Rapid growth produces transient cortical weakness: A risk factor for metaphyseal fractures during puberty
Abstract
Fractures of the distal radius in children have a similar incidence to that found in postmenopausal women but occur more commonly in boys than in girls. Fractures of the distal tibia are uncommon in children and show no sex specificity. About 90% of lengthening of the radius but only 30% of lengthening of the tibia during puberty occur at the distal growth plate. We speculated that more rapid modeling at the distal radial metaphysis results in a greater dissociation between growth and mineral accrual than observed at the distal tibia. We measured the macro- and microarchitecture of the distal radial and tibial metaphysis using high-resolution peripheral quantitative computed tomography in a cross-sectional study of 69 healthy boys and 60 healthy girls aged from 5 to 18 years. Bone diameters were larger but total volumetric bone mineral density (vBMD) was lower at the distal radius (not at the distal tibia) by 20% in boys and by 15% in girls at Tanner stage III than in children of the same sex at Tanner stage I (both p < .05). In boys at Tanner stage III, total vBMD was lower because the larger radial total cross-sectional area (CSA) had a thinner cortex with lower vBMD than in boys at Tanner stage I. In girls at Tanner stage III, the larger total radial CSA was not associated with a difference in cortical thickness or cortical vBMD relative to girls in Tanner stage I. Cortical thickness and density at both sites in both sexes after Tanner stage III were greater than in younger children. Trabecular bone volume fraction (BV/TV) was higher in boys than in girls at both sites and more so after puberty because trabeculae were thicker in more mature boys but not in girls. There was no sex- or age-related differences in trabecular number at either site. We infer that longitudinal growth outpaces mineral accrual in both sexes at the distal radius, where bone grows rapidly. The dissociation produces transitory low cortical thickness and vBMD in boys but not in girls. These structural features in part may account for the site and sex specificity of metaphyseal fractures during growth. © 2010 American Society for Bone and Mineral Research
Introduction
The incidence of distal forearm fractures during the peripubertal years is comparable with the incidence in postmenopausal women but is higher in boys than in girls.1-3 By contrast, the incidence of distal tibia fracture is low in children and does not differ by sex.1 Boys are more likely participate in risk-taking activities than girls. However, the heterogeneity in regional growth that produces the diversity in size, shape, and distribution of bone mass along and around the perimeter of a bone also may contribute to the site and sex specificity of fracture incidences.4, 5 For example, lengthening of a long bone is achieved by growth at the proximal and distal growth plates, but 90% of the increase in radial length and only 30% of the increase in tibial length occur at the distal growth plate during puberty.6, 7 Thus the distal metaphysis undergoes more rapid modeling and remodeling at the radius than at the tibia.
The rapid increase in long bone length and diameter is accompanied by little change in total volumetric bone mineral density (vBMD) during growth because the increase in size is matched by a commensurate increase in cortical and trabecular bone accrual within the periosteal envelope of the enlarging bone.8 However, several studies suggest that total vBMD decreases at the distal radius during early puberty owing to a dissociation between growth in size and mineral accrual, as reflected in the 7- to 8-month earlier peak growth velocity in length rather than in mineral accrual. That is, accrual of bone within the periosteal envelope does not “keep up” with the increase in the bone's external dimensions.9-11
While diaphyseal cortical bone is assembled by membranous bone formation, metaphyseal cortical bone is formed by endochondral ossification. Adjacent trabeculae arising from the periphery of the growth plate coalesce by bone formation on their surfaces to form the metaphyseal cortex.12, 13 As this “corticalization” of trabecular bone thickens the cortex on the endocortical surface, concurrent periosteal resorption thins the metaphyseal cortex adjacent to the diaphysis, producing in-wasting to “fit” the metaphysis to the more narrow diaphysis14, 15 (Fig. 1).
