Maternal Transmission Ratio Distortion of GNAS Loss-of-Function Mutations
ABSTRACT
Pseudohypoparathyroidism type 1A (PHP1A) and pseudopseudohypoparathyroidism (PPHP) are two rare autosomal dominant disorders caused by loss-of-function mutations in the imprinted Guanine Nucleotide Binding Protein, Alpha Stimulating Activity (GNAS) gene, coding Gsα. PHP1A is caused by mutations in the maternal allele and results in Albright's hereditary osteodystrophy (AHO) and hormonal resistance, mainly to the parathormone (PTH), whereas PPHP, with AHO features and no hormonal resistance, is linked to mutations in the paternal allele. This study sought to investigate parental transmission of GNAS mutations. We conducted a retrospective study in a population of 204 families with 361 patients harboring GNAS mutations. To prevent ascertainment bias toward a higher proportion of affected children due to the way in which data were collected, we excluded from transmission analysis all probands in the ascertained sibships. After bias correction, the distribution ratio of the mutated alleles was calculated from the observed genotypes of the offspring of nuclear families and was compared to the expected ratio of 50% according to Mendelian inheritance (one-sample Z-test). Sex ratio, phenotype of the transmitting parent, and transmission depending on the severity of the mutation were also analyzed. Transmission analysis was performed in 114 nuclear families and included 250 descendants. The fertility rates were similar between male and female patients. We showed an excess of transmission from mother to offspring of mutated alleles (59%, p = .022), which was greater when the mutations were severe (61.7%, p = .023). Similarly, an excess of transmission was found when the mother had a PHP1A phenotype (64.7%, p = .036). By contrast, a Mendelian distribution was observed when the mutations were paternally inherited. Higher numbers of females within the carriers, but not in noncarriers, were also observed. The mother-specific transmission ratio distortion (TRD) and the sex-ratio imbalance associated to PHP1A point to a role of Gsα in oocyte biology or embryogenesis, with implications for genetic counseling. © 2019 American Society for Bone and Mineral Research.
Introduction
Pseudohypoparathyroidism type 1A (PHP1A, MIM #103580) is a rare disorder characterized by hormonal resistance and variable developmental features referred to as Albright's hereditary osteodystrophy (AHO):1 a round shaped face, short stature, early-onset obesity, ectopic ossifications, brachymetacarpia type E, and global developmental delay. PHP1A is caused by heterozygous loss-of-function mutations in the maternal allele of the Guanine Nucleotide Binding Protein, Alpha Stimulating Activity (GNAS) gene.2 GNAS encodes the ubiquitous alpha-subunit of a heterotrimeric stimulatory G protein (Gsα) that couples many G protein-coupled receptors, including the parathyroid hormone receptor (PTHR1) and thyroid-stimulating hormone (TSH) receptor, and which stimulates adenylyl cyclase and cyclic AMP (cAMP) formation.3 A related disorder, pseudopseudohypoparathyroidism (PPHP, MIM #612463), is caused by inactivating mutations on the paternal allele of GNAS. PPHP is characterized by some, but not all, AHO features, subcutaneous ossification—or progressive osseous heteroplasia (POH)–and intrauterine growth retardation (IUGR) with no hormonal resistance observed.4, 5
Those discrepancies in the phenotype depending on the transmitting parent are explained by a tissue-specific imprinting of GNAS.6, 7 In most tissues, GNAS is expressed in a biallelic manner: a loss-of-function mutation leads to haploinsufficiency, causing AHO features. In some endocrine tissues (renal proximal tubules, thyroid, gonads, and pituitary gland) GNAS is paternally imprinted.8 When the mutation is carried by the maternal allele, there is a partial to complete deficit in Gsα depending on the severity of the mutation, resulting mainly in PTH resistance with hypocalcemia, hyperphosphatemia, high or ill-adapted PTH, and TSH resistance with hypothyroidism.7, 9-11 Previous studies of PHP1A-causing and PPHP-causing mutations have focused on the correlations between phenotype and genotype, showing a more severe clinical and laboratory phenotype in the case of more deleterious mutations, in relation to the parental inheritance.5, 11-18 Because of their singular mode of transmission, the two diseases can be observed in the same family, but never in the same sibship, and the parental origin of the mutated allele can be inferred from the patient's phenotype. In our experience, carriers appear to represent a high proportion of the descendants in large pedigrees (Supplemental Fig. S1). In addition, our attention was drawn to a higher number of positive prenatal diagnoses than expected. These data prompted us to study the transmission of GNAS mutations in a systematic manner.
