YAP and TAZ Mediate Osteocyte Perilacunar/Canalicular Remodeling
ABSTRACT
Bone fragility fractures are caused by low bone mass or impaired bone quality. Osteoblast/osteoclast coordination determines bone mass, but the factors that control bone quality are poorly understood. Osteocytes regulate osteoblast and osteoclast activity on bone surfaces but can also directly reorganize the bone matrix to improve bone quality through perilacunar/canalicular remodeling; however, the molecular mechanisms remain unclear. We previously found that deleting the transcriptional regulators Yes-associated protein (YAP) and transcriptional co-activator with PDZ-motif (TAZ) from osteoblast-lineage cells caused lethality in mice due to skeletal fragility. Here, we tested the hypothesis that YAP and TAZ regulate osteocyte-mediated bone remodeling by conditional ablation of both YAP and TAZ from mouse osteocytes using 8 kb-DMP1-Cre. Osteocyte-conditional YAP/TAZ deletion reduced bone mass and dysregulated matrix collagen content and organization, which together decreased bone mechanical properties. Further, YAP/TAZ deletion impaired osteocyte perilacunar/canalicular remodeling by reducing canalicular network density, length, and branching, as well as perilacunar flourochrome-labeled mineral deposition. Consistent with recent studies identifying TGF-β as a key inducer of osteocyte expression of matrix-remodeling enzymes, YAP/TAZ deletion in vivo decreased osteocyte expression of matrix proteases MMP13, MMP14, and CTSK. In vitro, pharmacologic inhibition of YAP/TAZ transcriptional activity in osteocyte-like cells abrogated TGF-β-induced matrix protease gene expression. Together, these data show that YAP and TAZ control bone matrix accrual, organization, and mechanical properties by regulating osteocyte-mediated bone remodeling. Elucidating the signaling pathways that control perilacunar/canalicular remodeling may enable future therapeutic targeting of bone quality to reverse skeletal fragility. © 2019 American Society for Bone and Mineral Research.
Introduction
Skeletal fragility diseases are characterized by decreased bone strength. Bone strength is determined by both the quantity and quality of the bone, both of which are necessary to explain fracture susceptibility.1-3 Decreased bone mass is a hallmark of osteoporosis, but defects in bone geometry, microarchitecture, porosity, or matrix material properties are significant contributors to bone fragility.4 Both bone quantity and quality are influenced by bone remodeling, a tightly coordinated process by which old, damaged bone is resorbed and new, strong bone is deposited. Imbalanced bone remodeling, either by excessive bone resorption or decreased bone formation, can impair bone quantity and quality.5, 6 Understanding the mechanisms that control bone remodeling is both scientifically and therapeutically valuable.
The most abundant cell type in bone, osteocytes regulate bone remodeling.7, 8 As terminally differentiated osteoblasts, osteocytes reside within lacunae in the mineralized bone matrix and extend dendritic processes through an interconnected network of micro-channels called canaliculi that permeate the bone matrix.9-11 In humans, this lacunar/canalicular network contains an estimated 3.7 trillion dendritic projections and covers more than 215 m2 of bone surface area.12 Through this widespread network, osteocyte-derived molecules reach the bone surfaces and regulate bone remodeling by coordinating osteoblast/osteoclast coupled remodeling.13, 14 In addition, osteocytes directly resorb and deposit bone in their surrounding bone matrix via perilacunar/canalicular remodeling.15-17 However, the molecular mechanisms by which osteocytes control bone remodeling remain poorly understood.