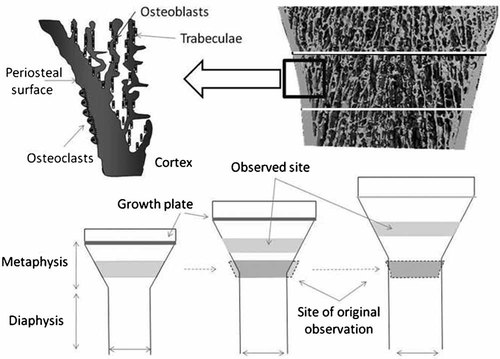
The metaphyseal cortex is formed by “corticalization” of trabeculae of growth plate origin. Trabeculae coalesce as bone formation on their surfaces proceeds forming a compact cortex. Bone length increases by endochondral bone formation moving the metaphysis further from the centre of the diaphysis. Periosteal resorption produces in-wasting of the metaphysis to accommodate the narrow diaphysis.
Unlike aging, where intracortical remodeling produces porosity by resorptive removal of bone,16 we proposed that the rapidity of growth in length outpaces corticalization of trabeculae of growth plate origin leaving intracortical porosity which is reflected in a decline in cortical vBMD. We also proposed that this porosity will be transient as completion of longitudinal and circumferential growth allows cortical consolidation by trabeculae coalescence in both sexes,12, 13 but later in boys because longitudinal and circumferential growth continues longer in boys as they reach maturity later than girls.
Subjects and Methods
The recent development of noninvasive methods for measuring the microstructure of cortical and trabecular bone in vivo,16, 17 gave us the opportunity to explore the structural development of the metaphyseal region in a cross sectional study of 69 healthy Caucasian boys and 60 girls aged from 5 to 18 years. Participants were recruited from metropolitan Melbourne and had no history of disease or medication use affecting bone metabolism. The study was approved by the Austin Hospital Ethics Committee for Human Research. Informed consent was obtained from the subjects and their parents. Standing height was measured using stadiometer to the nearest 0.1 cm. Body weight was measured using electronic scale to the nearest 0.1 kg. Pubertal stage was self-evaluated by providing the participants with images depicting the development of pubic hair in both sexes and breast in female and the genital size (using Prader Orchidometer) in males.
High-resolution peripheral quantitative computed tomography (HR-pQCT, Xtrem CT, Scanco Medical AG, Basserdorf, Switzerland, resolution 82 µm) was used to scan the non-dominant distal radius and tibia. The scanned site was 4% of the forearm length proximal to the lateral edge of the radial joint surface of the wrist and 7% of the lower leg length proximal to the centre of the tibial joint surface of the ankle. Forearm length was measured from the styloid process to the olecranon of ulna with the forearm neutrally rotated and the elbow flexed at 90°. The lower leg length was measured from the medial condyle to the medial malleolus of tibia with the knee flexed at 90°. Both regions contain secondary trabeculae and were proximal to the growth plate and excluded the region of primary trabeculae. The region of interest (ROI) was 9.02 mm in length with 110 slices, each 82 µm thick. Attenuation data were converted to equivalent hydroxyapatite (HA) densities. Quality control, based on Shewart rules, was monitored by daily scans of a phantom containing rods of HA (of 0, 100, 200, 400, and 800 mg HA/cm3) embedded in a soft-tissue equivalent resin (QRM, Moehrendorf, Germany).
Directly measured parameters were the total bone volume (TV), bone tissue volume (BV), total volumetric bone mineral density (vBMD) and trabecular vBMD. Total vBMD is the bone mass per unit of total bone volume including void spaces, and trabecular vBMD is the bone mass per unit of total trabecular bone volume including the void spaces (apparent vBMD).17 Cortical bone volume was identified automatically as the volume containing 5 consecutive voxels filled with bone tissue. The cortical thickness was calculated as cortical volume/outer bone surface.18 Cortical vBMD of the distal and proximal 20 slices in each scan were also analyzed and compared in 24 randomly selected children to demonstrate its change within a short range along the metaphysis.
Trabecular bone volume fraction (BV/TV) was calculated as trabecular vBMD/1.2g HA/cm3, assuming vBMD of any voxel filled with mineralized tissue was 1.2 g HA/cm3. Trabecular number (Tb.N) was defined as the inverse of the mean spacing of between trabeculae identified by a mid-axis transformation method which identifies the three-dimensional ridges (centre points of the trabeculae).19 The trabecular thickness (Tb.Th) and trabecular separation (Tb.Sp) were obtained using standard methods in histomorphometry: TbTh = (BV/TV)/TbN and TbSp = (1 – BV/TV)/TbN. The coefficient of variation of repeated measurements with repositioning, which was done only in radius, is < 1.5% for vBMD and BV/TV, <3.5% for trabecular structural parameters and 1.4% for cortical thickness.
