Hyperparathyroidism in Patients With X-Linked Hypophosphatemia
ABSTRACT
X-linked hypophosphatemia (XLH) is characterized by increased activity of circulating FGF23 resulting in renal phosphate wasting and abnormal bone mineralization. Hyperparathyroidism may develop in XLH patients; however, its prevalence, pathogenesis, and clinical presentation are not documented. This observational study (CNIL 171036 v 0) recruited XLH adult patients in a single tertiary referral center. Each patient was explored in standardized conditions and compared with two healthy volunteers, matched for sex, age, and 25-OH vitamin D concentrations. The primary endpoint was the proportion of patients with hyperparathyroidism. The secondary endpoints were the factors influencing serum parathyroid hormone (PTH) concentrations and the prevalence of hypercalcemic hyperparathyroidism. Sixty-eight patients (51 women, 17 men) were enrolled and matched with 136 healthy volunteers. Patients had higher PTH concentrations compared with healthy controls (53.5 ng/L, interquartile range [IQR] 36.7–72.7 versus 36.0 ng/L, IQR 27.7–44.0, p < .0001). Hyperparathyroidism was observed in 17 patients of 68 (25%). In patients, a positive relationship between PTH and calcium concentrations and a negative relationship between PTH and phosphate concentrations were observed. Seven (10%) patients (3 premenopausal women, 1 postmenopausal woman, and 3 men) were diagnosed with hypercalcemic hyperparathyroidism. All underwent parathyroid surgery, with consecutive normalization of calcium and PTH concentrations. Hyperparathyroidism is a frequent complication in XLH adult patients. Disruption of the physiological regulation of PTH secretion contributes to parathyroid disease. Early-onset hypercalcemic hyperparathyroidism can be effectively and safely cured by surgical resection. © 2020 American Society for Bone and Mineral Research.
Introduction
X-linked hypophosphatemia (XLH) is an inherited disease characterized by renal phosphate wasting, resulting from increased activity of circulating fibroblast growth factor FGF23. Mutations in the phosphate regulating endopeptidase homolog X-linked (PHEX) gene represent the main cause of the disease.1 Symptoms suggestive of rickets, e.g., leg bowing and short stature, often lead to diagnosis of XLH during childhood. Until recently, treatment relied on oral phosphate replacement several times daily, combined with active vitamin D analogs until full growth.2
Secondary and tertiary hyperparathyroidism have been described in XLH patients, mainly in case reports and small cohort studies.3-10 In two recent retrospective cohorts, elevated serum parathyroid hormone (PTH) concentrations were detected in 59% and 83% of the pediatric and adult patients evaluated.11, 12 Hyperparathyroidism has been mostly linked to long-term phosphate supplementation. However, it has been also noted in untreated patients, questioning the pathophysiology of this complication as, in healthy subjects, hypophosphatemia and FGF23 inhibit PTH production.5, 8, 10 Prevention, diagnosis, and treatment of hyperparathyroidism are crucial for clinical management of adult patients as it increases the risk of renal and bone complications, which are already high in this population.2 However, the prevalence, pathogenesis, and clinical presentation of hyperparathyroidism in XLH adults are not known because large controlled studies are lacking.
This study evaluated parathyroid function in a large and well-phenotyped cohort of XLH adult patients in standardized conditions. Patients were compared with healthy volunteers matched according to sex, age and 25-OH vitamin D levels. We describe secondary hyperparathyroidism in patients and analyze parameters influencing PTH levels in this population. We further describe the phenotypic characteristics of patients with hypercalcemic hyperparathyroidism, collected prospectively and retrospectively, and report outcomes of parathyroid surgery.
