Real-world evidence of heparin and citrate use in extracorporeal photopheresis: A hypothesis-generating data review of device settings and performance
Abstract
Extracorporeal photopheresis (ECP) is widely used for the treatment of cutaneous T-cell lymphoma, graft-vs-host disease, and other immune-related conditions. To avoid clotting during treatment, the ECP system used must be effectively primed with an anticoagulant. Heparin is the recommended anticoagulant for the THERAKOS CELLEX System, but acid citrate dextrose-A (ACDA) is often used. We compared system performance between these two anticoagulants for this ECP system. Deidentified data for ECP device performance were obtained at each treatment session, from automatically logged Smart Cards or labels completed by device operators. We compared the effects of ACDA or heparin on overall treatment duration, buffy coat (leukocyte) collection time, photoactivation time and the number of alarms and warnings. The variability in these parameters was also assessed. Data from 23 334 treat sessions were analyzed; ACDA was used in 34.4% and heparin in 65.6%. Overall, the ECP procedure duration, buffy coat collection time and photoactivation time were numerically similar regardless of whether ACDA or heparin was used, and regardless of needle mode. Photoactivation time variability was lower with ACDA compared with heparin in all needle modes. Among treatments that were completed automatically without any operator intervention, total treatment duration and photoactivation time were significantly reduced with ACDA use in both the double- and single-needle modes. The data presented indicate that, in both double- and single-needle modes, the THERAKOS® CELLEX® integrated ECP system performed similarly with ACDA compared to heparin, although ACDA demonstrated potential benefits in reducing variability in photoactivation time.
1 INTRODUCTION
Extracorporeal photopheresis (ECP) is an immunotherapy in which white blood cells are separated from whole blood by a photopheresis system1, 2; a photoactivating agent is then added to the separated cells, which are subsequently exposed to ultraviolet-A (UVA) irradiation prior to reinfusion into the patient. Although the exact mechanism of action is not fully established, it is understood that the photopheresis process promotes immunomodulatory effects. Extracorporeal photopheresis is a well-established treatment for skin manifestations of advanced cutaneous Tcell lymphoma (CTCL) and is also widely used for conditions such as acute and chronic graft-versus-host disease (GvHD), organ rejection following a solid organ transplant, autoimmune diseases, and dermatologic disorders.1, 3, 4
To avoid clotting and maintain blood flow during ECP treatments, the ECP system is primed with saline and an anticoagulant prior to treatment initiation.5 Anticoagulant is also added to the whole blood that is collected during the treatment.5 Unfractionated heparin is effective for this purpose and is specified in the US Food and Drug Administration (FDA)-approved protocol for the THERAKOS® CELLEX® system considered in this analysis.6 However, the use of heparin can be associated with bleeding complications, bone loss, and heparin-induced thrombocytopenia (HIT).5, 7 Citratebased anticoagulants such as acid citrate dextrose-A or -B (ACDA or ACDB) can also be used for ECP and are generally well tolerated, although they have been associated with risk of developing symptomatic metabolic complications such as hypocalcemia, hypomagnesemia, or metabolic alkalosis.5 The use of citrate-based anticoagulants is necessary for patients with contraindications for heparin use, such as those at risk of HIT,8, 9 and in recent years, many operators also report a preference for citrate-based anticoagulants over heparin.5
ECP systems have several performance characteristics that may be affected by the type of anticoagulant used. There are three main phases of the ECP treatment: leukapheresis, photoactivation, and reinfusion. Leukapheresis involves the collection and separation of the buffy coat from other blood components. The THERAKOS® CELLEX® ECP system is designed to end the buffy coat collection at a set hematocrit threshold. However, if this threshold is not reached, the operator must manually end the buffy coat collection.6, 10 Furthermore, the buffy coat collection can be ended manually based on operator preference. The buffy coat then undergoes photoactivation where a photo-sensitizing agent is added and it is exposed to UVA before the treated cells are reinfused into the patient.10 Optimal device performance for an ECP system is characterized by a short and consistent procedure duration, including consistent photoactivation times, and limited warnings and alarms throughout the procedure.11 Choice of anticoagulant may also have differential effects when the ECP system is used in singleneedle mode (cycles of periodic blood collection, continuous separation of buffy coat and periodic return of red blood cells and plasma) compared with doubleneedle mode (simultaneous blood collection, continuous separation of buffy coat and return of red blood cells and plasma with shortened treatment duration) or when both needle modes are used (when the patient gains or loses a venous access site during the procedure).6
The main objective of this hypothesis-generating data review was to examine real-world data of device performance characteristics of the THERAKOS® CELLEX® integrated ECP system utilizing either ACDA or heparin. Specifically, the impact of the anticoagulant type on total treatment time, buffy coat collection time, photoactivation time, and the number of alarms and warnings based on double-needle mode, singleneedle mode, or both needle modes, were assessed. Variation in these parameters was also evaluated by needle mode.