Osteoblast/osteoclast coordinated bone remodeling is regulated in part through the CCN family of matricellular growth factors, cysteine-rich angiogenic inducer-61 (CYR61, CCN1), and connective tissue growth factor (CTGF, CCN2).18, 19 CYR61 and CTGF have been implicated in activation of osteoblastogenesis20-23 and inhibition of osteoclastogenesis.24 Both CYR61 and CTGF are downstream gene targets of the paralogous transcriptional regulators Yes associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ).25, 26 YAP and TAZ are the key effector proteins of the Hippo signaling pathway and regulate important biological functions including organ size determination, tissue regeneration, and cancer.27, 28 Transcriptional complex formation of nuclear YAP and/or TAZ with the transcriptional enhancer activator domain (TEAD) family proteins is required to induce expression of CYR61 and CTGF.25, 29
In addition to paracrine regulation of osteoblast/osteoclast activity, osteocytes directly remodel the bone matrix through perilacunar/canalicular remodeling. Osteocyte perilacunar/canalicular remodeling is regulated by the TGF-β signaling pathway.30 The TGF-β and YAP/TAZ signaling pathways are known to interact in a variety of cell types, including cancer cells, fibroblasts, and epithelial cells,31-34 and the mechanisms by which TGF-β regulate YAP and TAZ continue to emerge. For example, YAP/TAZ form complexes with the R-SMAD proteins to co-activate TGF-β/SMAD-target gene expression.33, 34 Independent of the R-SMAD proteins, YAP/TAZ interact with additional transcriptional co-factors, such as AP-1 and MRTF, to regulate TGF-β/SMAD-target gene expression.31, 32 Taken together, these observations position YAP and TAZ as potential mediators of TGF-β-mediated bone remodeling in osteocytes.
Here, we conditionally ablated both YAP and TAZ from DMP1-expressing cells and evaluated bone remodeling. In vivo, we found that osteocyte YAP and TAZ regulate perilacunar/canalicular remodeling as well as coordinate osteoblast/osteoclast activity. In vitro, YAP/TAZ inhibition abrogated TGF-β-induced expression of matrix proteases required for osteocyte perilacunar/canalicular remodeling and expression of the matricellular growth factors Cyr61 and Ctgf. Together, these data demonstrate that osteocyte YAP and TAZ control bone matrix accrual, organization, and mechanical properties by regulating osteocyte-mediated bone remodeling.
Materials and Methods
Animals
Mice harboring loxP-flanked exon 3 alleles in both YAP and TAZ on a mixed C57BL/6J genetic background were kindly provided by Dr Eric Olson (University of Texas Southwestern Medical Center). Osteocytes were targeted using Cre-recombination under the control of an 8 kb fragment of the dentin matrix protein-1 promoter (8 kb-DMP1-Cre).35 All mice were fed regular chow (PicoLab Rodent Diet, cat #: 0007688, LabDiet, St. Louis, MO, USA) ad libitum and housed in cages containing 2 to 5 animals each. Mice were maintained at constant 25°C on a 12-hour light/dark cycle. Mice with homozygous floxed alleles for both YAP and TAZ (YAPfl/fl;TAZfl/fl) were mated with double heterozygous conditional knockout mice (YAPfl/+;TAZfl/+;DMP1-Cre) to produce eight possible genotypes in each litter, but only the genotypes in Table 1 were compared. Mice were tail or ear clipped after weaning or before euthanasia and genotyped by an external service (Transnetyx Inc, Cordova, TN, USA). Both male and female mice were evaluated with YAPfl/fl;TAZfl/fl mice serving as littermate wild-type (WT) controls. The different analyses were performed in both male and female young mice at either postnatal day 28 (P28) or postnatal day 84 (P84) as indicated. All protocols were approved by the Institutional Animal Care and Use Committees at the University of Notre Dame and the University of Pennsylvania. All animal procedures were performed in adherence to federal guidelines for animal care and conform to the Animal Research: Reporting of in vivo Experiments (ARRIVE) guidelines.
| Genotype | Abbreviation |
|---|---|
| Yapfl/fl;Tazfl/fl | YAPWT;TAZWT |
| Yapfl/+;Tazfl/+;DMP1cre/+ | YAPcHET;TAZcHET |
| Yapfl/fl;Tazfl/+;DMP1cre/+ | YAPcKO;TAZcHET |
| Yapfl/+;Tazfl/fl;DMP1cre/+ | YAPcHET;TAZcKO |
| Yapfl/fl;Tazfl/fl;DMP1cre/+ | YAPcKO;TAZcKO |
Microcomputed tomography
Micro-computed tomography (micro-CT) was performed according to published guidelines.36 Harvested femurs from P84 mice were stored at −20°C until evaluation. Frozen specimens were thawed and imaged using a vivaCT 80 scanner (Scanco Medical, Bruttisellen, Switzerland) to determine trabecular and cortical femoral bone architecture before mechanical testing. The mid-diaphysis and distal femur were imaged with an X-ray intensity of 114 μA, energy of 70 kVp, integration time of 300 ms, and resolution of 10 μm. Mid-diaphyseal and distal femoral 2D tomograms were manually contoured, stacked, and binarized by applying a Gaussian filter (sigma = 1, support = 1) at a threshold value of 250 mg HA/cm3. Eight mice were analyzed per group. Investigators were blinded to animal genotype during scan quantification.