Subjects and Methods
Study oversight
We conducted an observational study in XLH patients in a single tertiary referral medical center with cross-sectional comparison of patients with controls. The patient's cohort was registered with French “Commission nationale de l'informatique et des libertés” (CNIL 2171036 v 0). All patients were informed before testing that their data could be used anonymously for clinical research purposes, and written informed consent was signed for genetic testing, biobanking, as well as for the use of their medical data. Consent was obtained during the VARIETE study (NCT01831648) for healthy volunteers.13
Subjects
Patients were adult subjects diagnosed with XLH, defined as hypophosphatemia due to renal phosphate wasting with either documented PHEX mutation and/or family history of rickets and/or increased FGF23 concentrations. Patients with tumor-induced hypophosphatemia were not included. The study was carried out between October 2011 and January 2017 at the Department of Endocrinology and Reproductive Diseases at the Bicêtre Hospital (Le Kremlin-Bicêtre, France), which is part of the French National Reference Center for Rare Diseases of Calcium and Phosphate Metabolism. Patients were either (i) previously followed at the Pediatric Endocrinology Department in the same hospital and referred for transition; or (ii) relatives of affected children followed at the referral center; or (iii) referred by other French specialists. Some patients had been lost to follow-up for several years.
Each subject underwent a 2- to 3-day inpatient admission in our department. Treatment with oral phosphate salts and vitamin D was discontinued at least 1 week before evaluation. Subjects were on standardized diet with calcium and phosphate contents as recommended for healthy adults 3 days before admission and during their hospital stay. All these procedures were part of routine clinical care. Medical history, in particular age, clinical and biochemical findings at diagnosis, treatment, and complications were documented; weight, height, body mass index (BMI), and blood pressure were measured.
Healthy controls, matched for sex, age (±5 years), and 25OHD concentration (±15 ng/mL), were selected from a large cohort of French healthy adults (VARIETE, NCT01831648).13 Each patient was matched with two healthy subjects.
Biochemical and hormonal workup
Laboratory data including serum levels of phosphate, calcium, albumin, creatinine, PTH, 25OHD, 1,25(OH)2D, FGF23 and urinary phosphate, calcium, and creatinine excretion (24-hour urine collection or morning urine sample) were measured. These data were used to calculate the ratio of tubular maximum reabsorption of phosphate to glomerular filtration rate (TmP/GFR). Each patient was sampled at 8:00 a.m. after an overnight fast. Approximately 50 mL of blood per patient were collected for these analyses.
Imaging
Patients underwent renal evaluation to search for lithiasis and nephrocalcinosis with renal ultrasound or renal CT scans. Measure of bone mineral density (BMD) was performed with dual-energy X-ray absorptiometry (DXA). Parathyroid imaging, performed in subjects with autonomous hyperparathyroidism, included neck ultrasound as well as technetium-99m Sestamibi SPECT imaging.
Assays
Biochemical analyses were performed according to Bicêtre hospital laboratories' current standard methods. PTH was measured with a chemiluminescent immunometric assay (ADVIA Centaur, Siemens, Deerfield, IL, USA; adult reference range: 14 to 74 ng/L). 25OHD was measured by immunochemiluminescent assays on the Liaison XL (DiaSorin, Saluggia, Italy; reference range for adults: 30 to 100 ng/mL). Between October 2011 and January 2014, calcitriol (1,25(OH)2D) was measured by RIA after immunoextraction (IDS, Boldon, UK). After January 2014, 1,25(OH)2D was determined by a sandwich assay that used the ligand binding domain (LBD) of the vitamin D receptor (VDR) as a 1,25(OH)2D capture molecule on the Liaison XL (DiaSorin; reference range for adults: 20 to 70 pg/mL). For intact FGF23, blood was sampled and promptly centrifuged (2000g, 4°C). Serum was aliquoted, frozen, and stored at −80°C. Measure was performed by an immunochemiluminescent sandwich assay on an automated platform (Liaison XL; Diasorin; reference values for adults are 23.2 to 95.3 ng/L).
Genetic analysis
Genomic DNA was extracted from white blood cells. If the PHEX mutation status was unknown, all exons and intron–exon boundaries of the gene were sequenced. In PHEX mutation-negative patients, mutations in FGF23 were searched for. All exons and intron–exon boundaries of Multiple Endocrine Neoplasia type 1 (MEN1), Cell Division Cycle protein 73 homolog (CDC73), and Calcium Sensing Receptor (CaSR) genes were sequenced in subjects with hypercalcemic hyperparathyroidism.
Statistical analyses
Primary outcome measure was the percentage of patients with secondary hyperparathyroidism, defined by calcium concentrations within the normal range (2.20 to 2.55 mmol/L) and PTH concentrations above the upper normal limit (>74 ng/L). Secondary outcome measures were parameters influencing PTH concentrations in the patients' cohort and the percentage of patients with hypercalcemic hyperparathyroidism, defined by calcium concentrations above 2.55 mmol/L and PTH concentrations above the upper normal limit.
