Intracellular Accumulation of Advanced Glycation End Products Induces Osteoblast Apoptosis Via Endoplasmic Reticulum Stress
ABSTRACT
Osteoporosis is an aging-associated disease that is attributed to excessive osteoblast apoptosis. It is known that the accumulation of advanced glycation end products (AGEs) in bone extracellular matrix deteriorates osteoblast functions. However, little is known about the interaction between intracellular AGE accumulation and the induction of osteoblast apoptosis. In this study, we investigated the effect of intracellular AGE accumulation on osteoblast apoptosis in vitro and in vivo. In vitro, murine osteoblastic MC3T3-E1 cells were treated with glycolaldehyde (GA), an AGE precursor. GA-induced intracellular AGE accumulation progressed in time- and dose-dependent manners, followed by apoptosis induction. Intracellular AGE formation also activated endoplasmic reticulum (ER) stress-related proteins (such as glucose-regulated protein 78, inositol-requiring protein-1α (IRE1α), and c-Jun N-terminal kinase) and induced apoptosis. In agreement, treatment with the ER stress inhibitor 4-phenylbutyric acid and knocking down IRE1α expression ameliorated osteoblast apoptosis. Furthermore, the ratio between AGE- and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL)-positive osteoblasts in human vertebral bodies was significantly higher in an elderly group than in a younger group. A positive linear correlation between the ratio of AGE-positive and TUNEL-positive osteoblasts (r = 0.72) was also observed. Collectively, these results indicate that AGEs accumulated in osteoblasts with age and that intracellular AGE accumulation induces apoptosis via ER stress. These findings offer new insight into the mechanisms of osteoblast apoptosis and age-related osteoporosis. © 2020 American Society for Bone and Mineral Research.
Introduction
Bone remodeling is a lifelong process that depends on a correct balance between bone resorption by osteoclasts and bone formation by osteoblasts. In contrast, remodeling imbalance, stemming from a decrease in osteoblasts or an increase in osteoclasts, results in osteoporosis. Osteoblasts promote bone formation by synthesizing new bone collagen matrix and mineralization.(1) Under physiological conditions, osteoblast apoptosis is necessary to maintain bone mass.(2) However, excessive osteoblast apoptosis is associated with reduced osteoblast numbers and inhibited bone formation.(3) Moreover, decreased osteoblast numbers contribute to age-related bone loss.(4) Therefore, excessive osteoblast apoptosis is a crucial pathogenetic mechanism underlying age-related osteoporosis.
Advanced glycation end products (AGEs) are generated from the non-enzymatic reaction of glucose, ketose, or carbonyl compounds (such as glyoxal [GO], methylglyoxal [MG], or glycolaldehyde [GA]) with proteins. AGEs are known to accumulate in the bone matrix with age.(5, 6) However, few reports have shown whether intracellular AGEs accumulate in osteoblasts with age. AGEs are thought to deteriorate cell functions via two mechanisms: (i) interactions between extracellular AGEs and cell-surface receptors of AGEs, especially the receptor for advanced glycation end products (RAGE), and (ii) disturbed homeostasis caused by AGE modification of intracellular proteins. Extracellular AGEs can induce harmful effects on bone formation by inducing osteoblast apoptosis via AGE–RAGE signaling.(7, 8) In contrast, little is known regarding the association between intracellular AGEs and osteoblast apoptosis.
Mounting evidence has suggested that endoplasmic reticulum (ER) stress induces osteoblast apoptosis.(9, 10) In eukaryotic cells, the ER plays an important role in protein biosynthesis, folding, assembly, and post-translational modifications.(11) ER stress is a condition in which the functions of the ER are disrupted by various stimuli. Under the ER stress condition, misfolded or unfolded proteins accumulate in the ER lumen, which modulates various cellular activities. To relieve this cellular stress, cells activate ER-specific adaptation response, ie, the unfolded-protein response (UPR). Initially, the UPR can protect cells and maintain protein homeostasis under mild stress.(12) When severe ER stress persists, the UPR is insufficient for protecting cells and apoptosis is induced.(13) Recent reports have shown that extracellular AGEs induce apoptosis via ER stress in diverse cell types.(14, 15) However, little is known regarding the effects of intracellular AGEs on the activation of ER stress in osteoblasts.
In this study, we investigated the effect of intracellular AGE accumulation on ER stress-induced apoptosis in osteoblastic cells. We also investigated associations between the AGE accumulation and apoptosis induction in osteoblasts of human bone samples, due to the influence of aging.