In a retrospective study, we investigated the transmission and distribution of the mutated alleles in a large number of families with PHP1A and PPHP, genotyped in our reference center, after correction for ascertainment bias. We aimed to determine whether the autosomal mutations that cause PHP1A and PPHP follow classical Mendelian inheritance and sex ratio.
Patients and Methods
All family members that were genotyped, or the parents or legal guardians in the case of children, gave written informed consent before the genetic investigations, in accordance with the French bioethics laws and the Declaration of Helsinki.
This retrospective study involves pedigrees of patients with a molecular diagnosis of PHP1A or PPHP established in our National Reference Center for rare calcium and phosphate metabolism disorders. Most patients were white. To differentiate between PHP1A and PPHP, we relied on clinical information provided by the prescribing physician. A questionnaire was sent out when relevant information was lacking. It included serum and urinary calcium and phosphate levels, PTH and TSH values, biologic activity of the Gs protein where available, treatments, birth weight and length, current height and weight, and inquiries about the clinical presentation: moon face, intellectual deficit, subcutaneous ossifications. Hand X-rays were also requested to evaluate for brachymetacarpia. In case of hormone resistance (elevated TSH, hypocalcemia, hyperphosphatemia, high or inappropriate PTH levels) and/or of maternal transmission, a PHP1A diagnosis was made. When patients exhibited AHO features with no sign of hormone resistance (normal TSH and PTH, normocalcemia and normophosphatemia without treatment), subcutaneous ossifications or POH, IUGR, a low birth weight, and/or when the father was the transmitting parent, PPHP was diagnosed.
Family members without available DNA who were described as asymptomatic, or for whom clinical data was lacking, were considered free of GNAS mutations.
GNAS exons 1 to 13 (RefSeq NM_8001077488.2) including intron/exon boundaries were analyzed by conventional dideoxy sequencing (Sanger sequencing) using the BigDye Terminator v.1.1 Cycle Sequencing Kit (Thermo Fisher Scientific, Waltham, MA, USA) and application of sequencing products onto an ABI 3500 Genetic Analyzer (Thermo Fisher Scientific), or by next-generation sequencing (NGS) with a custom Ampliseq panel (Thermo Fisher Scientific) for genes involved in calcium and phosphate metabolism covering GNAS exons 2 to 13 using an Ion Torrent PGM Sequencer (Thermo Fisher Scientific). In case of novel variants, the segregation with the phenotype was studied and prediction scores were used to interpret and classify them as disease-causing mutations, according to American College of Medical Genetics and Genomics (ACMG) guidelines.19
GNAS mutation, variants were classified into two groups according to their type and location: (i) severe mutations included nonsense mutations, mutations of canonical splicing sites or predicted to modify splicing, and mutations leading to a frameshift; (ii) non-severe mutations included missense mutations and insertions and deletions within the reading frame predicted to have a milder impact on Gsα function.12, 14, 20, 21
All mutations that were not previously described in the literature were implemented into the Leiden Open Variation Database (www.lovd.nl/GNAS).
All pedigrees with an identified GNAS mutation were included. Pedigrees of patients with de novo mutations or with no family history (sporadic cases), and with no offspring were excluded, because it was a transmission analysis. The smallest families were therefore composed of one parent with a GNAS mutation and his or her offspring. The larger pedigrees were divided into nuclear families, consisting of a parent, with a GNAS mutation or an obligate carrier, and his/her offspring.22
To prevent ascertainment bias toward a higher proportion of affected children due to the way in which data were collected, we excluded from transmission analysis all probands in the ascertained sibships.23, 24 This leads to calculating ratios from analysis of the relatives who are assumed to be ascertained with less bias.
