than WT.
Syk Is Required for Cell Spreading and Osteoclast Differentiation
The protein tyrosine kinase Syk binds to the phosphorylated ITAM domain of Dap12, undergoes its own phosphorylation and mediates Dap12 dependent intracellular events [Lanier and Bakker, 2000]. Thus, to further explore Dap12 downstream signals required for efficient cell spreading and in turn osteoclastogenesis, we analyzed Syk function. Since Syk is phosphorylated in response to integrin engagement [Obergfell et al., 2002] we asked if the tyrosine kinase is activated during cell adhesion to an αvβ3 integrin substrate. Figure 7A shows that within 30 min of adhesion to osteopontin, wild type pre-osteoclasts induce phosphorylation whereas their Dap12 deficient counterparts fail to do so. With this information in hand, we turned to the impact of Syk on osteoclast differentiation. Syk−/− BMMs generated by transplantation of fetal liver stem cells into lethally irradiated Syk+/− mice [Mocsai et al., 2002], fail to differentiate into mature osteoclasts in the presence of the two cytokines regardless of the dose of M-CSF (Fig. 7B). Attachment of Syk−/− pre-osteoclasts to OPN is delayed and, similar to Dap12−/− cells, those Syk−/− pre-osteoclasts that adhere to the matrix protein fail to spread properly (Fig. 8A). In keeping with this observation, phosphorylation of c-Src and Pyk2, two signaling molecules which are central to organization of the osteoclast cytoskeleton, is defective in OPN-adherent, Syk deficient cells (Fig. 8B). Thus, Syk participates in osteoclast differentiation and cytoskeletal organization.
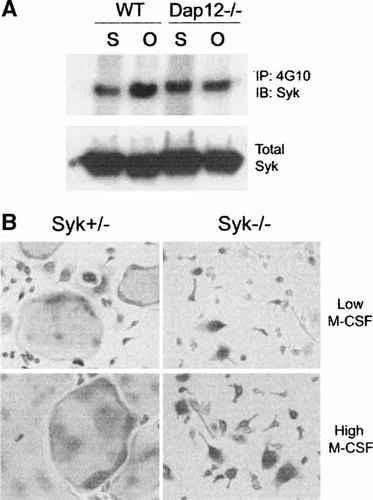
Syk regulates intracellular signaling and osteoclast formation. A: WT and Dap12−/− pre-osteoclasts were maintained in suspension (S) or plated on OPN (O) for 30 min and Syk phosphorylation was analyzed by immunoblot. Cell adhesion increases Syk phosphorylation in WT but not in Dap12−/− cells. B: Syk+/− and Syk−/− BMMs were maintained in the presence of RANKL and low or high dose M-CSF. Syk+/− osteoclasts form within 5 days in both conditions. In contrast, Syk−/− cells, independent of M-CSF concentrations, form TRAP-expressing cells with few nuclei, which fail to spread normally.
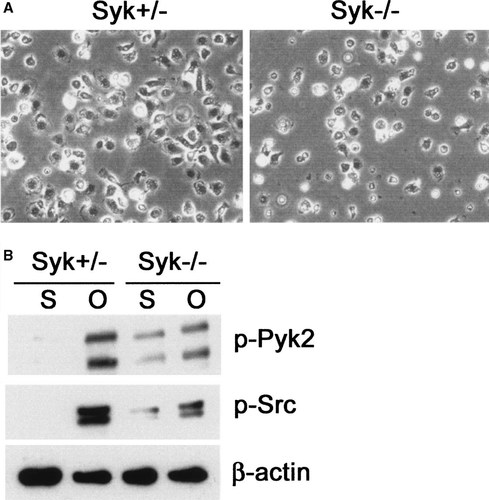
Syk−/− pre-osteoclasts adhere poorly and fail to activate Src and Pyk2. A: Syk+/− and Syk−/− pre OCs were plated on OPN coated dishes. While Syk+/− cells spread onto OPN within 30 min, Syk−/− pre-osteoclasts do not spread on the protein. B: Syk+/− and Syk−/− pre-osteoclasts were maintained in suspension (S) or plated on OPN (O) for 30 min and Src and Pyk2 phosphorylation was analyzed by immunoblot. Cell adhesion increases Src and Pyk2 phosphorylation in Syk+/− but not in Syk−/− cells. β-Actin serves as loading control.
