Estrogen-Responsive Gene MAST4 Regulates Myeloma Bone Disease
YC and FW contributed equally to this work.
ABSTRACT
Our previous data showed that young female multiple myeloma (MM) patients had a low frequency of osteolytic lesions. Based on this clinical observation, we found that estrogen cell signaling played a regulatory role in MM bone disease (MMBD), and the estrogen-responsive gene microtubule-associated serine/threonine kinase family member 4 (MAST4) was a critical factor. The presence of estrogen in cell cultures promoted MAST4 expression in MM cells, while knocking down estrogen receptor 1 (ESR1) inhibited MAST4 expression. Chromatin immunoprecipitation assay suggested a binding site of ESR1 on the MAST4 promoter. Bisphosphonates, such as zoledronic acid (ZOL), which was widely used in MMBD control, could stimulate MAST4 expression in MM cells by promoting ESR1 expression. MAST4 interacted with phosphatase and tensin homolog (PTEN), therefore regulating the PI3K-Akt-mTOR pathway and the expression of downstream cytokines, such as CCL2/3/4. MAST4 knockdown (MAST4-KD) or ESR1 knockdown (ESR1-KD) MM cells had repressed PTEN activity, elevated PI3K-Akt-mTOR activity, and increased CCL2/3/4 expressions. Coculture of MAST4-KD or ESR1-KD MM cells with pre-osteoclasts (pre-OCs) stimulated OC formation in vitro, whereas neutralizing antibodies of CCL2/3/4 attenuated such stimulation. In mouse models, mice inoculated with MAST4-KD or ESR1-KD MM cells had severer MMBD than control knockdown (CTR-KD). The correlations between MAST4 and ESR1 expressions in MMBD, as well as related cell signaling pathways, were confirmed in analyses using gene expression profiles (GEPs) of patients' MM cells. The negative correlation of MAST4 expression and occurrence of MMBD was further validated by patients' immunohistochemical tissue array. Overall, our data suggested that estrogen cell signaling negatively regulated MMBD through MAST4. © 2022 American Society for Bone and Mineral Research (ASBMR).
Introduction
Multiple myeloma (MM) is hematological cancer characterized by malignant accumulation of monoclonal plasma cells in the bone marrow (BM). Lytic bone lesions (BL) are the most commonly observed clinical complication in MM patients, which seriously deteriorate the patient's quality of life by causing substantial bone pain and skeletal-related events, such as pathological fractures. It has been well established that MM bone disease (MMBD) is caused by increased osteoclast (OC) activity and suppressed osteoblast (OB) activity, both of which promote bone mass loss in patients.(1) Therefore, the treatment strategy for MMBD is to inhibit aberrant OC activation or promote OB-mediated bone formation. At present, bisphosphonates, such as zoledronic acid (ZOL), are widely used for MMBD prevention and treatment in patients. Mechanistically, bisphosphonates act by selectively inducing OC apoptosis and inhibiting OC activation.(2) More recently, the RANK ligand monoclonal antibody, denosumab, has been used in clinics to treat MMBD.(3, 4)
Our previous study showed less incidence of MMBD in young female MM patients compared with other MM patients.(5) Such observational study inspired our speculation on the role of estrogen in MMBD. Considerable evidence showed that estrogen is involved in bone metabolism, and serum estrogen levels significantly correlated with bone mineral density and bone turnover markers in both males and females.(6, 7) A bioinformatics based on pathway exploration suggested that microtubule-associated serine/threonine kinase family member 4 (MAST4), an estrogen-responsive gene, might contribute to the lower incidence of MMBD in young female patients.(5) The human MAST4 gene product was first reported by Sun and colleagues.(8) Functional studies demonstrated that MAST4 is involved in the pathological process of the central nervous system.(9-11) However, the role of MAST4 in MM, as well as its upstream regulation, has not yet been determined. Herein, we show for the first time that MAST4 is a negative regulator of MMBD. In MM, MAST4 links upstream estrogen cell signaling and downstream PI3K-Akt-mTOR pathway, which mediates OC regulatory cytokine expression. The level of MAST4 expression in MM cells negatively correlates with the severity of MMBD in patients.
Materials and Methods
Patients and healthy samples
Bone-marrow biopsies of newly diagnosed MM patients were obtained from the tissue bank of the Department of Hematology, West China Hospital of Sichuan University. Peripheral blood mononuclear cells (PBMCs) were obtained from noncancer donors. Written informed consent was obtained from all patients and donors. This study was conducted following the Declaration of Helsinki and approved by the Ethical Committee of West China Hospital of Sichuan University.
Cell culture
Human MM cell lines AMO.1 and ARD and murine MM cell line 5TGM1 were cultured in RPMI-1640 (HyClone, Cytiva, Logan, UT, USA) supplemented with 10% fetal bovine serum (GeminiBio, West Sacramento, CA, USA), 100 U/mL penicillin (GeminiBio), and 100 g/mL streptomycin (GeminiBio) at 37°C and 5% CO2.