2 MATERIALS AND METHODS
2.1 Study design
This was a retrospective analysis of device-generated data from ECP sessions across 27 sites throughout the United States (US) that was undertaken to evaluate the performance of the integrated ECP system with different anticoagulants.
2.2 Data source
The THERAKOS® CELLEX® Photopheresis System (Mallinckrodt Pharmaceuticals, Bridgewater, NJ, USA) is US FDAapproved and widely used for ECP.6, 8 Data were obtained from the CELLEX® System Smart Card data program, a US-based initiative to collect deidentified treatment and device operation data at the point of care, between December 2011 and July 2020. Sites participating in the Smart Card data program were obligated to submit all smart card data to reduce selection bias confounding the results. A unique, factory-programmed Smart Card, included in every THERAKOS® CELLEX® Photopheresis System Procedural Kit, was inserted into the machine during each treatment session to log event records and performance metrics such as duration of the ECP procedure, photoactivation time, anticoagulant volume and the number of alarms and warnings.6 Additionally, during each session, printed labels were generated on which the operator was to log venous access type, indication for ECP, and the anticoagulant used (Figure S1). Only data specific to the procedure were collected, with no patient demographic data and identifying information available from the Smart Card or label. After treatment completion, Smart Cards with attached operator-filled labels were submitted to the manufacturer for data download and analysis.
2.3 Endpoints
Data were analyzed to assess the relative effects of different priming anticoagulants (ACDA or heparin) on device performance. The duration and variability of the overall ECP procedure time, buffy coat collection time, photoactivation time, and number of alarms and warnings during individual treatment sessions were evaluated, taking into consideration the needle mode used, double, single, or both needle modes. Rates of operator intervention to manually stop the buffy coat collection process were also recorded.
2.4 Statistical analysis
Descriptive statistics for the duration of the total ECP procedure, buffy coat collection time, photoactivation time, and the number of alarms and warnings were performed and are presented within the results sections as box and whisker plots, based on the type of anticoagulant and needle mode. Each ECP treatment session was considered a single data point, since individual patient records were not collected; multiple entries from individual patients were allowed given the objective was to assess machine function and not patient response/outcome. Records were excluded from the analyses if treatment was incomplete or if required entries on the printed labels were left blank.
Given that operators could terminate the buffy coat collection based on their personal preference and not because of alarms or warnings generated by the ECP device, operator intervention could have introduced variability into the data and artificially impacted the timing of the ECP procedure. Therefore, a logistic regression analysis was performed to investigate differences in the proportion of treatments that had operator intervention.
A multivariable linear regression analysis was performed to investigate differences in total treatment time, buffy coat collection time, photoactivation time, and warnings and alarms associated with anticoagulant type. Only treatment sessions that were automatically stopped by the device, rather than through operator intervention, were considered. We fit separate regression models for double-needle mode and single-needle mode treatment. Both the logistic regression model for operator intervention and the multivariable linear regression model controlled for the following confounder factors: diagnosis of the patient, age of the lamp light, computed white blood cell hematocrit, and site number.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) using two-sided testing; nominal P-values <.05 were considered statistically significant.