The distribution ratio of the mutated alleles was calculated from the observed genotypes of the offspring of nuclear families and was compared to the expected ratios under Mendel's second law of inheritance. For autosomal dominant inheritance, 50% of children of carriers should inherit the mutated allele, the frequencies of maternal and paternal transmission should be similar, and the sex ratio balanced among the affected siblings.25 Statistically significant departure from the Mendelian inheritance ratio with a preferential transmission of alleles defines transmission ratio distortion (TRD).24, 26
We also evaluated the transmission ratios according to the phenotype of the transmitting parent (PHP1A or PPHP) by looking at two-generation families where the information was available and at three-generation families: the transmitted parent was deduced to have a PHP1A phenotype when the deleterious allele was the grand maternal one, PPHP when it was the grand paternal one.27
For the statistical analysis, we used the one-sample Z-test to compare the observed distributions with the expected population proportions under Mendel's laws.28 All p values were two-sided. A p value of <.05 was used to indicate statistical significance.
Results
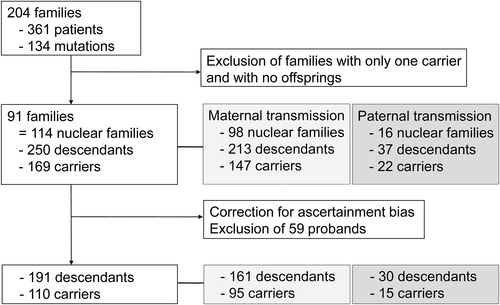
The study population comprised 204 unrelated-families with 361 patients carrying 134 different mutations causing PHP1A or PPHP/POH, including a deletion of exons 1 and 2 (Fig. 1, Supplemental Table S2). A total of 113 patients presenting as sporadic cases and with no offspring, who were on average 13.3 years old, were excluded. Ninety-one pedigrees were included in the transmission study, divided into 114 nuclear families. A total of 385 family members were genotyped, and 247 were carriers; 250 descendants were identified, including 169 carriers. The number of children per affected parent was similar between male and female patients: GNAS mutated mothers had 2.17 children (2.00 for mothers with PHP1A; 2.35 for mothers with PPHP); spouses of GNAS mutated fathers had 2.31 children. Ten females affected by PHP1A (n = 5), PPHP (n = 4) or with an unknown phenotype (n = 1), and one female whose husband is affected by PPHP, underwent prenatal testing (Table 1). The parental GNAS mutation was identified in seven cases; in five of five females affected by PHP1A, but not in four of four females affected by PPHP.
| Family mutation | Sex of the transmitting parent | Phenotype of the transmitting parent | Result of the prenatal diagnosis | Phenotype of the child |
|---|---|---|---|---|
| c.1042-1G>C (S) | F | PPHP | No mutation | No phenotype |
| c.568_571del (S) | F | PPHP | No mutation | No phenotype |
| c.568_571del (S) | F | PPHP | No mutation | No phenotype |
| c.1009C>T (NS) | F | PPHP | No mutation | No phenotype |
| c.1-4_2delCGCCAT (S) | F | PHP1A | Parental mutation identified | PHP1a |
| c.481C>T (NS) | F | PHP1A | Parental mutation identified | PHP1a |
| c.742G>A (NS) | F | PHP1A | Parental mutation identified | PHP1a |
| c.742G>A (NS) | F | PHP1A | Parental mutation identified | PHP1a |
| c.695G>A (NS) | F | PHP1A | Parental mutation identified | PHP1a |
| c.843-2del (S) | F | Not available | Parental mutation identified | PHP1a |
| c.136_137delinsGG (NS) | M | PPHP | Parental mutation identified | PPHP |
- S = severe mutation; NS = non-severe mutation; F/M = sex of the transmitting parent.
Among the 114 nuclear families studied, 98 were families with a maternal transmission and 16 with a paternal transmission (86.0%; p < .001; 95%CI, 79.6% to 92.3%). The deleterious allele was inherited from the mother in 147 children as opposed to 22 from the father. With regard to the 250 descendants, 169 carried a GNAS mutation, with a ratio of 67.6% (p < .001; 95%CI, 61.8% to 73.4%). Correction of ascertainment bias led to the exclusion of 59 probands from the analysis (Fig. 1).
When only considering the 101 nuclear families out of 114 for which all the descendants’ dates of birth were available, the first child was affected in 80 families (Supplemental Fig. Supplemental Fig. S3). Among those 80 families, 68 cases had PHP1a (maternal transmission) and 12 cases had PPHP (paternal transmission).
Among the 191 remaining siblings, 110 were carriers, which was a higher proportion than expected (57.6%; p = .036; 95%CI, 50.6% to 64.6%; Fig. 2 A).