PBMCs were obtained from noncancer donors through density-gradient centrifugation. Cells were incubated with RPMI-1640 for 2 hours to adhere in culture plates. Nonadherent cells were removed and the adherent cells were cultured in OC medium, which was composed of αMEM medium supplemented with 10% FBS, 25 ng/mL M-CSF (R&D Systems, Minneapolis, MN, USA), and 50 ng/mL RANKL (Sino Biological, Beijing, China). Human OCs were generated as previously described.(12) Details are provided in Supplemental Materials and Methods.
Murine OCs were derived from murine BM cells of C57BL/KaLwRijHsd (C57BL/Ka) mice.(13) BM cells were obtained through crushing the hind leg bones in a mortar and then resuspended cells with RPMI-1640. BM cells were collected through filtration and erythrolysis and incubated in culture plates with RPMI-1640 for 2 hours. Nonadherent cells were removed and the adherent cells were cultured in OC medium.
TRAP assay and bone resorption assay
Mature OCs were examined using tartrate-resistant acid phosphatase (TRAP) stain kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).(12) TRAP staining was performed according to the manufacturer's instructions. Briefly, OCs were washed with 1× phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde. Afterward, the cells were stained with TRAP dye solution at 37°C for 60 minutes. The stained cells were observed under microscope (Zeiss, Jena, Germany). Alternatively, TRAP staining was used for formalin-fixed, paraffin-embedded slides to visualize OCs in experimental mice biopsies.
The function of OC was examined using bone resorption assay in vitro.(14) PBMCs were cultured on the bovine bone slices with OC medium for 14 days. The bovine bone slices were washed with 1× PBS and then subjected to ultrasonic cleaning for 10 minutes in 0.25 M ammonium hydroxide solution. Next, bone slices were examined under a scanning electron microscope (Zeiss) to visualize and calculate the resorption pits generated by OCs.
Surface plasmon resonance (SPR) assay
SPR assay was used to examine the protein–protein interaction between MAST4 and PTEN according to the manufacturer's protocol. See Supplemental Materials and Methods for details.
Lentivirus packaging and infection
MISSION pLKO.1-puro Control Vector (catalog no. SHCOO2) and plasmids containing MISSION human-MAST4-shRNA (TRCN0000037874 and TRCN0000195364), mouse-MAST4-shRNA (TRCN0000360913), and human-ESR1-shRNA (TRCN0000003300) were used for transfection. The control virus and shRNA virus were produced in HEK293T cells. Lentiviral stocks were concentrated using a virus precipitation solution (System Biosciences, Palo Alto, CA, USA). For infection, cells were plated onto 6-well plates (1 × 105 cells/well) and then infected with lentiviral stocks in presence of polybrene. Infection efficiency was identified using Western blot.
Bioinformatics
MM gene expression profile (GEP) data sets GSE13591,(15) GSE755,(16) GSE8546,(17) GSE26760,18) and GSE9782(19) were downloaded from the NCBI Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/) and corresponding clinical information was obtained from Oncomine database (https://www.oncomine.org/). Gene Set Enrichment Analysis (GSEA) was performed according to the guidelines (https://www.gsea-msigdb.org/).(20)
Immunohistochemistry (IHC)
Formalin-fixed, paraffin-embedded samples of MM patients' BM or experimental mice were sectioned for immunohistochemistry analysis as described earlier.(5) Briefly, BM tissue sections were deparaffinized, antigen unmasked, and stained with anti-MAST4 antibody (Atlas Antibodies, Stockholm, Sweden). Then, sections were incubated with biotinylated goat anti-rabbit IgG and streptavidin-biotin complex (Boster Biological Technology, Pleasanton, CA, USA) and diaminobenzidine (DAB) detection solution (ZSGB-Bio, Beijing, China) following the manufacturer’s instructions. A microscope (Zeiss, Germany) was employed for imaging. Image J was used for the quantification of MAST4 expression.
RNA-sequencing (RNA-seq)
ARD-CTR-KD MM cells and ARD-MAST4-KD MM cells were used for RNA-seq. Total RNA of each sample was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) and RNA-seq was performed by GENEWIZ (Suzhou, China) on an Illumina Hiseq platform. The raw and normalized RNA-seq data are available through GEO accession number GSE179453. Details are provided in Supplemental Materials and Methods.
Western blots
Whole-cell lysates were extracted from MM cell lines, protein concentration was measured with a BCA protein assay kit (CoWin Biosciences, Taizhou, China) and then equal protein amounts were loaded in each lane of polyacrylamide gels. Blocking and primary antibody incubation were performed in Tris-buffered saline-Tween buffer containing 0.5% nonfat skimmed milk. Then the blots were incubated with secondary antibodies conjugated with horseradish peroxidase and enhanced chemiluminescence (MilliporeSigma, Burlington, MA, USA) was applied for signal generation.