3 RESULTS
3.1 Data collected
ECP session data were collected from a total of 85 devices at 27 treatment centers in the US; these sites participated in the Smart Card program and collected information on anticoagulant use. Data used were restricted to those collected between January 2018 and July 2020 because over 80% of procedure information, including anticoagulant use, was available for this data subset. In contrast, only 0.31% of procedures between 2011 and 2017 collected data on anticoagulant usage, which precluded any analysis for procedures completed during these years. A total of 28 587 deidentified treatment sessions were available during this time, of which 23 334 sessions had anticoagulant type or indication information available. Of sessions with diagnosis information, the following indications were included: acute or chronic GvHD (n = 12 263), lung transplant (n = 6815), CTCL (n = 3228), heart transplant (n = 379), scleroderma (n = 134), or bronchiolitis obliterans syndrome (n = 124; Table 1). Of the 23 334 sessions with known anticoagulant type, 8020 procedures (34.4%) used ACDA and 15 314 (65.6%) used heparin (Table 1). Approximately equal proportions of procedures used the double-needle mode (46.0%) compared to the single-needle mode (45.3%). Both needle modes were used in only 8.7% of procedures, therefore, the analyses reported here focus mainly on the double-needle and single-needle mode. The single-needle mode was used in 51.4% of the 15 314 procedures using heparin, and in 25.7% of the 8029 procedures using ACDA (Table S1). Site-specific information was only available from a small number of sites. Data from these sites on anticoagulant preference, needle mode, and the proportion of treatments in which buffy coat collection was terminated by operator intervention are presented in Table S2.
| Procedures (N = 28 587) | |
|---|---|
| AC use, n (%) | 23 334 (81.6) |
| ACDA | 8020 (28.1) |
| Heparin | 15 314 (53.6) |
| Needle mode, n (%) | |
| Double-needle | 13 141 (46.0) |
| Single-needle | 12 949 (45.3) |
| Both needle modes | 2497 (8.7) |
| Diagnosis, n (%) | |
| Bronchiolitis obliterans syndrome | 124 (0.4) |
| CTCL | 3228 (11.3) |
| GvHD, acute | 1896 (6.6) |
| GvHD, chronic | 10 367 (36.3) |
| Scleroderma | 134 (0.5) |
| Transplant—heart | 379 (1.3) |
| Transplant—lung | 6815 (23.8) |
| Others | 223 (0.8) |
| Unknown | 5421 (19.0) |
- Abbreviations: AC, anticoagulant; ACDA, acid citrate dextrose-A; CTCL, cutaneous T-cell lymphoma; GvHD, graftversus-host disease.
3.2 Incomplete treatment
A treatment was considered incomplete if the ECP procedure had to be interrupted or stopped for any reason, including loss of venous access or patient adverse events (such as dizziness); 1.9% of all procedures with ACDA and 0.8% of heparin procedures were incomplete.
3.3 Treatment duration, buffy coat collection time, photoactivation time, warnings/alarms for ACDA and heparin
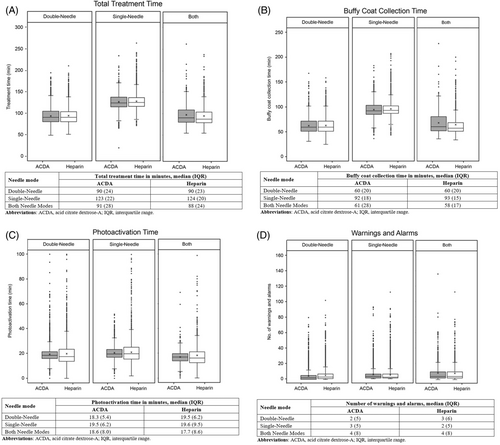
Total treatment duration (Figure 1A), buffy coat collection time (Figure 1B), photoactivation time (Figure 1C) and the number of warnings and alarms (Figure 1D) were numerically similar between treatments using ACDA or heparin for each of the needle mode. Variability across all parameters was generally similar with ACDA and heparin, although there appeared to be less variability in the photoactivation time when ACDA was used as the anticoagulant compared to when heparin was used.