The biological data were summarized with principal components analysis (PCA) used for identifying groups or clusters of variables. Shapiro–Wilk test was used to determine if the selected variables followed a Gaussian distribution in patient and control groups. Because most variables were not normally distributed, they were described with the median and interquartile range. Welch's t test and Wilcoxon rank-sum tests were used to test the hypothesis that both populations had equal means for quantitative variables. Standard linear models were used for the identification of explanatory variables, and regression analyses were performed using Spearman coefficient correlation.
Data were analyzed using R version 3.5.0 (R Core Team [2012]. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/). The packages FactorMineR14 and FactoextraR were used to extract and visualize the output of PCA. Statistical significance was defined as a p < .05 (two-tailed test).
Results
Patient characteristics
Seventy adult patients (52 women and 18 men) were referred for XLH during the study period. The diagnosis was confirmed in 68 (51 women and 17 men) patients (Supplemental Table S1). Hypophosphatemia and, when available, low TmP/GFR were noted in all patients. Fifty-six patients had a family history of hypophosphatemia; 12 patients were index cases without documented family transmission. PHEX gene was sequenced in all patients, with a mutation identified in 64 (94%) patients (Table 1 {TBL 1}and Supplemental Table S1). No mutations in FGF23 were identified in the four PHEX mutation-negative patients. One of 4 had a family history of rickets and 3 were sporadic cases. Median time elapsed since diagnosis was 30 years (interquartile range [IQR] 21–40). Fifty-one patients (75%) had been treated with oral phosphate salts and active vitamin D metabolites during childhood. Treatment with vitamin D and/or phosphate salts was still ongoing at assessment in 44 cases (65%) (Table 1). Patients were matched with 136 healthy volunteers according to age, sex, and 25OHD status. Pairing of male subjects was easier because of the high percentage of men in VARIETE cohort, resulting in a smaller range of differences in 25OHD concentration (±7.5 ng/mL) between male patients and selected controls.
| XLH patients (n = 68) | Control group (n = 136) | p Value for between-group difference | |
|---|---|---|---|
| Age (years) | 38 (31–48) | 38 (30–50) | 0.96 |
| Sex ratio (F/M) | 51/17 | 102/34 | |
| Height (cm) | |||
| Women | 149.5 (146–154) | 164 (158.5–169) | <0.0001 |
| Men | 165 (155–169) | 177.5 (172–183) | <0.0001 |
| Body mass index (kg/m2) | 26 (23–29) | 22 (21–24) | <0.0001 |
| PHEX mutation (n) (%) | 64 (94) | ||
| Age at diagnosis (years) | 2 (1.5–10) | ||
| Time since diagnosis (years) | 30 (21–40) | ||
| Treatment during childhood (n) (%) | 51 (75) | ||
| Ongoing treatment (n) (%) | 44 (65) | ||
| Oral phosphate salts | 31 (46) | ||
| Active vitamin D metabolites | 35 (51) | ||
| Vitamin D | 28 (41) | ||
| Calcium (mmol/L) | 2.25 (2.20–2.32) | 2.30 (2.20–2.40) | 0.24 |
| Albumin (g/L) | 41 (33–43) | 42 (39–45) | 0.0089 |
| Phosphate (mmol/L) | 0.57 (0.50–0.64) | 1.11 (1.03–1.21) | <0.0001 |
| Serum creatinine (μmol/L) | 52.5 (45–61) | 69.5 (62–80) | <0.0001 |
| eGFR CKD-EPI (mL/min/1.73 m2) | 116 (110–127) | 101 (87–110) | <0.0001 |
| TmP/GFR (n) | 0.46 (0.36–0.56) (54) | ||
| 25OHD (ng/mL) | 26.5 (19.7–32.0) | 25.8 (20.1–30.9) | 0.90 |
| 1,25(OH)2D (pg/mL) (n) | 49.0 (38.7–67.7) (60) | 52.0 (44.7–61.3) (120) | 0.70 |
| PTH (ng/L) | 53.5 (36.7–72.7) | 36.0 (27.7–44.0) | <0.0001 |
| FGF23 (ng/L) (n) | 34.2 (24.8–45.1) (40) | 17.4 (14.3–19.8) (73) | <0.0001 |
- Results are expressed as median (IQR) or numbers (%). Patients were compared with healthy volunteers matched according to age, sex, and 25OHD status. Wilcoxon rank-sum test with continuity correction has been performed to compare both groups, except for the normally distributed parameters albumin and eGFR, for which the Welch t test has been used. Affected subjects were untreated at the time of sample collection. (n) after TmP/GFR, 1,25(OH)2D, and FGF23 indicates the number of patients or control subjects in whom these parameters were available. Reference values: calcium 2.20–2.55 mmol/L; albumin 35–50 g/L; phosphate 0.80–1.45 mmol/L; serum creatinine 62–110 μmol/L; 25OHD 30–100 ng/mL; 1,25(OH)2D 20–70 ng/mL; PTH 14–74 ng/L; FGF23 23.2–95.3 ng/L.