Independent t-tests were used for sex comparisons for a given pubertal stage and ANOVA for comparison between maturational stages within a sex. Dependent t-tests were used to compare cortical vBMD between the distal and proximal 20 slices in each scan. Data were presented as mean ± SD unless otherwise stated. A p-value <0.05, two tailed, was considered statistically significant.
Results
There were no significant sex differences in age or weight at any maturational stage. Peri- and post-pubertal boys were taller and had longer radii and tibiae than girls. There were no sex differences in bone lengths in prepubertal subjects but total CSA was 26% greater at the distal radius and 11% greater at the distal tibia in boys than girls. The sex difference in total CSA was 36% and 31% respectively in postpubertal subjects and was 17% and 27% greater in boys than in girls after controlling for sex differences in bone length (p < 0.05) (Fig. 2, Table 1).
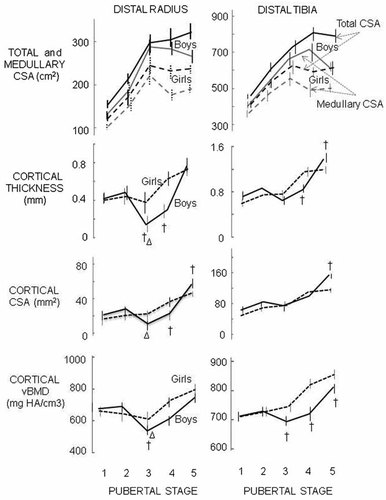
At the distal radius, medullary CSA approximated total cross-sectional area (CSA) more in boys than girls at Tanner stage III resulting in a thinner cortex with lower vBMD in boys. After Tanner stage III, total CSA differed little across pubertal stages while medullary area was smaller in both sexes so cortical thickness, cortical CSA and cortical vBMD were higher. At the distal tibia total and medullary areas were higher in more mature subjects with no differences in cortical thickness by sex. Full lines boys, broken lines girls (mean ± SE). †sex difference and Δ difference relative to Tanner stage I within a sex (p < 0.05).
| Pre-puberty (Tanner Stage I) | Peri-puberty (Tanner Stage II, III, IV) | Post-puberty (Tanner stage V) | ||||
|---|---|---|---|---|---|---|
| Male (n = 38) | Female (n = 28) | Male (n = 8, 8, 8) | Female (n = 7, 4, 9) | Male (n = 7) | Female (n = 12) | |
| Age (yr) | 9.0 ± 1.4 | 8.5 ± 1.5 | 13.0 ± 1.4 | 12.8 ± 2.1 | 16.7 ± 0.7 | 15.8 ± 1.4 |
| Height (cm) | 134.4 ± 9.3 | 130.0 ± 10.9 | 163.1 ± 14.7+ | 155.6 ± 8.9+ | 175.7 ± 4.5‡ | 164.2 ± 5.6‡ |
| Weight (kg) | 29.2 ± 6.9 | 29.7 ± 7.6 | 50.8 ± 11.6 | 46.9 ± 10.4 | 68.4 ± 10.2 | 62.0 ± 10.7 |
| Radial length (cm) | 20.4 ± 1.4 | 19.5 ± 1.7 | 25.7 ± 2.3+ | 24.2 ± 1.6+ | 27.6 ± 0.8‡ | 24.8 ± 1.0‡ |
| Tibial length (cm) | 30.1 ± 2.8 | 29.0 ± 3.6 | 37.8 ± 1.5+ | 35.5 ± 0.9+ | 38.4 ± 0.8‡ | 36.4 ± 1.5‡ |
| Radial CSA (mm2) | 153 ± 30‡ | 121 ± 21‡ | 266 ± 55‡ | 207 ± 41‡ | 322 ± 76‡ | 237 ± 31‡ |
| Tibial CSA (mm2) | 457 ± 101+ | 410 ± 97+ | 711 ± 99‡ | 565 ± 76‡ | 762 ± 98‡ | 615 ± 85‡ |
- +p < 0.05 and ‡ < 0.01, respectively, between sexes within a pubertal stage.