Antibodies used in this study were anti-MAST4 antibody (Bethyl, Montgomery, TX, USA), anti-PTEN antibody (Santa Cruz Biotechnology, Dallas, TX, USA), and anti-ESR1 antibody (Abcam, Cambridge, UK). All other antibodies were obtained from Cell Signaling Technologies (Danvers, MA, USA). Drugs used included 17β-estradiol (Dalian Meilun Biotechnology Co., Ltd., Dalian, China) and zoledronate (ZOL; Selleck, Houston, TX, USA). AZD9496, LY294002, and rapamycin were obtained from MedChemExpress (Monmouth Junction, NJ, USA).
Co-immunoprecipitation (Co-IP)
About 500 μg of the total cell lysates were harvested as described in the Western blot analysis, followed by incubation with protein A/G plus agarose beads (Santa Cruz Biotechnology) and moderate anti-PTEN or anti-MAST4 antibodies overnight at 4°C. Immunocomplexes were washed with 1× PBS. Bound proteins were detected by Western blot using anti-MAST4 or anti-PTEN antibodies.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from MM cell lines using TRIzol reagent (Invitrogen). Reverse transcription was performed using the Evo M-MLV RT kit (Accurate Biotechnology, Changsha, China) following the manufacturer's instructions. qRT-PCR was performed using SYBR Green qPCR Master Mix (Bimake, Changsha, China). Primer sequences are listed in Supplemental Table S1.
Duolink proximity ligation assay (PLA)
Proximity ligation assay was performed according to the manufacturer's protocol (Sigma-Aldrich, St. Louis, MO, USA). The primary antibodies used were anti-MAST4 (Atlas Antibodies) and anti-PTEN. Stained cells were captured using a confocal laser-scanning microscope (Nikon, Tokyo, Japan).
Immunofluorescence
Cells were washed with 1× PBS and fixed in 4% paraformaldehyde. The fixed cells were then incubated in 0.01% Triton X-100 in 1× PBS for permeabilization. Cells were blocked with 1× PBS containing 5% bovine serum albumin and then incubated with anti-PTEN antibody (Santa Cruz Biotechnology) and anti-MAST4 antibody (Atlas Antibodies) overnight at 4°C. Afterward, cells were incubated in fluorophore-conjugated secondary antibodies at room temperature for 1 hour. Slides were cover-slipped with mounting media containing DAPI. Stained cells were captured using a confocal laser-scanning microscope (Nikon).
Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was performed on ARD cells as previously described,(21) using the Magna ChIP G commercial kit (MilliporeSigma) and anti-ESR1 antibody (Abcam).
Enzyme-linked immunosorbent assay
The cell-conditioned supernatant was collected for detection of cytokine concentrations of CCL2, CCL3, and CCL4 using ELISA kits (Multi Sciences, Hangzhou, China). The assays were conducted following the manufacturer's instructions.
Animal experiments
Animal experiments were performed following approved protocols from West China Hospital Animal Ethics Committee (approval no. 20211248A). In some experiments, 7-week-old female NOD.CB17-Prkdcscid IL2rgtm1/Bcgen mice (B-NDG mice; Biocytogen, Beijing, China) were intravenously administered with ARD-CTR-KD, ARD-MAST4-KD, or ARD-ESR1-KD human myeloma cells (1 × 106 cells/mice) to establish a human MM xenograft mouse model. For the murine MM 5T model, 7-week-old female C57BL6/KaLwRij mice were implanted intravenously with 5TGM1-CTR-KD or 5TGM1-MAST4-KD mouse myeloma cells (1 × 106 cells/mice). To examine the effects of estradiol supplement, 7-week-old female immune-deficient B-NDG mice were intravenously administered with ARD MM cells. After MM establishment, the tumor-bearing mice were randomly divided into two groups: one group received subcutaneous estradiol (6 mg/d; Sigma-Aldrich) dissolved in corn oil, and the other group received the same amount of corn oil.(22-24) All mice received estradiol (or corn oil) for 3 weeks or until the mice became paraplegic.
Peripheral blood was collected once a week for detecting M-protein levels with human kappa ELISA Kit (Abcam). In vivo imaging system (Caliper Life Sciences, PerkinElmer, Waltham, MA, USA) was employed to verify the establishment of the MM model. After inoculation of MM cells, the skull and scapula were imaged under a micro-computed tomography (micro-CT) scanner (PerkinElmer) on days 14, 21, and 28 and analyzed by Quantum GX software (PerkinElmer) according to the manufacturer's instructions. The bone lesion area was quantified using Image J software. On day 28 after inoculation of MM cells or when the mice were paraplegic, femurs were harvested from mice for subsequent TRAP assay.
Statistical analyses
All in vitro experiments were performed thrice independently. Data were analyzed by a two-tailed Student's t test for the comparison between two groups using the GraphPad Prism 7.00 software (GraphPad, La Jolla, CA, USA). The results were presented as box plots with median and interquartile ranges. A p value <0.05 was considered statistically significant.