Comparison of patients and healthy controls
Demographic, clinical, and biological data of both groups are summarized in Table 1. Comparison of frequency distributions of phosphate, FGF23, calcium, PTH, 25OHD, and 1,25(OH)2D between patients and controls is illustrated in Fig. 1. {FIG1} As expected, phosphate concentrations were significantly lower in patients than in healthy controls. Total serum calcium levels did not differ between groups. Patients had higher PTH concentrations compared with healthy controls (median 53.5 versus 36.0 ng/L, p < .0001). Seventeen patients (25%) had a PTH level above the upper normal limit versus three healthy controls (2%) subjects (p < .0001). There was no sex effect. Significantly higher FGF23 concentrations were recorded in patients versus controls. 1,25(OH)2D concentrations in patients did not significantly differ between groups. Higher body mass index (BMI) values, lower serum creatinine concentrations, and higher estimated glomerular filtration rate (eGFR) values were observed in patients versus controls.
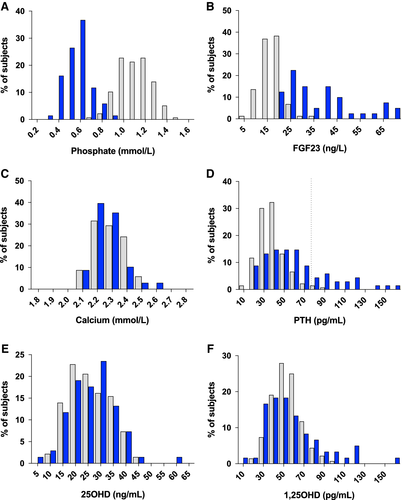
PCA was conducted to search for groups of associated variables and showed that calcium, phosphate, 25OHD, and 1,25(OH)2D represented specific components, distinct in patients and controls.
Determinants of serum PTH concentrations
Simple linear regression models showed a positive relationship between serum calcium and PTH concentrations in patients, which was not observed in healthy subjects (β1 = 112.5, R2 = 0.1238, p = .0033 versus β1 = − 18.1, R2 = 0.0194, p = .1054, Fig. 2A). {FIG2} However, three extreme points of serum calcium corresponding to patients with parathyroid autonomy (Fig. 2A) have a strong influence on the slope of the regression. After removal of patients with hypercalcemic hyperparathyroidism, the positive relationship between PTH and calcium was no longer significant (β1 = 5.21, R2 = 0.00025, p = .9033, data not shown).
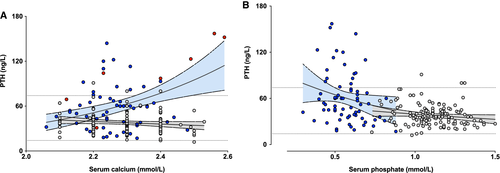
PTH levels were inversely correlated with serum phosphate levels in patients (R2 = 0.0575, p = .0489) but not in controls (R2 = 0.0242, p = .0708, Fig. 2B). Similar concentrations of calcitriol were observed in both groups despite higher PTH concentrations (Fig. 1D, F) in patients than in controls.