Materials and Methods
Cell culture
MC3T3-E1 cells, a clonal osteoblastic cell line isolated from calvariae of a late-stage mouse embryo, were purchased from RIKEN Cell Bank (Tsukuba, Japan). The cells were cultured in α-minimum essential medium (α-MEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. The cells were maintained in a humidified incubator with 5% CO2 at 37°C.
Measurement of AGEs by liquid chromatography–tandem mass spectrometry (LC–MS/MS)
MC3T3-E1 cells were seeded in a 6-well plate (4 × 104 cells/well) and incubated for 3 days before they became confluent. Subsequently, the cells were treated with GA (0 to 500 μM) and/or aminoguanidine (AMG) (0 to 20 mM) for up to 24 hours, after which they were collected, washed with cold PBS, and lysed in 0.1% Triton X. Cellular debris was removed from each sample by centrifugation at 10,000g for 5 minutes at 4°C, and then the supernatants were collected. AGE contents were measured by LC–MS/MS as described previously.(16) Briefly, protein samples (100 μg) were reduced with NaBH4 in 200 mM sodium borate buffer (pH 9.1) at room temperature for 4 hours. The protein samples were precipitated with an equal volume of 20% trichloroacetic acid. After adding standard [13C6] lysine (Cambridge Isotope Laboratories Inc., Tewksbury, MA, USA) and [2H2] (carboxymethyl)lysine (CML) (PolyPeptide Laboratories, Strasbourg, France) to the pellets, the protein samples were hydrolyzed with 1 mL of 6 N HCl at 100°C for 18 hours. The dried samples were added to 1 mL of distilled water and passed through a strata-X column (Phenomenex, Torrance, CA, USA), as described previously.(16) The pooled elution fractions were dried and resuspended in 1 mL of 20% acetonitrile containing 0.1% formic acid. The samples were subjected to an LC–MS/MS assay using a TSQ Quantiva triple-stage quadrupole mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). LC was conducted with a ZIC-HILIC column (150 × 2.1 mm, 5 μm; Merck Millipore, Billerica, MA, USA). The mobile phase was collected using solvent A (distilled water containing 0.1% formic acid) and solvent B (acetonitrile containing 0.1% formic acid). The flow rate was 0.2 mL/min, and the column temperature was maintained at 40°C. The retention times for CML and lysine were approximately 12 and 13 minutes. CML, lysine, and the standard were detected by electrospray ionization and positive-ion, mass spectrometric-multiple reaction monitoring.
Cell-proliferation assay
MC3T3-E1 cells were seeded in a 96-well plate (3000 cells/well). Then, the cells were incubated for 24 hours in medium containing GA (0 to 1000 μM) and/or AMG (0 to 20 mM). Next, the medium was changed to Hank's balanced salt solution, and cell viability was determined using Cell Counting Kit-8 (Doujin Chemical, Kumamoto, Japan), according to the manufacturer's protocol. Optical density values were measured using the SpectraMax i3x system (Molecular Devices, San Jose, CA, USA).
Fluorimetric caspase-3 assay
Caspase-3 activities were measured using the Amplite Fluorimetric Caspase 3/7 Assay Kit (AAT Bioquest, Inc., Sunnyvale, CA, USA), according to the manufacturer's protocol. Briefly, MC3T3-E1 cells were seeded on a 96-well plate at a density of 1 × 105 cells/well and cultured overnight. GA (500 μM) and/or AMG (10 mM) were then added to the cells. After a 24-hour incubation, caspase-3 activity was measured using SpectraMax i3x system.
Reverse transcriptase-PCR (RT-PCR) and real-time quantitative PCR
Total RNA was isolated using RNAiso Plus (Takara Bio, Inc., Shiga, Japan). RNA was reverse transcribed using a PrimeScript RT Reagent Kit (Takara Bio, Inc.). Real-time quantitative PCR was performed using TaqMan Polymerase with SYBR Green Fluorescence (Takara Bio, Inc.) and an ABI PRISM 7300 Sequence Detector (Applied Biosystems, Foster City, CA, USA). The following primers were used: for B-cell lymphoma-2 (Bcl-2), forward 5′-ATGCCTTTGTGGAACTATATGGC-3′, reverse 5′-GGTATGCACCCAGAGTGATGC-3′; for Bcl-2-associated X (Bax), forward 5′-TGAAGACAGGGGCCTTTTTG-3′, reverse 5′-AATTCGCCGGAGACACTCG-3′; for C/EBP homologous protein (CHOP), forward 5′-GACGCTTCACTACTCTTGACCCTGCG-3′, reverse 5′-GGATGTGCGT-GTGACCTCTGT-3′; for β-actin, forward 5′-TTTCCAGCCTTCCTTCTTGG-3′, reverse 5′-TGGCATAGAGGTCTTTACGGATG-3′. RT-PCR was performed using a TaKaRa Ex Taq Kit (Takara Bio Inc.), and electrophoresis was performed using a Mupid electrophoresis system (Mupid, Tokyo, Japan).
Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) staining
Apoptosis was measured by performing TUNEL assays to detect DNA fragmentation. TUNEL staining was performed using the Apoptosis In Situ Detection Kit (Wako, Osaka, Japan) according to the manufacturer's instructions. Briefly, the samples were incubated with TdT reaction solution for 60 minutes at 37°C. After the reaction of TdT, the samples were incubated with a horseradish peroxidase (HRP)-conjugated antibody for 10 minutes at 37°C. Immunoreactions were visualized using HistoGreen (Linaris, Wertheim, Germany).
Immunostaining and double staining
The monoclonal anti-CML antibody(17) and the anti-cleaved caspase-3 antibody (Cell Signaling Technology, Beverly, MA, USA) were used as primary antibodies for in vitro immunostaining experiments. MC3T3-E1 cells were washed with cold PBS and fixed with 10% neutral-buffered formalin. After incubation with the primary antibodies, the samples were incubated with secondary HRP-labeled goat anti-mouse or rabbit antibodies (Nichirei, Tokyo, Japan). Immunoreactions were visualized using a diaminobenzidine (DAB) substrate system (Nichirei) or HistoGreen. Double immunostaining for CML and cleaved caspase-3 was also performed. First, the samples were reacted with an anti-CML antibody, which was visualized with DAB. After the samples were washed with citrate buffer (pH 2.2), the samples were reacted with anti-cleaved caspase-3 antibody and visualized with HistoGreen.
The monoclonal anti-CML antibody(18) was also used as a primary antibody for in vivo immunostaining. After reaction with the anti-CML antibody, the sections were incubated with a secondary HRP-labeled goat anti-mouse antibody. Immunoreactions were visualized with DAB. Double staining with the anti-CML antibody and anti-osteocalcin antibody (Abcam, Cambridge, UK) was performed. The sections were reacted with the anti-CML antibody, which were visualized using DAB. After the samples were washed with citrate buffer (pH 2.2), they were incubated with anti-osteocalcin antibody or TDT reaction solution and visualized using HistoGreen. Double staining with the anti-osteocalcin antibody and TUNEL staining was also performed. The sections were reacted with the anti-osteocalcin antibody, which were visualized using DAB. After the samples were washed with citrate buffer (pH 2.2), they were incubated with TDT reaction solution and visualized using HistoGreen.
Western blotting
For Western blotting analysis, cells were lysed in ice-cold lysis buffer (50 mM Tris pH 8.0, 1 mM EDTA, 150 mM NaCl, 1% NP-40) with a phosphatase inhibitor cocktail (R&D Systems, Minneapolis, MN, USA) and a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). Equivalent amounts of protein lysates were loaded on an 8% to 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel and transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA, USA). PVDF membranes were blocked with 1% nonfat milk in Tris-buffered saline with 0.1% of Tween 20 for 1 hour at room temperature and incubated overnight at 4°C with primary antibodies against protein kinase RNA-like ER kinase (PERK), glucose-regulated protein 78 (GRP78), eukaryotic initiation factor 2 alpha (eIF2α), phosopho-eIF2α, CHOP, inositol-requiring protein-1α (IRE1α), c-Jun N-terminal kinase (JNK), phospho-JNK, p38-MAPK, phospho-p38, Bcl-2, Bax, cleaved caspase-3, cleaved caspase-8, caspase-9 (Cell Signaling Technology), phospho-PERK (BioLegend, San Diego, CA, USA), phospho-IRE1α (Abcam), pro-caspase-3 (PeproTech, Rocky Hill, NJ, USA), CML (6D12), and β-actin (Santa Cruz Biotechnology, Dallas, TX, USA). HRP-conjugated goat anti-mouse or anti-rabbit IgG (Invitrogen, Camarillo, CA, USA) was used as the secondary antibody. Immunoreactive bands were visualized using the Pierce Western Blotting Substrate Plus Kit (Thermo Fisher Scientific, Rockford, IL, USA) and an ImageQuant LAS-4000 mini (Fuji Film, Tokyo, Japan).
Small-interfering RNA (siRNA) experiments
MC3T3-E1 cells were transfected with siRNA against mouse IRE1α and CHOP (Santa Cruz Biotechnology), using Lipofectamine RNAi MAX (Thermo Fisher Scientific). Cells were transfected with control siRNA (Santa Cruz Biotechnology) as a negative control.