Results
Correlation between MAST4 expression and cell signaling in myeloma bone disease
Our previous work suggested the role of MAST4 in MMBD with undetermined mechanisms.(5) We analyzed differentially regulated genes in MM cells of patients with or without bone lesions (BL). The results showed that in three independent gene sets, MAST4 was overexpressed in MM patients without BL (p < 0.05 in all tested gene sets) (Supplemental Fig. S1A–C). Pathway enrichment analyses in two independent gene sets using GSEA showed that high MAST4 expression was associated with estrogen pathway activation (Fig. 1A; Supplemental Fig. S1D). Estrogen pathway activation was also found in MM patients without BL (Fig. 1B). Moreover, estrogen-responsive cell signaling was among the top enriched cell signaling in MM patients without BL (Fig. 1C; Supplemental Fig. S1E), whereas mTOR cell signaling was enriched in patients with BL (Fig. 1D, E; Supplemental Fig. S1F). Based on the above findings, we also analyzed the correlation between MAST4 expression and mTOR pathway activation and found that low MAST4-expressed MM cells had mTOR cell signaling enrichment (Fig. 1F; Supplemental Fig. S1G). In addition, we analyzed ESR1 expression in related cell signaling and MMBD. Similarly, MM cells with low ESR1 expression had mTOR cell signaling enrichment (Supplemental Fig. S1H). The MM patients with BL had lower ESR1 expression (Supplemental Fig. S1I). We also observed moderate co-expression of ESR1 and MAST4 in MM GEPs (Supplemental Fig. S1J). Besides, we found no sex difference in MAST4 and ESR1 expression (Supplemental Fig. S1K, L). All the above analyses were performed using GEPs of patients; therefore, they represented the expression of targeted genes and cell signaling in MM patients.
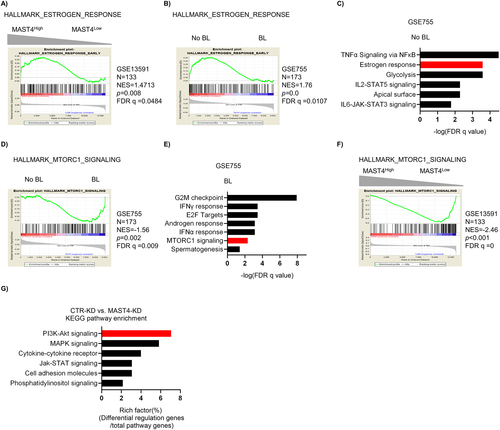
Next, we generated consistent MAST4-KD MM cells by shRNA lentiviral infection and selection (Supplemental Fig. S1M). By comparing the cell signaling in CTR-KD MM cells and MAST4-KD MM cells, we found that PI3K-Akt signaling was significantly altered after MAST4 was knocked down (Fig. 1G). The difference of estrogen downstream cell signaling in CTR-KD versus MAST4-KD was not significant (Supplemental Fig. S1N). This was probably because MAST4 was located downstream of estrogen cell signaling. Furthermore, MAST4 knockdown did not affect proliferation of MM cells in vitro (Supplemental Fig. S1O).
Overall, our data suggested that the expression of MAST4 and ESR1 correlated with MMBD. The related cell signaling included the PI3K-Akt-mTOR pathway. Because MAST4 is an estrogen-responsive gene,(5) we suggested that estrogen cell signaling was upstream of MAST4 and regulated MAST4 expression, whereas MAST4 regulated the PI3K-Akt-mTOR pathway and consequently affected the occurrence of MMBD.
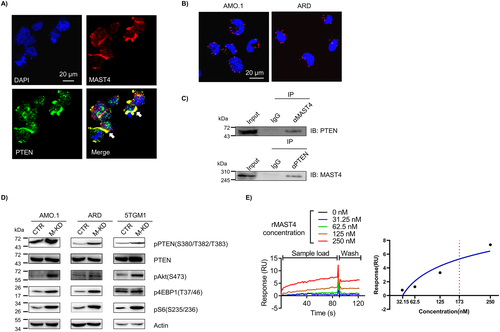
MAST4-PTEN interaction regulates PI3K-Akt-mTOR pathway activity in myeloma
Previously, we used cell signaling exploration to identify MAST4-centered cell signaling.(5) According to such a bioinformatics model, the functional cross-talk between MAST4 and PTEN bridged the interaction of MAST4 with the PI3K-Akt-mTOR pathway. PTEN suppressed the PI3K-Akt-mTOR pathway by dephosphorylating phosphatidylinositol 3,4,5-trisphosphate.(25, 26) Thus, PTEN inhibition promoted PI3K-Akt-mTOR pathway activity. Immunofluorescent staining showed colocalization of MAST4 and PTEN in MM cells (Fig. 2A). Results of PLA assay revealed that MAST4 interacted with PTEN in MM cell lines (Fig. 2B). In addition, immunoprecipitation assay showed that using either PTEN or MAST4 antibodies could precipitate each other in MM cell lysates (Fig. 2C). In MAST4-KD cells, PTEN phosphorylation at S380/T382/T383 residues was upregulated, whereas the total PTEN level remained consistent after MAST4 was knocked down (Fig. 2D). Previous studies suggested that phosphorylation at these sites downregulated PTEN activity,(27-29) therefore abrogating PTEN's inhibition of PI3K and promoting PI3K-Akt-mTOR activation. Western blotting confirmed that MAST4-KD MM cells had elevated pAkt, p4EBP1, and pS6 levels, all of which indicated upregulation of PI3K-Akt-mTOR signaling (Fig. 2D). Different MAST4-KD MM cells, generated using different shRNAs of MAST4, had similar results (Supplemental Fig. S2A). In addition, the SPR assay showed that MAST4 was bound to PTEN with affinity constant KD = 173 nM (Fig. 2E). Lastly, we performed a structural simulation to exhibit the interaction between MAST4 and PTEN(30, 31) (Supplemental Fig. S2B).