Hypercalcemic hyperparathyroidism in patients
Seven patients of 68 (10%) had hypercalcemic hyperparathyroidism (3 premenopausal women, 1 postmenopausal woman, and 3 men) but none of the control subjects. Their clinical and biochemical characteristics versus those of patients without hypercalcemia are summarized in Table 2 {TBL 2}and Fig. 3. {FIG3} Three patients had been diagnosed with hypercalcemia and treated before referral to our department; their data regarding hypercalcemic hyperparathyroidism have thus been collected retrospectively. In addition to these patients, one 75-year-old female patient presented with normocalcemic hyperparathyroidism, cured by selective parathyroidectomy. She had never been treated with oral phosphate salts and/or active vitamin D metabolites. This case, considered as primary hyperparathyroidism unrelated to XLH, was not included in further analyses.
| Patients with HHPT (n = 7) | Patients without HHPT (n = 61) | p Value for between-group difference | |
|---|---|---|---|
| Age at HPT diagnosis (years) | 33 (25–45) | NA | |
| Sex ratio (F/M) | 4/3 | 47/14 | 0.35 |
| Treatment during childhood (n) (%) | 6 (86) | 45 (74) | 0.67 |
| Treatment duration before HPT (years) | 13 (10–19.5) | NA | |
| Ongoing treatment (n) (%) | 4 (57) | 39 (64) | 0.70 |
| Oral phosphate salts | 3 (43) | 28 (46) | 0.99 |
| Active vitamin D metabolites | 3 (43) | 31 (51) | 0.99 |
| Vitamin D | 2 (28.5) | 25 (41) | 0.69 |
| Biochemical parameters | |||
| Calcium (mmol/L) | 2.70 (2.64–2.84) | 2.25 (2.20–2.31) | 0.0001 |
| PTH (ng/L) | 159 (139.5–210) | 52 (36–66) | 0.0093 |
| Phosphate (mmol/L) | 0.42 (0.37–0.42) | 0.59 (0.50–0.64) | <0.0001 |
| Urinary calcium/creatinine ratio (mmol/mmol) | 0.84 (0.50–1.02) | 0.40 (0.17–0.53) | 0.0461 |
| 25OHD (ng/mL) | 26 (18.8–32.5) | 26 (18–32) | 0.83 |
| Complications | |||
| Lithiasis (n) (%) | 1 (12.5) | 6 (10) | 0.55 |
| Nephrocalcinosis (n) (%) | 2 (25) | 5 (8) | 0.14 |
| Osteoporosis (n) (%) | 1 (12.5) | 8 (13) | >0.99 |
- n = number of patients; HHPT = hyperparathyroidism; NA = not applicable. Results are expressed as median (IQR) or numbers (%). Statistical significance was evaluated by Welch's t test. For patients with hypercalcemic hyperparathyroidism, the highest values of serum calcium and PTH concentrations, and urinary calcium/creatinine ratio and the minimal values of serum phosphate concentrations were used. For patients without hypercalcemic hyperparathyroidism, values are those collected at the time of evaluation in our department. Reference values: calcium 2.20–2.55 mmol/L; phosphate 0.80–1.45 mmol/L; PTH 14–74 ng/L.
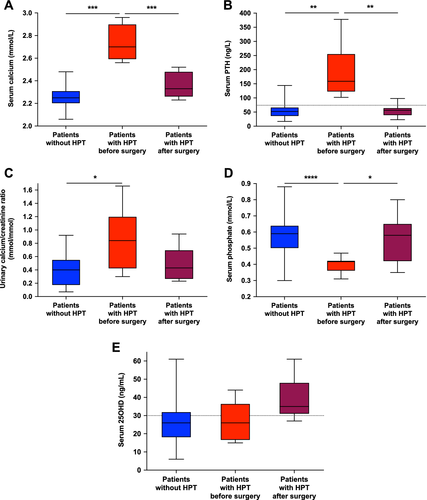
Median age at diagnosis of hypercalcemic hyperparathyroidism was 33 years (range 11 to 57 years). Six patients of seven (86%) had been treated with oral phosphate salts and active vitamin D metabolites during childhood and 4 (57%) were currently treated at diagnosis with autonomous hyperparathyroidism. Median duration of treatment before diagnosis of hypercalcemia was 13 years.