Immunoprecipitation
For immunoprecipitation assays, cells were lysed in an ice-cold lysis buffer with a protease inhibitor cocktail. Each protein lysate (1 mg) was incubated overnight at 4°C with 1 μg anti-GRP78 antibody (Abcam), anti-IRE1α antibody (1:50), or without the antibodies. Immunocomplexes were precipitated with protein G Dynabeads (Thermo Fisher Scientific) for 10 minutes, washed, and eluted by boiling for 5 minutes at 94°C with 1X SDS sample buffer. Each sample was tested with Western blotting against antibodies with GRP78, IRE1α, and CML.
Preparation of human vertebral bodies
Human vertebrae were acquired from cadavers with pathological anatomy. Donors were excluded if they had a medical history of diabetes mellitus or chronic kidney disease. In total, there were 18 cadaver donors: 5 in the younger group (2 males and 3 females, average age 19 years, ranging from 11 to 27 years) and 13 in the elder group (7 males and 6 females, average age 77.5 years, ranging from 71 to 86 years). Specimens were decalcified, delipidated, fixed with paraformaldehyde, and then embedded in paraffin. Paraffin-embedded bone tissues were sliced into 3-μm-thick sections for immunostaining. This study was conducted according to the Declaration of Helsinki. The Institutional Review Board at Kumamoto University approved this retrospective study (approval number 2224).
Statistical analysis
All data are representative of at least three independent experiments. The data are expressed as the mean ± standard deviation (SD) or median and interquartile range with minimal and maximum values indicated. Differences between groups were examined for statistical significance using Student's t test, the Mann–Whitney U test, or one-way analysis of variance with Bonferroni's test. Any p values <0.05 were considered statistically significant. Statistical analyses were conducted with SAS, University Edition (SAS Institute Inc., Cary, NC, USA).
Results
Effect of GA on AGE accumulation and apoptosis in osteoblastic cells
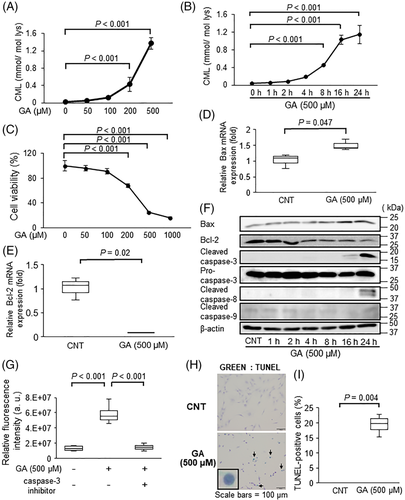
CML is well known as an abundant AGE and a biomarker of long-term protein modification.(19, 20) In addition, Hein and colleagues reported that the serum levels of CML were higher in osteoporotic patients than in healthy subjects.(21) Therefore, we focused on quantifying CML in osteoblasts among several AGEs. Because estimates of the physiological concentration of GA, a reactive hydroxyaldehyde known as a famous CML precursor,(22) have ranged from 1 to 1000 μM,(23, 24) we first measured the effect of the indicated concentration of GA (50 to 1000 μM) on CML accumulation in MC3T3-E1 cells using LC–MS/MS. CML contents significantly increased in MC3T3-E1 cells treated with GA in a dose- and time-dependent manner (Fig. 1A, B), reaching a plateau 16 hours after the GA treatment began (Fig. 1B). GA also induced CML accumulation in both differentiated MC3T3-E1 and human osteoblastic hFOB1.19 cells (Supplemental Figs. S1A and S2A, B), indicating that GA markedly induced CML accumulation in osteoblastic cells. In MC3T3-E1 and hFOB 1.19 cells and at concentrations of at least 200 μM, GA significantly inhibited cell proliferation (Fig. 1C; Supplemental Fig. S2C). In both MC3T3-E1 and hFOB1.19 cells treated with GA, the expression of Bcl-2, an anti-apoptotic protein, decreased, whereas the expression of Bax, a pro-apoptotic protein, increased (Fig. 1D–F; Supplemental Figs. S1B and S2D). Additionally, in MC3T3-E1 cells, caspase-9, the mitochondria disruption-induced apoptotic protein, was not activated by the GA treatment, whereas GA slightly activated caspase-8, the death receptor-related apoptotic protein (Fig. 1F). The number of cells positive for cleaved caspase-3 expression and TUNEL staining were also significantly enhanced by the GA treatment in MC3T3-E1 and hFOB 1.19 cells (Fig. 1F–I; Supplemental Figs. S1B, S2D, and S2E), indicating that GA induced apoptosis in osteoblastic cells.