The endocortical surface approximated the periosteal surface more closely in boys resulting in a thinner cortex at Tanner stage III than stage I (p < 0.01). This was not observed in girls (Fig. 2). After Tanner stage III, total CSA differed little by Tanner stage but medullary area was smaller so that more mature children had a thicker cortices (Fig. 2). Cortical vBMD was also lower at Tanner stage III vs. stage I in boys (p < 0.02) (Fig. 2). Thus, boys had a transiently thinner cortex with lower cortical vBMD during puberty than girls. Differences in cortical properties between Tanner stages and sexes were independent of bone length.
At the distal tibia, total and medullary CSAs enlarged similarly until Tanner stage III in both sexes resulting in little difference in cortical thickness in subjects across Tanner stage I to III (Figs 2). In children beyond Tanner stage III in maturity, total CSA differed little while medullary CSA was smaller resulting in a thicker cortex and larger cortical CSA in postpubertal subjects than younger subjects. These traits differences were independent of bone length (data not shown). In contrast to the radius, cortical vBMD showed no diminution in early puberty (Tanner stage I to III) then was higher in Tanner stage V (Fig. 2). Postpubertal boys had thicker cortices and greater cortical CSA than girls in a larger tibial CSA but there was no sex difference in ratio of cortical/total CSA. Boys had lower cortical vBMD than girls from Tanner stage III to V.
Metaphyseal cortical vBMD of the distal 20 slices in both radius and tibia was lower than the proximal 20 slices even though the region spanned only 9 mm (Fig. 3). In addition, the distal-proximal cortical vBMD difference was greater at radius than tibia independent of bone length (147 ± 41 vs. 81 ± 16 mg HA/cm3, p < 0.01).
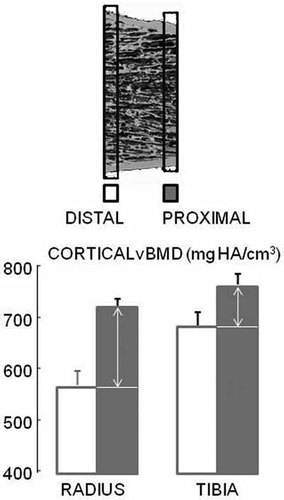
Cortical vBMD of the distal 20 slices in both radius and tibia was lower than the proximal 20 slices and the distal-proximal cortical vBMD difference was greater in radius than tibia independent of bone length (mean ± SE)(all p < 0.01).
In boys, trabecular BV/TV at both sites was higher in those more maturely advanced due to thicker trabeculae (p < 0.05). Tb.N did not differ in boys at different pubertal stages (Fig. 4). In girls, trabecular BV/TV, Tb.Th and Tb.N were independent of age at both sites. Thus, trabecular BV/TV was higher in boys than girls due to differences in Tb.Th, not Tb.N. At maturity, half of the 24% sex difference in trabecular BV/TV at distal radius and almost all of the 7% sex difference at the distal tibia was observed in prepubertal subjects.
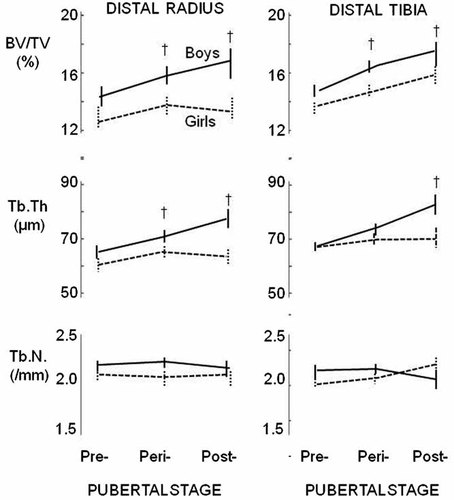
Trabecular BV/TV was higher in boys than girls throughout pubertal at both sites. Trabecular thickness was higher in boys but not in girls in more mature subjects. Trabecular number did not differ by sex or pubertal stage. †p < 0.05 for sex difference (mean ± SE).