Estrogen-responsive cell signaling positively regulates MAST4 expression in myeloma
It has been suggested that MAST4 is an estrogen-responsive gene. The evidence was obtained from the GSEA molecular signatures database (MSigDB),(20) in which MAST4 was listed as a member of the HALLMARK_ESTROGEN_RESPONSE_EARLY data set. To the best of our knowledge, there is no wet experimental validation of estrogen regulation on MAST4. In MM cell lines, the addition of estrogen (β-estradiol or E2) stimulated MAST4 expression, whereas the ESR1 inhibitor attenuated this stimulation (Fig. 3A). In addition, compared with CTR-KD, estrogen could not stimulate MAST4 overexpression in ESR1-KD MM cells (Fig. 3B). This finding suggested that the binding of estrogen to its receptor ESR1 is necessary for estrogen cell signaling to mediate MAST4 regulation. ESR1 was known as a transcription factor. A cell fractionation study showed that the addition of estrogen resulted in translocalization of ESR1 from the cytoplasm to the nucleus in MM cells (Fig. 3C). Next, we searched the MAST4 promoter region for a potential ESR1 binding site (Supplemental Fig. S3A, B). There were four potential ESR1 binding sites within 1000 nt upstream of the MAST4 transcription start site. ChIP assay suggested that ESR1 could bind to these potential sites. However, the addition of estrogen only resulted in increased binding of ESR1 to the fourth binding site on the MAST4 promoter region, which is located about 675 nt upstream of the MAST4 transcription start site (Fig. 3D). Estrogen could not stimulate ESR1 binding to other potential binding sites on the MAST4 promoter (Supplemental Fig. 3C). Lastly, the results showed that MAST4 expression was lower and PI3K-Akt-mTOR pathway activity was higher in ESR1-KD MM cells than in CTR-KD cells (Fig. 3E).
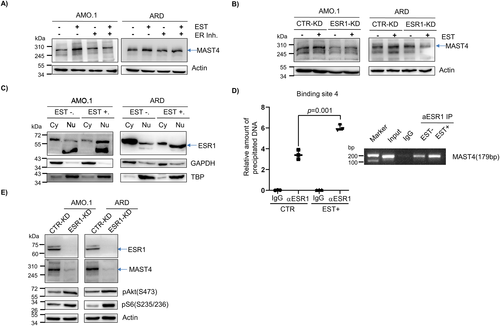
In summary, our data showed that after priming with estrogen, ESR1 translocated into the nucleus and functioned as a transcription factor in upregulating MAST4 expression.
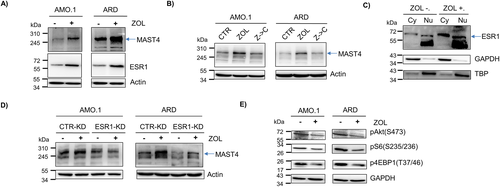
ZOL promotes MAST4 expression in myeloma cells
Bisphosphonates, such as zoledronic acid (ZOL), have been widely used in clinics for MMBD prevention and treatment. Previous studies suggested that bisphosphonates mainly targeted OC.(2) In our study, we found that ZOL treatment upregulated both MAST4 and ESR1 expressions in MM cells (Fig. 4A). The effect of ZOL on MAST4 was reversible: removing ZOL from the cell culture medium retained MAST4 expression at an ordinary level (Fig. 4B). Cell fractionation showed that ZOL treatment not only upregulated ESR1 expression but also caused more nuclear translocation of ESR1 (Fig. 4C). In ESR1-KD MM cells, the addition of ZOL could not stimulate MAST4 overexpression (Fig. 4D). This finding indicated that upregulation of MAST4 by ZOL was mediated by ESR1. Lastly, in agreement with our mechanistic model, MAST4 downstream PI3K-Akt-mTOR pathway was inhibited in MM cells after ZOL treatment (Fig. 4E). Overall, our data suggested that ZOL promoted MAST4 expression by upregulating ESR1. These findings indicated an alternative mechanism of ZOL function in MMBD treatment.