Interaction between intracellular AGE accumulation and apoptosis in GA-treated osteoblastic cells
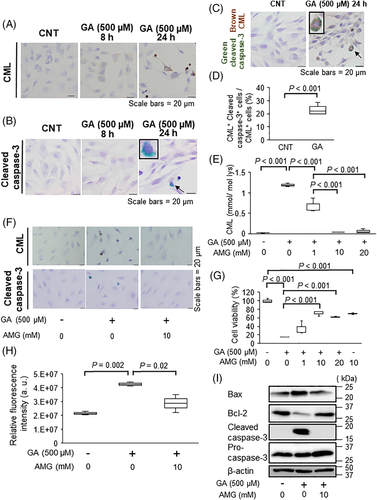
To determine whether GA-derived AGEs influence apoptosis in MC3T3-E1 cells, AGE accumulation was measured by immunostaining analysis using an anti-CML antibody. We found that CML accumulation increased in a time-dependent manner (Fig. 2A) and that caspase-3 activation occurred after CML accumulation (Fig. 2B). Co-localization of cells with CML and cleaved capase-3 was observed by double immunostaining (Fig. 2C). In addition, 28.1% of CML-positive cells expressed cleaved caspase-3 (Fig. 2D), suggesting the possibility that intracellular CML accumulation directly induces caspase-3 activation. Therefore, we next measured the effects of aminoguanidine (AMG), a well-known inhibitor of AGE formation, on GA-induced CML formation and apoptosis in MC3T3-E1 cells. As shown in Fig. 2E, F, AMG significantly inhibited CML formation in a dose-dependent manner. In addition, AMG significantly improved cell viability (Fig. 2G) by inducing Bcl-2 expression and suppressing Bax expression and caspase-3 activation (Fig. 2F, H, I). These findings suggest that GA-promoted intracellular CML accumulation was strongly involved in osteoblastic cell apoptosis.
Involvement of ER stress with GA-induced AGE accumulation within the ER of osteoblastic cells
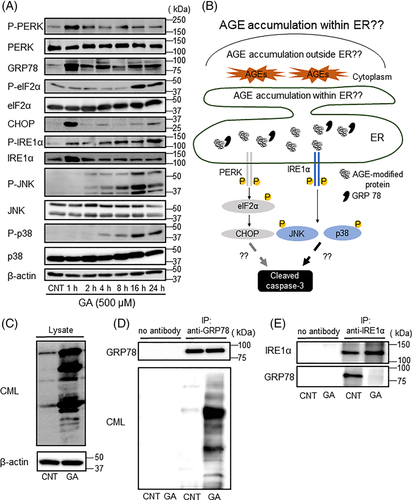
Because ER stress plays important roles in osteoblast apoptosis and osteoporosis,(15, 25) we hypothesized that intracellular AGEs induced osteoblast apoptosis via ER stress. Therefore, we examined the effect of GA on ER stress signaling in MC3T3-E1 cells. GA stimulation increased the expression of GRP78, a chaperone protein, and phosphorylated PERK (Fig. 3A). Phosphorylated eIF2α was also increased by GA at 16 and 24 hours, whereas CHOP expression was increased at 1 and 24 hours (Fig. 3A). In addition, GA stimulation also enhanced IRE1α, JNK, and p38 phosphorylation. Similar results were observed in differentiated MC3T3-E1 and hFOB 1.19 cells (Supplemental Figs. S1C and S2F). These results suggest that both PERK and IRE1α signaling contributed to CML-related apoptosis in osteoblastic cells (Fig. 3B). However, it remains unclear whether the accumulation of CML-modified proteins within the ER lumen activated UPR signaling (Fig. 3B). It is known that GRP78 is located in the ER lumen and binds to misfolded proteins.(26) To assess whether GA-induced AGEs accumulate within the ER, the accumulation of CML-modified proteins bound to GRP78 was examined. As shown in Fig. 3C, CML-modified proteins were observed in MC3T3-E1 cells after GA treatment. Under this condition, several proteins in the cell lysates were co-immunoprecipitated using an anti-GRP78 antibody. These immunoprecipitated proteins were detected with an anti-CML antibody, suggesting that GA-induced CML-modified proteins bound to GRP78 (Fig. 3D). In contrast, GRP78 was remarkably decreased in immunoprecipitated protein with anti-IRE1α antibody under GA treatment condition (Fig. 3E), suggesting that GRP78 binding to IRE1α in the ER was reduced by GA-induced accumulation of CML-modified proteins. Furthermore, CML-modified proteins were detected within the ER in GA-treated MC3T3-E1 cells by immunoelectron microscopy analysis (Supplemental Fig. S3). These findings indicate that CML-modified proteins in the ER affected UPR activation by interfering with GRP78-IRE1α binding.