Total vBMD (a function of the total CSA and cortical and trabecular morphology within the periosteal envelope) at the distal radius was 20% lower in boys and 15% lower in girls at Tanner stage III vs. stage I (both p < 0.05) (Fig. 5). However, in boys, the lower total vBMD was the result of a larger total CSA, a lower cortical thickness and cortical vBMD despite greater Tb.Th. In girls, this was due to a larger total CSA with no difference in cortical thickness, cortical vBMD or trabecular BV/TV. At the distal tibia, total vBMD was independent of age until Tanner stage III after which it increased in both sexes.
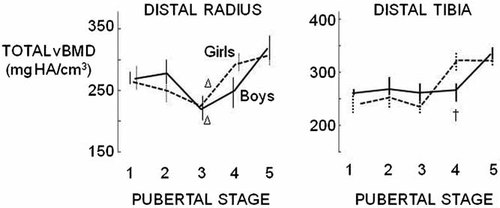
Total volumetric bone mineral density (vBMD) was the net result of total CSA and the cortical and trabecular morphology within the periosteal envelope. At the distal radius, total vBMD diminished at Tanner stage III (p < 0.05 comparing to Tanner stage I) in both sexes. At the distal tibia, total vBMD was independent of Tanner stage until Tanner stage III when it was higher. †sex difference and Δ difference relative to Tanner stage I within a sex (p < 0.05).
Discussion
We report that sex differences in radial and tibial morphology were predominantly in macro- architecture. Sex difference in total CSA of the radius and tibia at maturity in favor of males was present before puberty and magnified after puberty. By contrast, there were either no sex differences or only small sex differences in total vBMD, cortical thickness before or after puberty. During the peripubertal period there was a temporary lower total vBMD at the distal radius but not distal tibia than either less or more mature children in both sexes. In boys, this was the result of larger total CSA containing less cortical area due to a thinner cortex with lower vBMD despite a greater trabecular BV/TV than prepubertal subjects. In girls, this was due to a larger bone size containing cortical bone of the same area and density and no higher trabecular BV/TV than observed in prepuberal girls. Boys had greater trabecular BV/TV due to thicker trabeculae. Trabecular numbers did not differ by sex.
Appendicular growth proceeds at a similar rate in prepubertal boys and girls but slows earlier in girls as they enter puberty earlier.20 Although bone diameter is greater in boys than girls before puberty,20, 21 the two years longer prepubertal growth in boys accounts for most of their greater limb length and width.22, 23 Despite the increasing bone size, cortical thickness was lower in boys in puberty than before puberty as medullary area more closely approximated total CSA. There was no difference in cortical thickness in girls across early puberty as incremental increases in periosteal and medullary areas were similar.
We and others have reported a decrease in total vBMD of distal radius in girls during puberty based on prospectively derived data.9, 10, 24, 25 However, the morphological basis for this reduction has not been reported. The data presented here suggest the dissociation between growth in size and mineral accrual is accompanied by the occurrence of thinner cortex with lower cortical vBMD. In peripubertal boys, a larger total bone CSA, smaller cortical area and lower cortical vBMD reduced total vBMD than prepubertal subjects. In peripuberal girls, a larger total bone CSA without an observed change in cortical area or cortical vBMD produced the lower total vBMD. Total vBMD did not differ by sex at Tanner stage III because trabecular thickness was higher in males offsetting the thinner cortex than in females. Thus, the lower total vBMD in boys and girls is likely the result of different structural changes. This dissociation between growth in size and mineral accrual occurred at the distal radius and less so at the distal tibia, perhaps because of the more rapid growth of the distal radius given most of its length is achieved by growth distally whereas most tibia growth occurs proximally.5, 6
The decline in cortical density was likely the result of cortical porosity. The endocortical surface is a bone forming surface upon which trabeculae of growth plate origin thicken and coalesce forming the cortex – “corticalizing” trabeculae.12, 13 In this region, the cortex consists of compacted or condensed trabeculae.12, 13 We suggest that the rapidity of growth in length outpaced corticalization of trabeculae at the periphery of the growth plate. This relative delay produces incomplete coalescence of trabeculae on the endocortical surface leaving cortical porosity and a reduction in vBMD of cortical bone of the distal radial metaphysis but not so at the distal tibial metaphysis because of regional difference in growth velocity.6, 7 This view is supported by a data from a recent study by Kirmani et al.24 Thus, this temporary decrease in cortical thickness and cortical vBMD is likely the result of incomplete consolidation by bone formation on adjacent trabecular surfaces. Finding lower cortical vBMD at more distal sites of the metaphyses (adjacent to the growth plate) than distally (adjacent to the diaphysis) and greater proximal-distal cortical vBMD difference at the site of more rapid growth (distal radius) than site of slower growth (distal tibia) support this view and is consistent with the report by Tanck et al.12 However, the distal-proximal difference was likely overestimated due to partial volume effect which underestimated the cortical vBMD more at the distal than at the proxmial site because of their difference in cortical thickness. The technique we used in this study, however, cannot rule out the possiblity that the redueced cortical vBMD during puberty might be due to reduced mineralization of newly formed cortices, although the change in tissue mineral density across early puberty is small.26 The partial volume effect may also artifically overestimated the matruational difference in cortical vBMD because of thinner cortex in boys at Tanner stage III. Thus, cautions must be exercised and further investigation is needed to test our inference.