Mechanical testing
After micro-CT scanning, femurs from P84 mice were tested in three-point bending to failure. Femurs were loaded with the condyles facing down onto the bending fixtures with a support span length of 4.4 mm, which attenuated the effect of femur length on the measured mechanical properties. The upper fixture was aligned with the mid-diaphysis. The femurs were loaded to failure at a rate of 0.5 mm/s using the ElectroForce 3220 Series testing system (TA Instruments, New Castle, DE, USA). Stiffness, maximum load to failure, work to maximum load, and work to failure were quantified using a custom MATLAB script.37 Eight mice were analyzed per group. Investigators were blinded to animal genotype during data quantification.
Osteocyte lacunae visualization and quantification
Nano-computed tomography (nano-CT) was used to evaluate the morphological characteristics of osteocyte lacunae in the proximal tibia (Xradia Versa XRM-520, Zeiss, Dublin, CA, USA). Tibias from P84 mice were harvested and removed of all non-osseous tissue. Samples were scanned in 70% EtOH, in custom-made sample holders that oriented the samples vertically on the stage. Tibias were originally scanned with a 4× objective to determine regions of interest. A 650 μm3 region of bone was located on the anterior medial aspect of the tibia, 8 mm from the tibiofibular junction (TFJ). Nano-computed tomography images image were collected in the region of interest using a 20× objective with energy settings of 40 V, 3.0 W, using the Air Filter and 3201 projections. To obtain a constant resolution of 0.6 μm voxel for all the samples, the source and detector distance were varied between 5.8 and 8.5 mm from the sample, and excitation ranged from 6 to 8 seconds contingent on resulting intensity values.
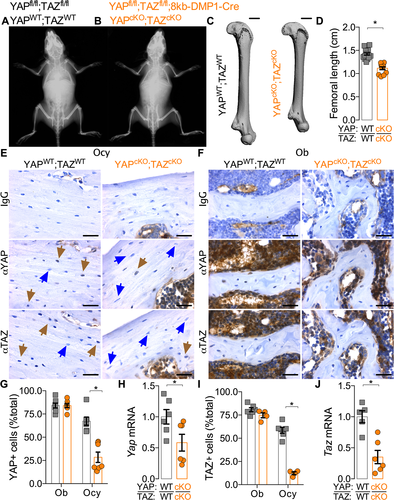
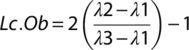 (1)
(1)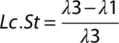 (2)
(2)Histology, immunohistochemistry, and immunofluorescence
Femurs and tibias from P28 and P84 mice were fixed with 10% neutral-buffered formalin for 48 hours at 4°C and decalcified for 4 weeks with 0.25 M EDTA (pH 7.4) at 4°C. Paraffin sections (5 μm thickness) were processed for either immunohistochemistry or histology. Primary antibodies were compared with both normal rabbit sera IgG control sections. For immunostaining, anti-CTSK (1:75, ab19027; Abcam, Cambridge, MA, USA), anti-MMP13 (1:100, ab39012; Abcam), anti-MMP14 (1:100, ab38971; Abcam), anti-YAP (1:200, 14074; Cell Signaling, Danvers, MA, USA), and anti-TAZ (1:200 NB110-58359; Novus Biologicals, Centennial, CO, USA) primary antibodies were applied overnight. Next, sections were incubated with corresponding biotinylated secondary antibody, avidin-conjugated peroxidase, and diaminobenzidine substrate chromogen system (329ANK-60; Innovex Biosciences, Richmond, CA, USA), which allowed for immunohistochemical detection of positively stained cells. Hematoxylin and eosin stains (H&E), tartrate-resistant acid phosphatase (TRAP), picrosirius red, and silver nitrate stains were used to stain for osteoblasts, osteoclasts, collagen, and the osteocyte lacunar/canalicular network as previously shown.44, 45
Femurs from P28 mice were fixed with 10% neutral-buffered formalin for 48 hours at 4°C, transferred to 30% sucrose in PBS overnight at 4°C, and then embedded in OCT compound (Tissue-Tek, Sakura Finetek USA, Torrance, CA, USA). Thin sections (7 μm thickness) were made from undecalcified femurs using cryofilm IIC tape (Section Lab Co. Ltd., Hiroshima, Japan) as previously described46 and processed for immunofluorescence. Taped sections were glued to microscope slides using chitosan adhesive glue, rehydrated, and then decalcified with 0.25 M EDTA (pH 7.4) for 5 minutes before immunofluorescence staining. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays were then performed using the Click-iT Plus TUNEL Assay kit (C10618; Invitrogen, Carlsbad, CA, USA) according to the manufacturerʼs instructions. Thick sections (20 μm thickness) were processed similarly and stained for F-actin with Alexa Fluor 488-conjugated phalloidin (1:50, Life Technologies, Carlsbad, CA, USA) for confocal imaging.