Effect of IRE1α knockdown on GA-induced apoptosis in osteoblastic cells
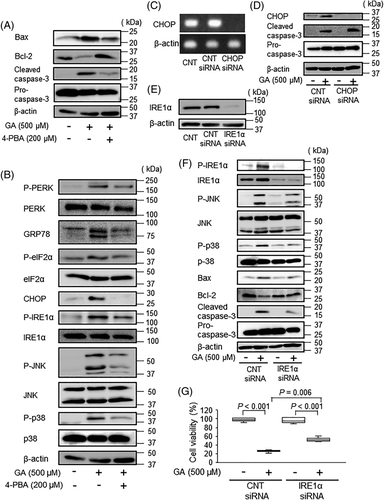
To determine whether ER stress contributes to intracellular AGE-induced apoptosis, we measured the effects of 4-phenylbutyric acid (4-PBA), an inhibitor of ER stress, on osteoblastic cell apoptosis. Our results showed that 4-PBA inhibited GA-induced Bcl-2 downregulation, Bax upregulation, and caspase-3 and capase-8 activation (Fig. 4A; Supplemental Fig. S4). 4-PBA also reduced GA-induced GRP78 and CHOP expression, as well as GA-induced PERK, eIF2α, IRE1α, JNK, and p38 phosphorylation (Fig. 4B), indicating that 4-PBA suppressed GA-induced osteoblast apoptosis related to ER stress signaling. Therefore, the effects of CHOP siRNA and IRE1α siRNA on caspase-3 activation were examined to determine whether PERK or IRE1α signaling was associated with GA-induced apoptosis. CHOP knockdown did not affect GA-induced caspase-3 activation, when compared with that in control siRNA transfectants (Fig. 4C, D). In contrast, IRE1α knockdown inhibited GA-induced phosphorylation of IRE1α, JNK, and p38 compared with control siRNA-treated cells (Fig. 4E, F). In addition, IRE1α knockdown also inhibited GA-induced Bcl-2 downregulation, Bax upregulation, and caspase-3 activation (Fig. 4F). Furthermore, IRE1α knockdown significantly improved the cell viability compared with control siRNA-treated cells (Fig. 4G). These findings demonstrated that intracellular AGE accumulation induced osteoblast apoptosis by activating IRE1α-dependent signaling.
Involvement of intracellular AGE accumulation with apoptosis in human samples
The relationship between AGE accumulation and osteoblast apoptosis was analyzed in human vertebral bodies acquired from cadavers with pathological anatomy. To determine whether intracellular AGEs accumulated in osteoblasts with increasing age in humans, CML accumulation in a younger and an elder group was compared by immunostaining analysis using an anti-CML antibody (Fig. 5A). In addition, we identified CML-positive osteoblasts using the double immunostaining with anti-CML antibody and anti-osteocalcin antibody (Fig. 5B). The median percentage of CML-positive osteoblasts was significantly higher in the elder group than in the younger group (28% versus 9%, respectively, p = 0.002; Fig. 5A–C). The ratio of osteoblast apoptosis in the younger and elder groups was compared by TUNEL staining analysis (Fig. 5D). We also identified TUNEL-positive osteoblasts using the double staining with anti-osteocalcin antibody and for TUNEL assay (Fig. 5E). The median percentage of TUNEL-positive osteoblasts was significantly higher in the elder group than in the younger group (9% versus 2%, respectively, p = 0.003; Fig. 5D–F). Similarly, the median percentage of TUNEL-positive osteocytes was significantly higher in the elder group than in the younger group (10% versus 2%, respectively, p = 0.004; Supplemental Fig. S5A, B). Furthermore, the results of statistical analysis demonstrated that the proportions of CML-positive and TUNEL-positive osteoblasts correlated significantly (r = 0.72, p = 0.0008; Fig. 5G). Double CML and TUNEL staining also revealed that CML-positive osteoblasts in the elder group were partially TUNEL-positive (Fig. 5H). Moreover, the median percentage of TUNEL-positive osteoblasts within the population of CML-positive osteoblast was significantly higher in the elder group than in the younger group (16% versus 0.5%, respectively, p = 0.003; Fig. 5H–I). These results suggest that intracellular AGE accumulation is associated with human osteoblast apoptosis.