MAST4 regulates the expression of cytokines associated with OC activation in myeloma cells
The above findings suggested that estrogen cell signaling regulated MMBD through MAST4, and MAST4 regulated the PI3K-Akt-mTOR pathway. To further elucidate MAST4 downstream events in MMBD, we examined a panel of OC regulatory cytokine expression in CTR-KD versus MAST4-KD MM cells by qPCR at the mRNA level. Our data suggested that CCL2/3/4 were upregulated in MAST4-KD MM cells (Fig. 5A). Previously, CCL2,(32) CCL3,33) and CCL4(34) had each been suggested to regulate OC activity. Consistent with the results of the qPCR array, the overexpression of the three cytokines in MAST4-KD and ESR1-KD cells at the protein level was further confirmed by ELISA assay (Fig. 5B). ELISA assay also showed that estrogen or ZOL inhibited CCL2/3/4 expression in CTR-KD MM cells (Fig. 5C). This inhibitory effect, however, was attenuated in MAST4-KD and ESR1-KD MM cells: There was no significant decrease in the expression of CCL2/3/4 in MAST4-KD and ESR1-KD MM cells in the presence of estrogen or ZOL (Supplemental Fig. S4A–C). These data indicated that ESR1 and MAST4 were required for the inhibitory effect of estrogen or ZOL on CCL2/3/4 expression in MM cells. In addition, blocking the PI3K-Akt-mTOR pathway, downstream of MAST4, with LY294002 (PI3K inhibitor) or rapamycin (mTOR inhibitor) also inhibited CCL2/3/4 expression (Fig. 5D). These data suggested that cytokines CCL2/3/4 were downstream of the ESR1 → MAST4 → PI3K-Akt-mTOR signaling transduction axis. Overall, these findings indicated that ESR1 and MAST4 were upstream of those OC regulatory cytokines.
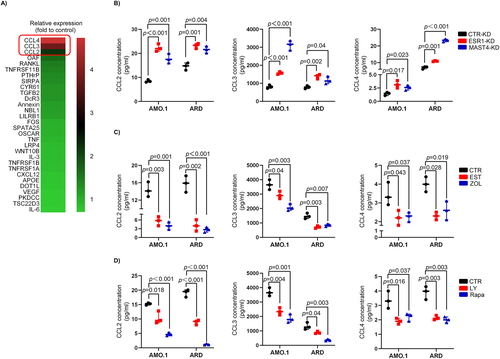
Expressions of MAST4 and ESR1 regulate OC activity in vitro via CCL2/3/4 cytokines in myeloma cells
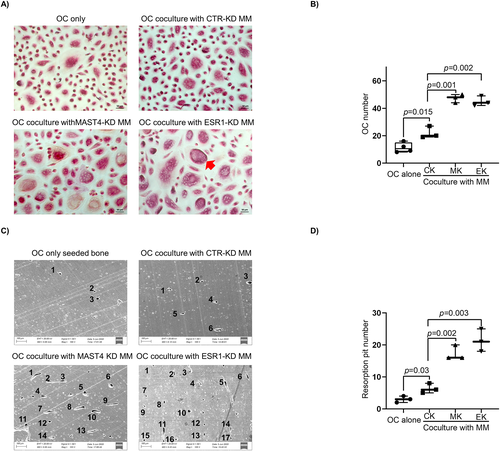
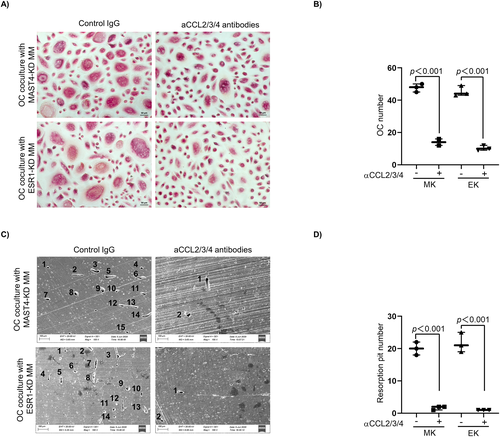
We next investigated how MAST4 or ESR1 expression in MM cells affected OC activity under the coculture model as described in Materials and Methods. As shown in the TRAP assay, more human monocytes-derived pre-OCs cocultured with MAST4-KD or ESR1-KD MM cells differentiated into mature OCs than those pre-OCs cocultured with CTR-KD MM cells (Fig. 6A, B). An in vitro bone resorption assay showed that more resorption pits by OCs on bone slices were formed when pre-OCs were cocultured with MAST4-KD or ESR1-KD MM cells, indicating that these pre-OCs had enhanced osteolytic activity (Fig. 6C, D). We repeated the same experiments using murine MM cells and murine BM-derived pre-OCs and found similar results (Supplemental Fig. S5A, B). More importantly, the addition of mixture neutralizing antibodies of CCL2/3/4 attenuated MAST4-KD- or ESR1-KD-induced OC activation. A fewer mature OCs (Fig. 7A) and lower osteolytic activity (Fig. 7B) were observed in the presence of CCL2/3/4 neutralizing antibodies. These results provided in vitro evidence that ESR1 and MAST4 negatively regulated OC activity through CCL2/3/4 cytokines.