Patients had mild hypercalcemia with a maximal calcium concentration 2.70 mmol/L (range 2.56 to 2.96 mmol/L, Fig. 3) and increased PTH concentrations with a maximal PTH concentration 159 ng/L (range 102 to 378 ng/L, Fig. 3). Severe hypophosphatemia and high urinary calcium/creatinine ratio were observed.
Four patients of 7 (57%) had renal or skeletal abnormalities. The 3 patients with lithiasis or nephrocalcinosis had been treated with oral phosphate salts and active vitamin D metabolites during childhood. The one with osteoporosis was a postmenopausal woman.
Two patients were siblings with no other known affected family members (2 sisters aged 44 and 46 years at time of diagnosis of hypercalcemia). We searched for a genetic predisposition and sequenced MEN1, CDC73, and CaSR genes in these 2 sisters and 3 other patients with early onset hypercalcemic hyperparathyroidism. No mutations were found in these patients.
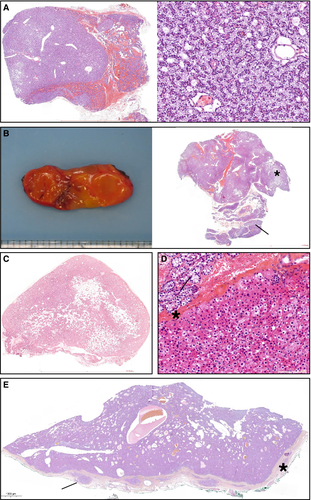
Imaging results are presented in Table 3. {TBL 3} Parathyroid ultrasound and technetium-99m Sestamibi SPECT imaging, performed in 5 patients, gave consistent results. In 3 cases, parathyroid ultrasound and scintigraphy showed a single image suggestive of parathyroid adenoma. In 2 cases, two images compatible with pathological parathyroid glands were found. In 1 of these 2 cases, 18F-fluorocholine PET imaging was also performed and showed focal uptakes compatible with global parathyroid autonomy. All patients underwent surgery. Surgical outcomes and representative images of histological findings are summarized in Table 3 and Fig. 4, {FIG4} respectively.
| Patients who underwent parathyroid surgery (n = 7) | |
|---|---|
| Results of preoperative imaging (n) | |
| Parathyroid ultrasound | 6 |
| Single adenoma | 3 |
| Multiple adenomas | 3 |
| Hyperplasia | 0 |
| Not done | 1 |
| Technetium-99m Sestamibi SPECT imaging | 6 |
| No lesion detected | 0 |
| Single focal uptake | 3 |
| Multiple focal uptakes | 3 |
| Not done | 1 |
| Type of surgery (n) | |
| Cervical exploration | 7 |
| Selective parathyroidectomy (1 or 2 resected glands) | 5 |
| Subtotal parathyroidectomy | 2 |
| Parathyroid reimplantation | 1 |
| Histological findings (n) | |
| Adenoma | 3 |
| Hyperplasia | 3 |
| « Adenomatous hyperplasia » | 1 |
- Results are expressed as numbers (n).
None of the patients developed symptomatic postoperative hypocalcemia. Follow-up duration after surgery was 44 months (IQR 18.5–81). All patients had prolonged normal serum calcium concentrations at the last follow-up visit (2.70 mmol/L before surgery versus 2.33 mmol/L after, p = .0002, Fig. 3). PTH serum concentrations decreased by nearly 65% (159 ng/L before surgery versus 56 ng/L after, p = .0086, Fig. 3). Normal PTH concentrations were noted several months after surgery except in one case. There was a significant increase in serum phosphate concentrations (0.42 mmol/L before surgery versus 0.58 mmol/L after, p = .0410, Fig. 3).
Discussion
Here, we present the results of a large cohort study of the parathyroid function in XLH adult patients. We show that these patients have increased PTH compared with healthy controls matched for sex, age, and vitamin D status. Hyperparathyroidism in XLH is associated with disruption of the physiological regulation of parathyroid hormone secretion. Ten percent of patients in our study developed hypercalcemic hyperparathyroidism with mild hypercalcemia, which normalized after parathyroidectomy.
Considering the lack of controlled data on parathyroid dysfunction in XLH patients, this cohort study was designed to investigate the prevalence, pathogenesis, and clinical presentation of hyperparathyroidism in these patients. Sixty-eight consecutive XLH adult patients were evaluated in standardized conditions and compared with 136 healthy matched controls. The sex ratio of 3 women for 1 man is consistent with the mode of disease transmission, with PHEX mutations found in 90% of the patient population. However, the overrepresentation of females may also be due to easier recruitment of affected women at adult age.