Discussion
GA is a highly reactive hydroxyaldehyde and is well known to react with proteins to produce AGEs, such as CML and GA-pyridine.(22) The accurate measurement of physiological GA concentrations is difficult because carbonyl compounds such as GO, MG, and GA, in a free form, are highly reactive and have short half-life under physiological conditions.(27, 28) The physiological concentration of GA is controversial and estimates have ranged from 1 to 1000 μM.(23, 24) Therefore, we used a GA concentration of up to 1000 μM as a precursor for AGE in vitro and demonstrated that GA promoted AGE formation in murine osteoblastic MC3T3-E1 cells and human osteoblastic hFOB 1.19 cells. GA also induced apoptosis in both mouse and human osteoblastic cells, whereas GA had no effect on the expression of osteoblast markers (Supplemental Fig. S1D, E). GA also slightly increased the cleaved caspase-8 expression, thus suggesting that death receptor may be partially associated with GA-induced apoptosis. On the other hand, AGE-positive cells were strongly positive for cleaved caspase-3 after incubation with GA. Since AMG is a well-known inhibitor of AGE formation, we determined the effects of AMG on CML formation and apoptosis in GA-treated cells to elucidate the relationship between intracellular AGE accumulation and osteoblast apoptosis. Given that, in mammals, high concentrations of AMG may inhibit cell proliferation by elongating the M-phase of the cell cycle,(29) a slight decrease in cell viability was observed in 10 mM AMG-treated cells when compared with control cells. On the other hand, AMG significantly inhibited AGE accumulation and ameliorated apoptosis in GA-treated cells. These results demonstrate that intracellular AGE accumulation induced osteoblast apoptosis.
We also found that AGE-modified bovine serum albumin (BSA) did not affect cell proliferation or cleaved caspase-3 expression in MC3T3-E1 cells (Supplemental Fig. S6A, B; Supplemental Table S1). Extracellular AGEs have been considered to induce osteoblast apoptosis via AGE–RAGE signaling.(13, 14) AGE–RAGE interactions lead to cellular dysfunction through the generation of reactive oxygen species(30) and the activation of NF-κB, the famous downstream effector of AGE-RAGE signaling.(31) In particular, AGE-induced NF-κB upregulation is known to release the inflammatory cytokines and facilitate cell apoptosis.(31, 32) However, previous evaluations of RAGE expression in osteoblasts were limited to in vitro studies. Surprisingly, the present findings showed that RAGE was highly expressed on macrophages in human vertebral bodies, whereas we did not detect RAGE expression on osteoblasts (Supplemental Fig. S7A). Similarly, RAGE expression on human osteoblastic Saos-2 cells was much lower than that on human monocyte-derived macrophages (Supplemental Fig. S7B). Moreover, GA and AGE-modified BSA had no effect on RAGE expression and NF-κB activation in MC3T3-E1 cells (Supplemental Fig. S7C, D). These results suggest that extracellular AGEs, especially AGE–RAGE interactions, do not affect osteoblast apoptosis.
Several reports have demonstrated the effects of AGEs on the induction of ER stress response in various cell types.(14, 15) However, little is known regarding the associations between AGEs and ER stress responses in osteoblasts. We found that, in MC3T3-E1 cells, high concentrations of AGE-modified BSA did not affect the expression levels of GRP78 and CHOP proteins and the corresponding mRNA (Supplemental Fig. S8A–D). In contrast, the present data demonstrated that GA activated UPR signaling at 1 and 24 hours in osteoblastic cells. In addition, AGEs did not accumulate in osteoblastic cells after incubation with GA for up to 4 hours, whereas AGE contents increased markedly after incubation with GA for 24 hours. These results suggest that GA itself activated UPR signaling at 1 hour, whereas intracellular AGE formation induced ER stress responses at 24 hours. We then investigated whether the accumulation of AGE-modified proteins within the ER lumen was related to activation of the UPR (Fig. 3B). Three ER stress sensors, namely, IRE1α, PERK, and activating transcription factor 6, are known to initiate UPR. GRP78 not only binds to these UPR sensors but also inhibits their activation under non-stressed conditions.(33) Upon misfolded protein accumulation within the ER lumen, GRP78 can dissociate from the UPR sensors and target the misfolded proteins.(26) Our results showed that GA treatment remarkably increased the amount of AGE-modified proteins that bound to GRP78. Moreover, immunoelectron microscope analysis showed that CML-modified proteins were observed within the ER after GA treatment. These results suggest that GA promotes AGE accumulation within the ER in osteoblastic cells. We also demonstrated that GRP78 dissociated from IRE1α after GA exposure. It is known that protein glycations lead to alternations of secondary and tertiary protein structures.(34) In addition, the side chains of amino acids play important roles in the formation of these protein structures. Our previous findings showed that the positively charged side chains of lysine and arginine were converted into negatively charged side chains by AGE modifications.(35) This alternation of ionic charge can lead to protein denaturation. These findings imply that protein denaturation by AGE modifications may contribute to the dissociation between GRP78 and IRE1α, thereby promoting the association of AGE-modified proteins with GRP78, resulting in the activation of UPR signaling.