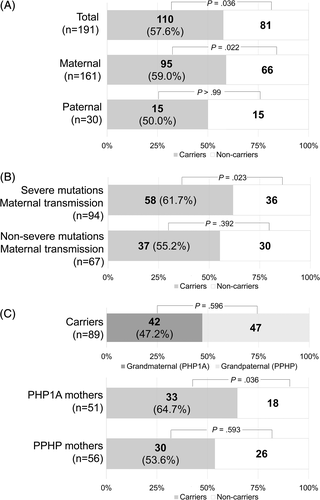
In nuclear families with maternal transmission, the proportion of carriers was 69.0% (147/213 siblings; p < .001; 95%CI, 62.8% to 75.2%). After bias correction the proportion was 59.0% (95/161 siblings; p = .022; 95%CI, 51.4% to 66.6%; Fig. 2 A). To verify if this result was not driven by a single large family, we re-analyzed maternal transmission after ascertainment bias correction, after excluding the descendants of one large family (pedigree with more than one nuclear family) after another. We found similar results in each case, with statistical significance and 95%CI that did not include 50% (Supplemental Fig. Supplemental Fig. S4). In contrast, in nuclear families with paternal transmission, the ratio was of 50.0% with 15 carriers in 30 descendants (Fig. 2 A).
Because of the low number of families and offspring with paternal transmission, further transmission analyses were performed either on the entire study population or on the nuclear families with a maternal transmission.
Out of the 71 observed mutations in the families included in the transmission study, 42 were classified as severe (Supplemental Table S2). Thirteen pedigrees (14.8%) were carrying the c.568_571delGACT mutation (p.Asp190Metfs*14), the most common loss-of-function GNAS mutation.11, 13, 16, 29
We aimed to evaluate whether the observed distortion depended on the severity of the mutation. Severe mutations were associated with a tendency to TRD, with 70 carriers of 119 descendants (p = .054). This distortion reached statistical significance when only nuclear families with a maternal transmission were considered (58 carriers of 94 descendants; 61.7%; p = .023; 95%CI, 51.9% to 71.5%; Fig. 2 B). By contrast, when the mutations were classified as non-severe mutations, no imbalance of transmission was observed, whether the whole population (40 carriers of 72 descendants; p = .346), or only the nuclear families with a maternal transmission (37 carriers of 67 descendants; p = .392; Fig. 2 B) were observed.
GNAS is an imprinted locus and phenotypes vary depending on the sex of the transmitting parent. Grandparental origin of alleles could have an influence on TRD. Under Mendel's law, the expected allele ratios from each grandparent are 0.25. Again, because of the low number of paternal transmissions, we only included nuclear families with a maternal transmission, and therefore only looked at alleles transmitted by the maternal grandmother and the maternal grandfather. As a result, the expected ratios were 0.5. The grandparental origin of the deleterious allele was known for 89 descendants with a mutation: 42 were from the maternal grandmother, ie, their mothers had PHP1A, and 47 from the maternal grandfather, ie, their mothers had PPHP (p = .596; Fig. 2 C). We performed a transmission analysis after correction of ascertainment bias. When the mother had PHP1A, there was an excess of transmission of the mutated allele with 64.7% of carriers (33/51 descendants; p = .036; 95% CI, 51.6% to 77.8%). When the mother had PPHP, 53.6% of descendants were carriers (30/56 descendants; p = .593).
A greater distortion was also seen in families with a severe mutation and where the mother has PHP1A, although statistical analysis was not performed because of the small size of the groups (Supplemental Fig. Supplemental Fig. S5).
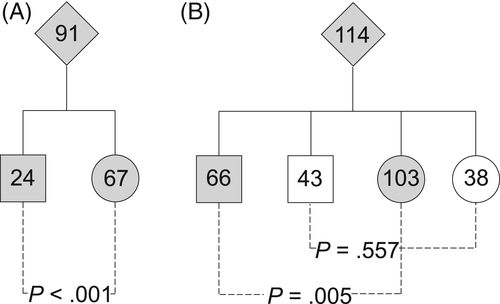
In the whole population of patients, 225 patients were females and 136 were males (sex ratio [SR] = 0.6; p < .001). Within the families included in the transmission analysis, 141 descendants were females and 109 were males (SR = 0.77; p = .043). Among the 91 probands, 67 were women, 24 men (p < .001; Fig. 3 A). Out of the 169 mutation carriers in the 114 nuclear families, 103 were females and 66 males (SR = 0.64; p = .005; Fig. 3 B). However, noncarriers showed a balanced SR with 38 females and 43 males (SR = 1.13; p = .577).