Femurs from P28 mice injected with Calcein (C0875-25G; Sigma-Aldrich, St. Louis, MO, USA) at P21 and Alizarin Complexone (A3882-25G; Sigma-Aldrich) at P26 were embedded in methyl-methacrylate and processed for dynamic bone histomorphometry. Using a diamond-embedded wire saw (Delaware Diamond Knives, Wilmington, DE, USA), transverse sections (40 μm) were cut from the midshaft and ground to a final thickness of 20 μm. The slice sections were mounted on slides, and three sections per limb were analyzed.
Imaging and histomorphometric analysis
Histological and immunohistochemical sections were imaged on either a 90i Upright/Widefield Research Microscope (Nikon Instruments, Melville, NY, USA) at the 20× and 40× objectives or an Axio Observer Z1 (Zeiss) at the 20×, 40×, and 63× objectives. Quantification of paraffin immunohistochemistry and histology was performed using ImageJ (NIH) and Osteomeasure (OsteoMetrics, Decatur, GA, USA). To determine the number of positively immunostained cells, three fields of view per mouse per antibody were manually scored as either positive or negative and reported as percentage positively stained lacunae per total number of lacunae using ImageJ (NIH). To determine canalicular length, primary canaliculi emanating from each lacuna and extending as a single, unbranched process30 were traced with ImageJ (NIH). The mean length was taken from at least 5 osteocytes per field within the mid-cortex, with 3 fields per mouse and 6 mice per group.30 Osteoblast number per bone surface, osteocyte number per bone area, and osteoclast surface per bone surface were quantified using Osteomeasure (OsteoMetrics) on H&E- and TRAP-stained sections in the metaphyseal cancellous bone with 8 mice per group.
The samples tested in three-point bending were stained with picrosirius red to construct a multivariate regression model that included collagen organization and content as a predictor of mechanical behavior. These samples were imaged both under polarized light using an Eclipse ME600 Microscope (Nikon Instruments) at the 20× objective and using second harmonic generated (SHG) microscopy. SHG images were taken on a multiphoton-enabled Fluoview Research Microscope (Olympus) at a fundamental wavelength of 875 nm with the 25× objective on sections oriented in the same direction for all groups. All SHG images were quantified using ImageJ (NIH) and reported as mean pixel intensity within the mid-cortical region relative to WT bone. Mean pixel intensities across four separate regions of interest within each image of the cortex were averaged as technical replicates for a given sample.
Confocal images were acquired using an LSM 710 confocal (Zeiss) with the 63× objective on 20-micron-thick sections. The 15-micron-thick z-stack images were acquired using a step size of 0.5 microns. Three separate regions of interest within the mid-cortex were averaged as technical replicates for a given animal sample. All confocal image stacks were quantified using ImageJ (NIH). Stacks were smoothed by applying a Gaussian filter (sigma = 0.3), then skeletonized using the Skeletonize3D function. The AnalyzeSkeleton2D/3D function was then used to quantify mean branch length, number of branches, and number of junctions.47 Six YAPWT;TAZWT and four YAPcKO;TAZcKO mice were analyzed.