Expressions of MAST4 and ESR1 regulate myeloma bone disease in vivo
To investigate MAST4 function in MMBD in vivo, we intravenously inoculated human ARD-CTR-KD or ARD-MAST4-KD MM cells in immune-deficient mice. The mice MMBDs were examined by micro-CT. We found that healthy immune-deficient mice without tumor cell inoculation had intact scapula under micro-CT imaging (Fig. 8A). MAST4-KD MM cell–inoculated mice had severer lesions on the bones than CTR-KD MM cell–inoculated mice (imaging results of all mice are shown in Supplemental Fig. S6A). We quantified mice MMBD based on the ratio of the BL area to the total scapula area (Supplemental Fig. S6B). Our results showed that MAST4-KD MM cell–inoculated mice had significantly severer MMBD compared with CTR-KD mice (Fig. 8B). Meanwhile, we examined the circulating M protein in mice and found that CTR-KD- and MAST4-KD-inoculated mice had a similar tumor burden (Supplemental Fig. S6C). Therefore, the difference in MMBD between the two groups was not caused by the tumor burden variance. A TRAP assay using mice hind leg bone biopsies showed that MAST4-KD-inoculated mice had more OCs (Fig. 8C). The differential MMBD was not limited to scapula. For example, MAST4-KD mice also had severer BL on skull bones than CTR-KD mice (Supplemental Fig. S6D). We also repeated the experiment in a murine 5T MM model and found the similar results (Supplemental Fig. S6E). In addition to MAST4 knockdown, ESR1-KD MM cell inoculation also resulted in severer MMBD in immune-deficient mice (Supplemental Fig. S6F, G). Similarly, a high number of OCs were detected in the bone from ESR1-KD MM-inoculated mice (Supplemental Fig. S6H). Furthermore, we carried out estradiol treatment in vivo in MM-bearing mice. Our result revealed that control mice without estradiol supplement had significantly severer MMBD than estradiol-treated mice, suggesting that estradiol treatment reduced the severity of MMBD in vivo (Fig. 8D; Supplemental Fig. S6I, J).
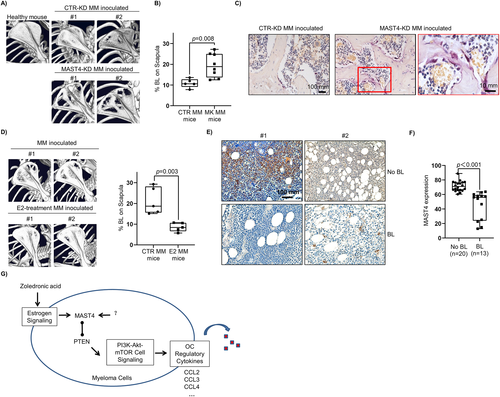
Lastly, we performed a tissue array to investigate MAST4 expression and MMBD in the patients. In this retrospective study, BM biopsies from 33 newly diagnosed MM patients were immunohistochemically examined for MAST4 expression. The patients' characteristics are summarized in Supplemental Table S2. The results revealed that patients with BL had lower MAST4 expression compared with those without BL (Fig. 8E; for more details of IHC data quantification, see Supplemental Fig. S7). These findings suggested that MAST4 or ESR1 expression inhibited MMBD in vivo.
Overall, our findings showed that estrogen cell signaling played a protective role in MMBD, and MAST4, as an estrogen-responsive gene, was a key regulator (Fig. 7G). The activation of estrogen cell signaling promoted MAST4 transcription and expression. MAST4 interacted with PTEN and inhibited PTEN activity, therefore affecting PTEN's inhibition of the PI3K-Akt pathway. MAST4low MM cells had a more activated PI3K-Akt-mTOR pathway, increased expression of OC regulatory cytokines CCL2/3/4, and thus increased OCs activation in the MM tumor microenvironment.
Discussion
It has long been recognized that estrogen cell signaling is essential for bone metabolism in both males and females.(35-37) In this study, we offered new insights into the role of estrogen cell signaling in MMBD and identified MAST4 as a key regulator. The biological effects of estrogen were mainly mediated by its receptors, ESR1 and ESR2.(38) ESR1 and ESR2 are members of the nuclear receptor superfamily of hormone receptors and function as transcription factors.(39) Wang and colleagues reported ESR1 expression in MM cells and demonstrated that ESR1 regulated the MM cell cycle.(40) However, Otsuki and colleagues reported that ESR2 was dominantly expressed in MM cell lines and might be used for targeted therapy.(41) In our analyses using MM patients' microarray data sets, the expression of ESR1, but not ESR2, correlated with MMBD. Importantly, ESR1 and MAST4 were similar in expression-associated pathway enrichments, indicating these two factors might belong to the same regulatory pathway. The binding of ESR1 on the MAST4 promoter, identified by ChIP assay, elucidated the regulatory hierarchy that estrogen cell signaling regulated MAST4 expression in MM cells. To identify the MAST4 downstream cell signaling that led to MMBD regulation, computational pathway exploration was applied.(5) The model suggested that functional cross-talk between MAST4 and PTEN played a key role in the interaction of MAST4 with the PI3K-Akt-mTOR pathway. We employed multiple methods to understand this cross-talk and demonstrated that MAST4 and PTEN had protein–protein interaction in MM cells. We also showed that MAST4-KD cells had decreased PTEN activity. These findings provided a molecular basis for understanding downstream PI3K-Akt-mTOR pathway activation and OC regulatory cytokine expression. We also provided evidence that MAST4 was required for estrogen cell signaling–initiated MMBD regulation. In MAST4-KD cells, estrogen priming could not inhibit downstream cytokine expression. Therefore, we considered MAST4 as a key mediator in this single-threaded signaling transduction. However, with all the above positive findings, the molecular basis of how MAST4 regulates PTEN activity remains to be determined. We had no evidence whether MAST4, a kinase, could directly phosphorylate PTEN at specific amino acid residuals. It is also possible that other factors were involved in the functional cross-talk between MAST4 and PTEN. This question should be addressed in future studies.