Lower cortical vBMD in postpubertal males than females has been reported.27, 28 However, the notion that this is likely to be the result of incomplete ‘corticalization’ of trabeculae which is both sex- and site-specific has not been reported. Porosity during growth is the result of incomplete or delayed apposition while porosity during ageing is the result of resorptive excavation and reduced formation within the excavated sites.16
The reduced cortical density and thickness was transitory. In late puberty, cortical vBMD and thickness increased, probably due to reduced porosity.24 We suggest that this is the result of slowing of external bone growth while trabeculae coalescence continues as bone formation proceeds on trabecular surfaces. Earlier exposure to sex steroids in peri- and post-pubertal girls may enhance the consolidation of metaphyseal cortex at the endocortical surface decreasing the residual cortical porosity. This is likely to explain the higher cortical density in girls than boys reported here and in other studies.27, 28 Trabecular coalescence is likely to form the morphological basis of postpubertal bone ‘consolidation’ referred to in the literature.29
We confirm a previous report that males had 26% greater trabecular BV/TV at distal radius due to thicker trabeculae than females.30 However, about half of this sex difference in trabecular BV/TV we observed in postpubertal boys and girls was present in those children who were prepubertal at the time of our study suggesting that differences in early exposure to sex steroids may be responsible.31 The greater trabecular BV/TV in males was the result of sex differences in trabecular thickness not number. Our data suggest that trabecular thickness increased as maturational age advanced in males, not females explaining the higher trabecular vBMD in postpubertal males.32 We have no explanation for the lack of increase in trabecular thickness in females. We confirm that trabecular number was independent of age in both sexes.33
This study had several limitations. The study was cross-sectional. Prospective follow-up studies are needed to test whether the observations and accompanying inference we made are robust. There may have errors in estimating the peripubertal stages II, III, IV as self-assessment using illustrations may overestimate the pubertal stage in boys and underestimate in girls. The small sample size across a broad age range and two sexes limited our ability to demonstrate sex differences in age at any given maturational stage. The reduction in cortical density may also be partly due to differences in tissue mineral density and partial volume effects (PVE). It is likely the sex- or maturational difference in cortical vBMD had been overestimated and difference in cortical thickness been underestimated due to PVE. Nonetheless, the influence of PVE and change in tissue mineral density on the results of sex- and maturational- differences is likely to be nonsubstantial because they affect both sexes in similar direction and the change in tissue mineral density across early puberty is small.26
In summary, sex differences of the radial and tibial metaphyseal region are found in macrostructure and microstructure. During early and mid puberty, dissociation between growth in length and mineral mass accrual results in transient reduction in cortical volumetric bone mineral density and thickness at the distal radius. This transitory deficit is likely to be the result of a delay in corticalization of trabeculae as they coalescence by bone formation on their surfaces, one factor likely predisposing pubertal children to fractures at this site.
Disclosures
All the authors state that they have no conflicts of interest.
Acknowledgements
The authors are grateful to the participants and their families. The study was made possible, in part, by a grant to Q.W, the recipient of ESCEO-Amgen fellowship awards in 2008.