Discussion
In this retrospective study, we analyzed the segregation of GNAS mutations in a large group of PHP1A and PPHP/POH patients and their families to assess transmission of the mutated allele from parents to offspring. We showed a statistically significant departure from Mendelian transmission ratios with a higher proportion of affected children than expected. The allelic transmission imbalance occurs in a sex-specific manner with an excess of transmission of mutated alleles from mothers to offspring not observed in fathers. The TRD is consistent over three generations. Globally, a higher number of females within the carriers, but not in noncarriers, was observed.
The effect seen in families with a maternal transmission is significant despite the fact that PHP1A patients might have been missed within the sibships, with a risk of underestimating the distortion, because family members for whom clinical, biological or genotypic data was lacking were assumed to be free of GNAS mutations. In fact, in young children with PHP1A, hormonal resistance and brachymetacarpia can be absent and not develop until later. We chose this stringent conduct in order to be able to include families for which information was missing.
In the literature pertaining to TRD, artificial transmission imbalance has been shown. Indeed, in the case of rare genetic disorders, a family with multiple cases would be more likely to come to the attention of researchers or physician (to be ascertained), and are therefore expected to be overrepresented, with the risk of spurious conclusion.30 Therefore, to perform the transmission analysis, we examined the nuclear families of pedigrees with a GNAS mutation after correction for single ascertainment bias.23 By excluding all probands in the ascertained sibships, we corrected for selection bias due to the nonrandom fashion in which families were included.
Another source of bias could have been an alteration of family planning after having an affected child. This, however, does not seem to be the case in our population because the number of children per affected parent was close to the national fertility rate31 despite a high number of families with a first child affected (80/101), and a low number of prenatal diagnoses (only 11 spanning over 10 years) with no termination of pregnancy for medical reason as a result. In the cases of nuclear families with only one affected child at the time of the diagnosis, 14 of 28 were excluded for the analysis when we corrected for ascertainment bias.
There was a large imbalance between nuclear families with a maternal transmission and families with a paternal transmission in our population, resulting in PHP1A and PPHP/POH, respectively. Similar results were found in a large multicentric cohort recently published, with 242 PHP1A and 64 PPHP patients included.20 Those larger numbers of PHP1A patients are probably because of an underdiagnosis of PPHP patients: the absence of hormonal and metabolic features in PPHP patients, subtle clinical signs or findings that are not considered significant enough to be recorded by a physician (mild brachymetacarpia or an occasional ossification can often be ignored), with less families with a paternal transmission brought to a geneticist's attention.4 This imbalance, with a small number of descendants from fathers with GNAS mutations prevented us from statistically analyzing transmission ratios from fathers with GNAS mutations to offspring.
Interestingly, although resistance to gonadotropin is described in PHP1A, fertility rates did not seem impaired, with similar rates for mutated women and mutated men. But because this was a transmission study, patients who are unable to have children are not factored in.
We observed a predominance of affected females. This is consistent with the literature, with SR ranging from 0.49 to 0.79.13, 14, 17, 20, 32 We can exclude a selection bias between males and females because symptoms do not differ between the two sexes; ossifications may even be more common in males.14 It could be due in part to an excess of nuclear families with a maternal transmission, because more transmitting mothers would mean more affected females in the GNAS pedigrees.
Although a rare phenomenon, TRD has already been described in other autosomal dominant disorders such as the long-QT syndrome24, 33 and trinucleotide repeat expansions,26, 34 but also in recessive35 and X-linked disorders.36 Similar transmission ratios were found: 57% for the long-QT syndrome,24 63% for myotonic dystrophy,34 60% for mutations on the ARX gene.36
Several biological mechanisms can contribute to TRD during the gametic or embryonic development stages, as were reviewed in 2013 by Huang and colleagues37: germline selection, meiotic gametic competition, and embryo lethality due to a deleterious genotype, an imprint resetting error, or a faulty imprint maintenance.