3.4 Rate of operator intervention
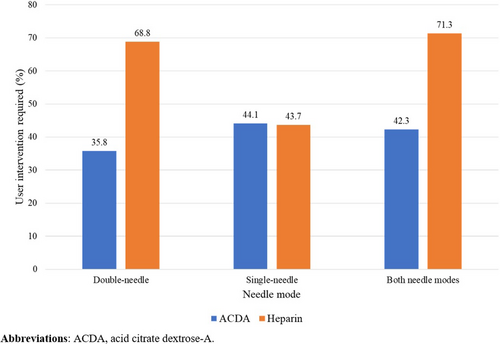
The rate of operator intervention in the termination of buffy coat collection varied among the sites (Table S2). Among the procedures with known information on anticoagulant use, there were a greater proportion of procedures with operator intervention when heparin was used compared with when ACDA was used in the double- and both needle modes (Figure 2). Meanwhile, for the single-needle mode, numerically similar proportions of procedures had operator intervention when the two anticoagulants were used. This association between the proportion of treatments that had operator intervention and the type of anticoagulant used was confirmed with the logistic regression analysis of the data. With the double-needle mode, the odds ratio for the proportion of treatments with operator intervention was 0.244 (95% CI 0.223, 0.267, P < −.001). With the single-needle mode, odds ratio for the proportion of treatments with operator intervention was 0.979 (95% CI 0.873, 1.099; P = .72).
Descriptive statistics on the total treatment time, buffy coat collection time, photoactivation time and the number of warnings and alarms based on needle mode, anticoagulant use, and whether there was operator intervention are presented in Tables S3 and S4.
3.5 Regression analysis of treatment times and warnings and alarms
When considering only treatment sessions that were automatically stopped by the device, rather than by operator intervention, a multivariable regression analysis indicated that total treatment time and photoactivation time was significantly reduced with ACDA use compared with heparin for both the double- and single-needle modes (Table 2). The buffy coat collection time was reduced only for the single-needle mode with ACDA compared with heparin. The number of warnings and alarms was significantly reduced with ACDA use only in the double-needle mode but not in the single-needle mode.
| Outcome variable | Adjusted | ||
|---|---|---|---|
| Estimate | 95% CI | p-value | |
| Double-needle | |||
| Total treatment time | −5.42 | (−6.47, −4.37) | <.001 |
| Buffy coat collection time | 0.06 | (−0.80, 0.91) | 0.898 |
| Photoactivation time | −5.42 | (−5.90, −4.93) | <.001 |
| Warnings and alarms | −0.57 | (−1.03, −0.12) | 0.013 |
| Single-needle | |||
| Total treatment time | −2.83 | (−4.07, −1.58) | <.001 |
| Buffy coat collection time | −1.39 | (−2.49, −0.28) | 0.014 |
| Photoactivation time | −1.99 | (−2.55, −1.43) | <.001 |
| Warnings and alarms | −0.04 | (−0.67, 0.60) | 0.909 |
- Note: Adjusted for diagnosis, lamp life remaining, computed whole blood hematocrit, site number. Reference group is heparin.
- Abbreviations: CI, confidence interval.
Importantly, it should be noted that while these differences were considered statistically significant, these data do not confirm the experiential clinical impact for patients, and it is also not known whether the magnitude of the differences observed translate into any clinically meaningful differences. Additionally, there may be additional factors for which data were not available for this analysis, yet which may have influenced the clinical results.
4 DISCUSSION
We present here the results of a real-world study of the use of ACDA versus heparin anticoagulants during ECP. Generally, the results of this real-world retrospective analysis demonstrated that treatment times, buffy coat collection times, and photoactivation times were similar among both anticoagulant groups, regardless of the needle mode used during the procedure. One key area of difference is the lower variability in photoactivation time with ACDA compared with heparin in all needle modes, although the potential advantage of this is not yet known.
Interestingly, there is a large numeric difference in operator intervention rate in the buffy coat collection between treatments using ACDA and heparin in the double-needle mode, and the rate of operator intervention between ACDA and heparin was similar in the single-needle mode. The reason for this difference is unclear; however, it should be noted that operator intervention includes both interventions triggered by alarms or warnings and interventions triggered by the operator's own personal preference, and the reason for operator intervention was not a variable captured in this study. Further research is required to investigate the reason for this difference.