Hyperparathyroidism in XLH patients has been reported in the literature, mainly in case reports and small retrospective cohorts. Hypercalcemia related to parathyroid gland autonomy is often reported in these cases.3-10 In two recent retrospective studies in pediatric and adult patients with XLH, elevated serum PTH concentrations with normal serum calcium were detected in 59% and 66.7% of the patients evaluated.11, 12
In our study, secondary hyperparathyroidism was noted in 25% of XLH adult patients. This increase in PTH concentrations was not related to 25OHD levels, as serum 25OHD concentrations were similar in patients and controls (Table 1; Fig. 1E). Renal function was not altered in patients. Actually, we observed lower creatinine levels and consequently higher eGFR in patients versus controls. However, lower serum creatinine may reflect muscular hypotrophy rather than increased glomerular filtration in XLH patients.15 Assessment of renal function with gold standard methods such as 51Cr-EDTA clearance would be more accurate than estimation of eGFR by usual creatinine-based equations (MDRD, CKD-EPI). For further clinical studies in XLH patients, estimation of GFR using cystatin C-based equations (independent of muscle mass) will be considered.16
In normal individuals, serum PTH is negatively correlated with ionized calcium, in hypo- and hypercalcemic states.17 In XLH patients, linear regression analysis showed a positive correlation between PTH and serum calcium, suggesting an altered regulation of parathyroid function. Similar changes in the PTH-calcium relationship have been reported in patients with primary hyperparathyroidism18 and with secondary hyperparathyroidism due to CKD.19 However, three extreme points of serum calcium corresponding to patients with parathyroid autonomy (Fig. 2) have a strong influence on the slope of the regression. After removal of patients with hypercalcemic hyperparathyroidism, the positive relationship between PTH and calcium was no longer statistically significant, but median PTH concentration in patients was still significantly higher than in controls (p < .0001). Parathyroid autonomy thus probably represents an evolved stage of previously altered parathyroid function in XLH patients.
Linear regression analysis showed a negative correlation between PTH and serum phosphate, suggesting a link between hyperparathyroidism and severity of the disease. However, these relationships explain only a small part of the variance in PTH values in the populations concerned, since the adjusted R-squared (R2) reached only 0.197 in the best multiple regression model integrating calcium, phosphate, and albumin levels.
The exact mechanisms leading to the rise in PTH levels are unknown. The recurrent stimulation of parathyroid glands by oral phosphate boluses was long thought to be the main physiopathological mechanism.2 Various hypothesis were proposed to explain the effect of oral phosphate salts; they include phosphate-mediated postprandial reduction in calcium levels, which stimulates PTH through activation of the parathyroid gland CaSR, phosphate-mediated reduction in calcitriol production at the renal tubule, and, finally, direct stimulation of phosphate ions on the parathyroid cell.7, 20-23 However, in our cohort, among the 17 patients with PTH concentrations above normal, 1 had never been treated with oral phosphate salts and 6 had discontinued treatment for 19 ± 17 years at the time of inclusion. Similarly, hyperparathyroidism has also been described in untreated patients in the literature.5, 6, 8, 10, 23-25 These observations argue against a major role of intermittent hyperphosphatemia in the occurrence of hyperparathyroidism.
Deficient production of calcitriol induced by FGF23 excess could contribute to the development of hyperparathyroidism as calcitriol is a known inhibitor of parathyroid cell proliferation.25 However, we and others8 did not observe any difference in calcitriol concentrations between patients and controls (Table 1, Fig. 1F). This could result from concomitant inhibitory action of FGF23 excess and stimulatory action of increased PTH (Fig. 1B, D) on 1α-hydroxylase activity.
Altered PTH secretory dynamics and abnormal PTH mRNA cleavage or degradation due to loss of PHEX function in the parathyroid gland have been also proposed to participate in the pathogenesis of hyperparathyroidism.5, 25 A positive correlation between FGF23 and PTH has been reported in XLH patients,8 suggestive of parathyroid resistance to FGF23 inhibition of PTH secretion. In our study, no such correlation between PTH and FGF23 was shown with Spearman's method, and FGF23 concentrations were not included in the regression analysis as concomitant FGF23 and PTH assessments were available in only 40 patients.