Of interest, estrogen cell signaling, as well as MAST4, was involved in bisphosphonates' mechanism of action in MMBD. Estrogenic agents and bisphosphonates are antiresorptive agents but have different cellular targets. In general, estrogenic agents promote OB, whereas bisphosphonates inhibit OC.(42) Tsubaki and colleagues reported that bisphosphonates inhibit CCL3 expression in MM cells.(43) The mechanistic cross-talk between bisphosphonates and estrogen cell signaling in MM has not been previously reported. The findings of the current study show that exposure to ZOL upregulated ESR1 expression in MM without affecting survival or proliferation of MM cells. Modulation of MAST4 expression by ZOL was mediated by ESR1. Moreover, ZOL inhibited expression of ESR1-MAST4 downstream OC regulatory cytokines in MM cells. Notably, this inhibition was abrogated in MAST4-KD or ESR1-KD MM cells. These findings provide new information on mechanisms of action of bisphosphonates in MMBD treatment. In addition to directly targeting OC, bisphosphonate ZOL may also inhibit MM cells expressing OC regulatory cytokines, thus indirectly suppressing OC. Estrogen cell signaling in MM cells was required for this regulation.
Although either estrogenic or anti-estrogenic therapies have been reported in MM treatment,(44) mainly at the preclinical stage, the majority of the studies report that anti-estrogenic agents have anti-MM cytotoxicity. Treon and colleagues explored estrogen agonists and antagonists and reported that estrogen antagonists induced MM cell apoptosis, whereas estrogen did not alter MM cell proliferation.(45) Chuanhan and colleagues reported that an estrogen derivative 2-methoxyestradiol (2ME) exhibited cytotoxicity toward MM cells.(46) However, studies report that antiproliferative and apoptotic activities of 2ME are independent of ESR1 and ESR2.(47) In addition, several studies report that the tamoxifen family of drugs, known as estrogen antagonists, have anti-MM activity.(48, 49) However, results of clinical trials on tamoxifen in MM patients returned disappointing.(50, 51) A 2ME clinical trial reported negative results.(52) Notably, all these studies mainly focused on the anti-MM effect of the drug. The status of MMBD in these trials was not reported. The findings of the current study show that estrogenic therapy may repress OC activity in the MM microenvironment, thus alleviating MMBD. Only a few studies have explored estrogenic therapy in MMBD. Li and colleagues conducted an in vitro study showing that 17β-estradiol reversed MM-conferred OB inhibition.(53) Here we proposed that estrogenic therapy might be beneficial for management of MMBD with a combination of anti-MM therapies for tumor treatment. However, the efficacy of such a treatment strategy should be explored further.
Disclosures
All authors state that they have no conflicts of interest.
Acknowledgments
The authors thank the tissue bank of the Department of Hematology, West China Hospital, Sichuan University, for patient samples. The authors thank Bo Su in Core Facilities of West China Hospital, Sichuan University, for critical suggestions of image data acquisition and analysis. We also appreciate critical suggestions from Dr Qing Yi of Methodist Hospital, Houston, TX, USA.
This work was supported by grants to YZ from the National Science Foundation of China (no. 81870157 and no. 82070219); grants to JH from the National Natural Science Foundation of China (no. 81800207) and the Health Commission of Sichuan Province (no. 18PJ357); and grants to TN from 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (no. ZYJC21007) and Translational Research Grant of NCRCH (no. 2021WWB03).
Authors’ roles
Y.Z. initiated this work, designed the study and wrote the manuscript. Y.C. and F.W. performed majority of the experiments and helped manuscript preparation. D.Z., J.H., Y.Y., J.X., Y.G., H.D., Y.Q. performed the experiments; W.Z., W.L., L.P., L.Z., Z.L., T.N., and T.L. provide samples and critical suggestions for this study.
Author Contributions
Yuhuan Zheng: Funding acquisition, Methodology, Supervision, Validation, Writing-original draft, Writing-review & editing.
Open Research
Data Availability Statement
Data available on request due to privacy/ethical restrictions