Regression analyses were performed on the data from treatments for which buffy coat collection was terminated automatically by the ECP device without operator intervention. A significant reduction in total treatment time and photoactivation time with ACDA use was consistently observed with both the double-needle and single-needle mode. The magnitude of reduction in the total treatment time and photoactivation time were similar in the double-needle mode, suggesting that the reduction in total treatment time was most likely a result of the reduction in photoactivation time with ACDA use. The use of the double-needle mode reduces the buffy coat collection time by approximately 30 min, compared with the single-needle mode. No reduction was seen in buffy coat collection time with ACDA use in the double-needle mode, but a significant reduction was observed with the single-needle mode. While the results of the regression analysis indicated a significant difference between use of ACDA and heparin in both the single- and double-needle modes in several treatment parameters, it cannot be determined whether these differences would translate into clinically meaningful differences for patients.
In this analysis, we did not consider patient platelet count. Low platelet counts (<20 × 109/L) are often considered a potential contraindication for ECP, and data suggest that heparin may better preserve platelet function compared with ACDA/citrate.12 However, citratebased anticoagulants may be safer for patients at risk of HIT.5, 8 Future research to assess whether platelet counts affect rates of operator intervention in ECP may be warranted.
Although heparin has been used as an anticoagulant for many years and was deemed generally safe and effective as the priming anticoagulant for the integrated ECP device considered in this analysis,5-7 any findings suggesting possible improvements in device performance are of particular interest. The data from the current study indicate that citrate-based anticoagulants are similar to heparin, suggesting that citratebased anticoagulants may be used in place of heparin without risking decreased device performance. This may be of particular interest for clinicians treating patients with a contraindication for heparin. In fact, the reduced variability in photoactivation time may suggest that citrate-based anticoagulants could improve ECP device performance as compared with heparin. Investigation into optimizing the duration of and variability between ECP treatments is important for both patients and device operators; further research into the differences in device performance and patient outcomes with different anticoagulants and in different geographical regions is needed.
4.1 Strengths and limitations
This analysis provides results based on a large data set that reflects the real-world implementation of ECP across the US. Due to the nature of real-world trials, studies may have been confounded by a possible imbalance in covariates. For instance, the choice of needle mode, anticoagulant and operator intervention to terminate buffy coat collection were often mostly based on operator preference and not a randomized parameter. The reason for operator intervention of buffy coat collection was also not collected. Some clinical data, such as adverse reactions, were also not included. As such, we cannot draw conclusions on how the use of different anticoagulants may affect patient outcomes. Moreover, as limited demographic data were collected, the potential influence of patient demographics, such as weight and age, on anticoagulant use cannot be established. Further research investigating patient-specific differences in anticoagulant use is needed. Furthermore, as photoactivation time is highly dependent on treatment volume and hematocrit, future research should assess the impact of these parameters on the results. Finally, all data analyzed pertain to the use of a specific integrated ECP technology and thus the results discussed are not meant to be applicable to other ECP devices and systems.
5 CONCLUSIONS
In this retrospective analysis, we compared the performance of one ECP system when using ACDA or heparin as the anticoagulant. Our results demonstrated that overall, ECP procedure duration, buffy coat collection time, and photoactivation time were similar among both groups regardless of the needle mode used in the procedure. Interestingly, reduced variability of photoactivation time irrespective of the needle mode used and operator intervention rates was observed with ACDA use. Data from this study suggest that from a device performance perspective, ACDA performance was generally similar to heparin, although ACDA was associated with potential benefits over heparin based on the observed reductions in both treatment duration and photoactivation time if the ECP device was operated automatically without operator intervention in the collection of buffy coat with the ECP system considered in this analysis.
FUNDING INFORMATION
This study was funded by Mallinckrodt Pharmaceuticals.
CONFLICT OF INTEREST STATEMENT
Laura S. Connelly-Smith and James Griffin have nothing to disclose. Albert T. Leung and Francesca Gennari are employees of Mallinckrodt Pharmaceuticals.
ETHICS APPROVAL
As patient level data were not collected and the device-related data collected were anonymous, IRB approval was not required.