Immunofluorescence sections of P28 femurs were imaged on an Axio Observer Z1 (Zeiss) at the 20×, 40×, and 63× objectives. To determine the number of positively TUNEL-stained lacunae, 3 fields of view per mouse in the metaphyseal cancellous bone were manually scored as either positive or negative and reported as percent positively stained per total number of lacunae scored in ImageJ (NIH). Dynamic bone histomorphometry parameters were quantified using Osteomeasure (OsteoMetrics). The following primary data were collected: total bone surface length (BS); single label perimeter (sL.Pm); double label perimeter (dL.Pm); and double label width (dL.Ith). From primary data, we derived mineralizing surface (MS/BS = [1/2sL.Pm + dL.Pm]/B.Pm × 100; %); mineral apposition rate (MAR = dL.Ith/5 days; μm/d) and bone formation rate (BFR/BS = MAR × MS/BS; μm3/μm2 per day). Six mice were analyzed per group. Investigators were blinded to animal genotype during image acquisition and quantification of all histology, immunohistochemistry, and immunofluorescent images.
Cell culture
IDG-SW3 cells were cultured in α-MEM supplemented with 10% fetal bovine serum, 1% antibiotic/antimycotic (15240062; Gibco) and 1 U/mL INF-γ (PMC4031; Invitrogen) at 33°C. OCY454 cells were cultured in α-minimum essential media (α-MEM) supplemented with 10% fetal bovine serum and 1% antibiotic/antimycotic (Gibco) at 33°C. Before treatment, OCY454 cells were differentiated for 12 days at 37°C, whereas IDG-SW3 cells were differentiated for 21 days at 37°C in α-MEM supplemented with 10% fetal bovine serum (100 to 106; Gemini Bio-Products, West Sacramento, CA, USA), 1% antibiotic/antimycotic (15240062; Gibco), 50 μg/mL ascorbic acid (A-4544; Sigma-Aldrich), and 4 mM β-glycerophosphate (G-9422; Sigma-Aldrich) without INF-γ. Before treatment on the last day of differentiation, both OCY454 and IDG-SW3 cells were treated with 3 μM verteporfin (SML0534; Sigma-Aldrich) overnight in 1% fetal bovine serum. The next morning, the media was changed and supplemented with 3 μM verteporfin and 5 ng/mL TGF-β1 (HZ1011; Humanzyme, Rosemont, IL, USA) for 6 hours before mRNA isolation.
RNA isolation and qPCR
P84 femur samples were dissected and removed of all non-osseous tissue. The ends were cut at the growth plate and marrow flushed before being snap-frozen in liquid nitrogen-cooled isopentane for 1 minute before storage at −80°C until processing. Tissues were then homogenized via mortar and pestle and RNA from the sample was collected using Trizol Reagant (15596026; Life Technologies) followed by centrifugation in chloroform. RNA from femur tissue and cell culture experiments were purified using the RNA Easy Kit (74106; Qiagen, Valencia, CA, USA) and quantified by spectrophotometry using a NanoDrop 2000 (Thermo-Fisher Scientific, Waltham, MA, USA). Reverse transcriptase polymerase chain reaction (RT-PCR) was performed on 0.5 μg/μL concentration of RNA using the High-Capacity cDNA Reverse Transcription Kit (4368814; Thermo-Fisher Scientific). Quantitative polymerase chain reaction (qPCR) assessed RNA amount using a StepOnePlus Real-Time PCR System (Thermo-Fisher Scientific) relative to the internal control of 18S ribosomal RNA (18S rRNA). Data are presented using the ΔΔCt method. Six mice per group were used. Specific mouse primer sequences are listed (Supplemental Table S1).
Statistics and regression
Sample sizes were selected a priori by power analyses based on effect sizes and population standard deviations taken from published data on YAPfl/fl;TAZfl/fl mice in other tissues,48 assuming a power of 80% and α = 0.05. All statistics and regression analyses were performed in GraphPad Prism (GraphPad, La Jolla, CA, USA) or using R (Version 3.5.1). Repeated-measures ANOVA with post hoc Tukey's HSD comparison test was performed using R (Version 3.5.1) for osteocyte lacunae quantification. All other comparisons between two groups were made using the two-tailed Student's t test, provided the data were normally distributed according to D'Agostino-Pearson omnibus normality test and homoscedastic according to Bartlett's test in GraphPad Prism. When parametric test assumptions were not met, data were log-transformed, and residuals were evaluated. If necessary, the nonparametric Mann–Whitney test was used. A p value < 0.05 was considered significant. Post hoc power analyses were performed using R (Version 3.5.1) for phenotypic results as indicated (Supplemental Table S2). Data are presented as bars and individual samples with lines corresponding to the mean and standard error of the mean (SEM).