DISCUSSION
DAP12 is a membrane-associated protein first identified as an adaptor molecule for a range of activating receptors found on cells of the lymphoid and myeloid lineage [Lanier and Bakker, 2000]. While its small external domain does not appear to bind ligands, two distinctive motifs provide the molecular basis for its activity. The transmembrane region contains a positively charged residue that facilitates non-covalent interactions with receptors. ITAM within the intracellular tail is phosphorylated following interaction between DAP12 and one of its cognate receptors, resulting to recruitment to the site of the src homology-2 (SH2) domains of ZAP-70 or Syk, tyrosine kinases that activate downstream signals.
Molecules that bind to and hence stimulate signal transduction through DAP12 are primarily on B or T cells. To examine the role of the adaptor in immunity two groups generated mice lacking a functional protein. In one instance the gene was targeted for deletion [Bakker et al., 2000], while the second approach yielded a knock-in mouse in which the ITAM motif was rendered inactive [Tomasello et al., 2000]. In both circumstances, the resulting animals are deficient in their innate immune response, a result consistent with the known functions of DAP12 and its activating receptors on lymphoid cells. Subsequent studies have uncovered defects in a number of cellular-based immune functions [Wu et al., 2000; Lucas et al., 2002; Sjolin et al., 2002].
In addition to its expression by immune cells DAP12 is also found on macrophages [Lanier and Bakker, 2000], where it modulates monocyte differentiation [Aoki et al., 2000]. The activating receptors in these circumstances are incompletely defined, but include myeloid-DAP12 associating lectin-1 (MDL-1), signal regulatory protein 1 beta (SIRPβ), and several members of the TREMs.
A potential role of DAP12 and TREMs in skeletal biology was first suggested by NHD. The major manifestations of this rare disorder, found in Japanese and Finnish populations, are a combination of demyelination, leading to early pre-senile dementia and the presence of lipid-filled bone cysts. The Finnish patients have a large deletion in the DAP12 gene while a mutation within the coding region characterizes the Japanese cohort [Paloneva et al., 2000]. In both instances the net result is DAP12 inactivation. Interestingly, a third mouse lacking DAP12 exhibits defects in osteoclast function resulting in osteopetrosis [Kaifu et al., 2003]. More recent studies reveal further heterogeneity in NHD in that a number of affected patients have normal DAP12 expression, but functionally relevant mutations in TREM2 [Paloneva et al., 2002; Cella et al., 2003]. The inability of the cells derived from TREM2 deficient patients to differentiate into osteoclasts suggests TREM2 is upstream of Dap12 in the osteoclastogenic process.
As part of their studies, Kaifu and co-workers cultured Dap12−/− bone marrow cells with M-CSF and RANKL but were unable to generate large numbers of osteoclasts. The few cells expressing TRAP contain only a small number of nuclei and fail to spread. These findings, plus mild osteopetrosis, are similar to the results of similar experiments involving β3 integrin deficient mice [McHugh et al., 2000]. This observation, plus the fact that much of the phenotype of β3 null osteoclasts is rescued by increasing the level of M-CSF in the culture medium [Faccio et al., 2003b] prompted us to ask if the same occurs in the absence of Dap12. We find this to be so and extended the study to examine selected markers of osteoclast differentiation induced by high dose M-CSF. Confirming our morphological observations, mRNA levels of CATK, MMP-9, and the calcitonin receptor are all enhanced with time in Dap12 null pre-osteoclasts, by the presence of additional M-CSF throughout the period of osteoclast differentiation.
We next asked if Dap12−/− osteoclasts so generated are functional. As part of their capacity to resorb mineralized matrix, osteoclasts form a resorptive organelle at the bone–cell interface, characterized by a ring of cortical actin. Cells lacking DAP12 fail to generate this marker of osteoclast polarization and this defect is not rescued by exposure to an increased concentration of M-CSF. Interestingly, the same high level of M-CSF restores the polarizing capacity of β3−/− osteoclasts but again, in this circumstance, the cells remain incapable of resorption [Faccio et al., 2003b].
M-CSF, in addition to functioning as an osteoclast differentiation factor, also regulates the proliferation, spreading, and migration of both early precursors, in the form of spleen- or bone marrow-derived macrophages, as well as cells committed to the osteoclast lineage by exposure to RANKL [Stanley et al., 1997; Feng et al., 2002; Faccio et al., 2003b]. Because M-CSF does not completely normalize Dap12−/− osteoclast number we reasoned the residual defect may reflect dampened precursor proliferation in response to an abundance of the cytokine. In fact, such is the case.