The interaction between ER stress and osteoblast apoptosis has been investigated previously. The chemical chaperone 4-PBA promotes protein folding and secretion, thereby reducing ER stress and UPR.(36) 4-PBA suppressed AGEs-induced ER stress and apoptosis in osteoblasts (Fig. 4A, B), demonstrating that intracellular AGE formation induces osteoblast apoptosis via ER stress. In addition, 4-PBA slightly inhibited GA-induced caspase-8 activation (Supplemental Fig. S4). Since it has been reported that caspase-8 is activated through the enhancement of ER stress,(37) our results suggest that GA-induced ER stress may be associated with caspase-8 activation. Among UPR sensors, PERK and IRE1α play important roles in ER stress-induced apoptosis.(38) PERK serves as an important apoptotic factor by enhancing eIF2α, ATF4, and CHOP expression.(39) IRE1α can also induce apoptosis by activating the downstream proteins, JNK and p38.(40, 41) Yamabe and colleagues(42) reported that intracellular AGEs induced apoptosis in rat chondrocytes by activating CHOP, the downstream effector of PERK signaling. However, the present findings showed that CHOP knockdown did not inhibit apoptosis induction, whereas IRE1α knockdown improved osteoblast apoptosis by inhibiting JNK and p38 phosphorylation. According to Chen and colleagues,(43) the modulation of IRE1α signaling ameliorates osteoporotic osteoblast apoptosis and targeting IRE1α signaling can potentially be applied for osteoporotic therapy. These findings suggest that intracellular AGE-induced IRE1α activation may be one of the mechanisms driving osteoblast apoptosis in age-related bone loss.
Our previous results demonstrated that AGEs accumulated in bone matrix with age,(9) whereas few studies have shown that AGEs accumulate in osteoblasts with age. Although the small sample size necessitates caution when interpreting our in vivo findings, our data indicated that AGEs accumulated in osteoblasts of human bones with age. Almeida and colleagues(44) reported that age-related bone loss in mice was related to osteoblast and osteocyte apoptosis resulting from enhanced oxidative stress. The number of apoptotic bone marrow stromal cells isolated from human femoral tissue increased in an age-dependent manner.(45) However, little is known regarding the in vivo association between osteoblast and osteocyte apoptosis in human bones and aging. In this study, we observed higher numbers of apoptotic osteoblasts and osteocytes in the elder group. Interestingly, a significant positive correlation was found between the ratio of AGE- and TUNEL-positive osteoblasts. Moreover, we found that AGE-positive osteoblasts were partially positive in TUNEL assays. These findings indicate that intracellular AGE accumulation is involved in human osteoblast apoptosis in vivo.
There are several limitations related to the interpretation of the results in this study. Since it is known that AGEs increase with diabetes mellitus and chronic kidney disease, cadaver donors with these diseases were excluded in our in vivo studies. Nonetheless, other underlying diseases may affect AGE accumulation in human osteoblasts that may have not been accounted for. In addition, further in vivo studies should be performed to assess the interaction between intracellular AGE-induced osteoblast apoptosis and age-related bone loss and fragility. Our in vitro findings suggest that intracellular AGE induced osteoblastic cell apoptosis via ER stress in osteoblastic MC3T3-E1 and human osteoblastic hFOB 1.19 cells. However, further in vitro studies using more cell lines and primary osteoblasts are needed to validate the relationship between intracellular AGE accumulation and osteoblast apoptosis. Although there are limitations, the present study demonstrated that intracellular AGE-induced ER stress is one mechanism underlying osteoblast apoptosis (Supplemental Fig. S9). Furthermore, our study also provided the first evidence that AGEs accumulate in human osteoblasts with age and that intracellular AGE formation is associated with apoptosis induction. This study may provide novel insight into the molecular mechanisms of age-related osteoblast apoptosis and the treatment of osteoporosis by reducing AGE accumulation in osteoblasts.
Disclosures
All authors state that they have no conflicts of interest.
Acknowledgments
The authors are grateful to Ms Yukie Takahashi for assistance with immunoelectron microscopy analysis and Ms Takana Motoyoshi (K.I. Stainer Inc.) for assistance with immunostaining analysis.
This work was supported by JSPS KAKENHI (grant no. 20K05895) to RN. This work was also supported in part by Japan Science and Technology Agency (JST), Adaptable and Seamless Technology Transfer Program through Target-Driven R and D (no. AS3015118U).
Authors' roles: Study design: RN, RS, and YF. Study conduct: RS, JS, SA, and YF. Data analysis: RS and YF. Drafting manuscript: RS. Revising manuscript content: MY, RN, YF, and MS. Supervision: MS, RN, and KM. All authors discussed the data and approved the submission.