In the case of GNAS loss-of-function mutations, a positive selection toward the pathologic genotype occurs, with a maternal TRD toward carriers. Because gametic competition to achieve fertilization concerns sperm, and because embryo lethality results in a negative selection of the pathological genotypes, those two mechanisms could be excluded. Germline selection occurs during mitosis of germ cells, which are sexually bipotential.38 The difference of transmission ratios between males and females, with a maternal TRD and a neutral paternal transmission ratio, could therefore be more consistent with meiotic drive in oogenesis.39, 40
Female meiotic drive can be the result of different processes. It can be due to chromosomal drive, in which some property of the general structure or size of a chromosome gives it a replication or orientation advantage on the meiotic spindle.39 Because most GNAS mutations are point mutations or small insertions/deletions, it does not seem plausible that they could have any effect on chromosome segregation. A specific allele at another locus close to the studied gene such as in the case of founder effects could be eliminated because many GNAS mutations described are de novo mutations, and have no linkage to a neighboring allele.40
Female meiotic drive could also be initiated by a direct or indirect effect of the mutation on meiosis in the oocytes. Recently, cAMP levels and the cAMP-protein kinase A (PKA) pathway have been implicated in the regulation of mammalian female meiosis. They were shown to have a role in both the oocytes and the ovaries, by controlling meiotic prophase I, primordial folliculogenesis, and asymmetrical division.41-44 GNAS is maternally expressed in the ovaries8 and therefore disruption of the Gsα protein subunit result in the subsequent perturbation of cAMP formation. The statistically significant TRD when the mother has a PHP1A phenotype; ie, a GNAS mutation on her maternal allele and the increased maternal TRD when GNAS mutations are severe supported a role in oocytes selection. Further investigations are required to better understand the specific mechanisms in humans.
Finally, we cannot exclude an imprinting effect. In fact, some TRD are described in imprinted regions. For example, in long-QT syndrome, an autosomal dominant disorder where sex-of-parent specific TRD has been well characterized, the main implicated locus KCNQ1 is imprinted.24, 33 GNAS locus has an impact on fetal growth mainly through the alternative extra-large transcript XLαs, which is exclusively paternally expressed.6, 7 When the embryo inherits the mutated allele from its father, severe IUGR and hypotrophic placenta have been observed.45 Conversely, in PHP1A, because the maternal allele is biologically deficient, XLαs could play a role in fetal growth and be involved in a positive effect on embryonic development.
Our study, with others in the literature, points to a female predominance in pseudohypoparathyroidism disorders, whereas the SR is normal when the descendants from the same pedigrees are not affected. The reason why more females than males are affected remains unknown, but could suggest a gametic competition at the fertilization stage between X-containing and Y-containing spermatozoids, or differences in chromosome segregation in the zygote, in the context of a disrupted cAMP pathway.41, 46, 47
Because of its retrospective design, this study could be subject to bias in population selection. The sample size, one of the largest groups of patients available in European centers, is, however, sufficient to achieve statistical significance and we observed TRD within two and three generations. We cannot exclude residual ascertainment bias inherent to retrospective transmission studies in rare genetic disorders, despite the use of appropriate methods to limit its effect.
In conclusion, we showed an excess of carriers of mutated GNAS alleles with a maternal TRD, which has important implications for genetic counseling. Our data suggest a role of Gsα in oocyte biology. Our study, with others in the literature, also points to a female predominance in pseudohypoparathyroidism disorders, requiring further investigations.
Disclosures
SS, AM, CC, NC, SP, HM, MG, ES, AG, JB, SO, SN, BD, LF, NG, MLK and NR state that they have no conflicts of interest.
Acknowledgments
We are grateful to the patients and their families for their cooperation in this study; we thank the physicians who have contributed to collect DNA samples, and clinical and laboratory data. We thank Charles Shyng, PhD, Paul Fontaine, PhD, and Antoine Resbeut for their help with the drafting of this manuscript, and Jean-Jacques Parienti, MD, PhD for his help with the statistical analysis.
Authors’ roles: Study design: NR, MLK, and SS. Study conduct: SS. Data collection: NC, CC, MG, ES, AG, JB, SO, SN, BD, LF, HM, NG, and SP. Data analysis: SS. Data interpretation: NR, MLK, AM, and SS. Drafting manuscript: SS. Revising manuscript content: AM, NR, and MLK. Approving final version of manuscript: NR and MLK. SS takes responsibility for the integrity of the data analysis.