In our cohort, 7 (10%) patients developed hypercalcemic hyperparathyroidism, which is similar to the prevalence of 16.7% reported in one recent retrospective study.12 In all cases, XLH had been diagnosed during childhood and duration of treatment with oral phosphate salts and active vitamin D metabolites was greater than 10 years. Hyperparathyroidism was characterized by early onset of the disease in 6 of 7 hypercalcemic patients. Genetic search for mutations in MEN1, CDC73, and CaSR in patients with early-onset hypercalcemic hyperparathyroidism and in 2 siblings with familial hyperparathyroidism did not identify any genetic predisposition. Despite mild hypercalcemia (below 3.0 mmol/L), 3 of 7 patients (43%) had renal complications. Of note, prevalence of nephrolithiasis and nephrocalcinosis was not different between patients with and without parathyroid autonomy (Table 2). A substantial number of patients without parathyroid autonomy presented with renal calcifications possibly linked to overtreatment with active vitamin D derivates.
All patients underwent parathyroid surgery. Diagnosis of hyperparathyroidism was confirmed histologically in all cases. Both hyperplasia and single adenoma were found (Fig. 4), as reported in literature.3, 7, 26, 27 Surgery in all 7 patients led to prolonged normalization of serum calcium level and a significant decrease in PTH concentrations still after 44 months (range 5 to 182 months) of follow-up. Similar efficacy of the surgical approach has been previously reported.3, 27, 28 Our results contrast with the high rate of recurrence in the recent Delacey and colleagues study,12 underlying the existence of regional differences in parathyroid surgery outcomes. Surgery in XLH patients requiring appropriate reduction of parathyroid tissue in the context of potential pluri-glandular disease should be provided in expert reference centers. Long-term benefits of surgery on kidney and bone complications remain to be demonstrated.
In centers without access to experienced surgeon, cinacalcet can be a therapeutic alternative as it seems to improve the biochemical profile in combination with standard therapy.21, 28 Paricalcitol, a vitamin D analog, could also be an effective adjunct to standard therapy as it efficiently suppressed PTH secretion in a prospective, randomized, placebo-controlled trial in XLH patients with previous evidence of secondary hyperparathyroidism.9 Burosumab, a recombinant human monoclonal IgG1 antibody directed against FGF-23, has recently proved its efficacy to improve phosphate homeostasis, vitamin D metabolism in XLH adult patients.30 PTH concentrations decreased in the burosumab-treated group and increased at week 24 in the placebo group, suggesting that burosumab could improve hyperparathyroidism in these patients.31
In conclusion, this is the first evaluation of parathyroid function in XLH adult patients, with cross-sectional comparison of patients with appropriate controls.30 Hyperparathyroidism is frequent, affecting 25% of patients. Loss of the physiological regulation of PTH secretion by serum calcium contributes to development of hyperparathyroidism. Hypercalcemic hyperparathyroidism affects young patients and can be effectively and safely cured by selective surgical resection of the pathological parathyroid glands.
Disclosures
KB, and PK were investigators on the UX023-CL303, UX023-CL304, and BUR02 trials. KB, AL, and PK received honoraria and travel grants from Kyowa Kirin.
Acknowledgments
We thank Hazar Haidar for her help in performing PTH assessment, Jean-Claude Souberbielle for his help in performing FGF23 assessment, Marie-Odile North for her help in performing genetic analysis, and Yahya Debza for his help with the CNIL declaration.
Authors’ roles: ALL and PK contributed to the conception and design of the research. AR, KB, AL, and PK referred the patients. PC and SB-T provided data from healthy volunteers. CS and BF performed genetic analysis. SB-T and MP performed hormonal tests. ED analyzed parathyroid imaging. BL operated the patients. MD performed the histological analyses of parathyroid tissue. ALL and EB collected data. ALL, PCR, and PK analyzed the data and interpreted the results. ALL, AB, and PK prepared the figures. ALL and PK drafted the manuscript. ALL, AB, PCR, AL, and PK edited and revised the manuscript. All authors approved the final version of the manuscript.