While increased concentrations of M-CSF markedly increases generation of large osteoclastic cells from Dap12−/− progenitors, the mutant polykaryons are incapable of actin ring formation. Thus, while M-CSF has a positive effect on Dap12−/− osteoclastogenesis, a profound defect in cytoskeletal organization persists.
Similar to Dap12, the αvβ3 integrin regulates the osteoclast cytoskeleton [Faccio et al., 2003a]. Furthermore, both Dap12 and αvβ3 interact with c-Cbl, a key osteoclastogenic signaling molecule, also known to mediate cell spreading [McVicar et al., 1998; Sanjay et al., 2001]. These observations prompted us to ask if like β3−/− pre-osteoclasts, those derived from Dap12 deficient mice, fail to spread on the αvβ3 ligand, osteopontin. While wild type cells are fully spread within 30 min even in the absence of M-CSF, such does not occur in those lacking Dap12, despite their capacity to attach to the matrix protein. Addition of M-CSF rescues the spreading defect, suggesting that signals emanating from DAP12 enhance the capacity of αvβ3 to ligate osteopontin. Haptotaxis and chemotaxis assays, using osteopontin as the coating ligand and M-CSF as the chemoattractant, reveal that once again cells lacking DAP12 are deficient in both parameters and can be partially rescued by the presence of M-CSF. Since attachment and spreading are both αvβ3 dependent and cross talk exits between c-Fms and αvβ3 [Faccio et al., 2003b], our findings reveal a complex interaction between the two receptors and Dap12.
To identify potential effectors of DAP12, we examined the activation of Syk, a tyrosine kinase interacting with and functioning downstream of the adaptor protein in myeloid cells [Lanier and Bakker, 2000]. In our first studies, we used wild type and DAP12 null pre-osteoclasts that we allowed to adhere to osteopontin, and then assessed the level of Syk phosphorylation. Only cells expressing DAP12 are capable of activating Syk. To confirm this observation, we used pre-osteoclasts generated from macrophages lacking Syk. In this instance, we treated the cells with RANKL and both low and high dose M-CSF. Irrespective of M-CSF concentration, absence of Syk precludes formation of fully mature, spread osteoclasts. Furthermore, absence of the enzyme blocks matrix-induced activation of c-Src and Pyk2, two tyrosine kinases essential to αvβ3 function in these cells [McVicar et al., 1998; Sanjay et al., 2001]. These morphological findings suggest that, as in other instances in myeloid differentiation, Syk appears to be downstream of DAP12. These studies led us to examine the intracellular signals that follow ligation of RANK and c-Fms, the receptors for RANKL and M-CSF, respectively. Despite the fact that Dap12−/− BMMs fail to become mature bone-resorbing osteoclasts, these cells normally activate major signals that follow binding of RANKL to RANK, including those involving NF-kB and Akt [Darnay et al., 1998; Galibert et al., 1998; Matsumoto et al., 2000; Ross, 2000; Lee et al., 2002]. Similarly, M-CSF dependent phosphorylation of ERKs and induction of their distal target c-Fos is intact, an observation that differs from that seen in the β3 null mice, where neither signal is activated by the cytokine [Kudo et al., 2002; Faccio et al., 2003b].
Finally, an important distinction exists between the numbers of osteoclasts present in vivo in DAP12 null mice and the capacity to generate the cells in vitro [Kaifu et al., 2003]. Thus, histomorphometry reveals that the mild osteopetrosis of these animals is accompanied by no deficiency of osteoclasts. This in vivo finding stands in contrast to in vitro data and our current observations, in which few mature osteoclasts are generated. One possible explanation lies in our studies of mice lacking NF-kB inducing kinase (NIK), where once again disparity exits between in vivo osteoclast number and in the capacity to generate these cells in vitro [Novack et al., 2003]. The fact that the in vitro phenotype can be rescued by TGFβ, a protein found in high concentration in bone matrix, suggests that complex mechanisms are in play in intact animals. Precisely what additional factors permit generation of normal numbers of albeit dysfunctional osteoclasts in Dap12 mice, is an issue which may provide important insights into the mechanisms of osteoclast recruitment and function.
Acknowledgements
We thank Dr. C.A. Lowell (University of California, San Francisco, CA) for providing BMMs Dap12+/+, Dap−/−, Syk+/−, and Syk−/−.