Multivariate regression analyses were performed as described previously,49 using R (Version 3.5.1) with some modifications.37 Briefly, we used an exhaustive best subsets algorithm to determine the best predictors of maximum load and stiffness from a subset of morphological parameters measured, which included moment of inertia (I) or section modulus (I/c), micro-CT-measured tissue mineral density (TMD), SHG intensity, and femur length. The best subsets algorithm selects the optimal model using the Akaike's information criterion (AIC), which gives preference to less complex models with fewer explanatory parameters to avoid overfitting the data.50 The overall “best” multivariate model for each predicted mechanical property was selected with the lowest relative AIC value, indicative of having the least variables with the greatest predictive power.
Results
DMP1-Cre conditionally ablates YAP and TAZ primarily in osteocytes
To determine the roles of YAP and TAZ in osteocyte-mediated bone remodeling, we used Cre-lox to selectively delete YAP and TAZ from 8 kb-DMP1-Cre expressing cells.35, 51 We used a breeding strategy that generated YAP/TAZ allele dosage-dependent DMP1-conditional knockouts.37 All genotypes (Table 1) appeared at expected Mendelian ratios. By early skeletal maturity (P84), YAP/TAZ allele dosage-dependent DMP1-conditional deletion did not significantly alter body mass in either males or females (Supplemental Fig. S1A). YAP/TAZ deletion reduced femoral length at P84 only in double homozygous knockouts, for both sexes (Fig. 1 A, B; Supplemental Fig. S1B). A single copy of either gene was sufficient to rescue this defect. Therefore, for further analyses, we selected littermate YAPfl/fl;TAZfl/fl WT (YAPWT;TAZWT) and YAPfl/fl;TAZfl/fl;8 kb-DMP1-Cre conditional double knockout (YAPcKO;TAZcKO) mice for comparison.
To analyze the specificity of 8 kb-DMP1-Cre-mediated YAP/TAZ deletion, we evaluated YAP/TAZ expression in osteocytes, osteoblasts, and growth plate chondrocytes. YAP mRNA expression was significantly reduced by 67% in YAPcKO;TAZcKO femoral bone preparations (Fig. 1 C). YAP protein expression was significantly reduced in osteocytes (58% reduction) but not osteoblasts (0% reduction; p = 0.98) (Fig. 1 D, E). Similarly, TAZ mRNA expression was significantly reduced by 72% in YAPcKO;TAZcKO femoral bone preparations (Fig. 1 F), and TAZ protein expression was significantly reduced in osteocytes (79% reduction) but not osteoblasts (6% reduction; p = 0.12) (Fig. 1 G, H). The reduction in YAPcKO;TAZcKO growth plate thickness did not reach statistical significance within the hypertrophic zone (16% reduction; p = 0.26), the proliferating zone (12% reduction; p = 0.39), or total growth plate thickness (13% reduction; p = 0.27; Supplemental Fig. S1C). Further, YAPcKO;TAZcKO growth plate chondrocytes did not show differential expression of YAP or TAZ in either proliferating or hypertrophic zones (Supplemental Fig. S1D–G).
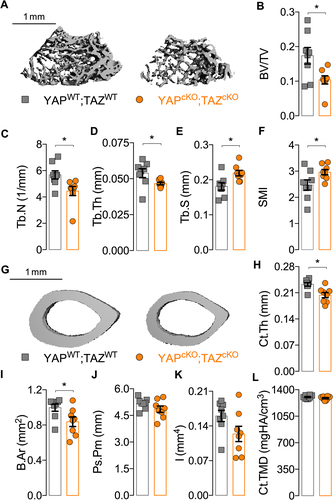
DMP1-conditional YAP/TAZ deletion impaired bone accrual
Dual homozygous YAP/TAZ deletion from DMP1-expressing cells reduced bone accrual and microarchitectural parameters in both the cancellous and cortical compartments of P84 femurs. In metaphyseal cancellous bone, conditional YAP/TAZ deletion significantly reduced bone volume fraction, trabecular number, and thickness and increased trabecular spacing and structural model index (Fig. 2 A–F). In mid-diaphyseal femoral cortical bone, conditional YAP/TAZ deletion reduced cortical thickness and bone area without changing medullary area (Fig. 2 G–I; Supplemental Fig. S2). Differences in TMD (p = 0.24), periosteal perimeter (p = 0.15), and moment of inertia (p = 0.08) were not statistically significant (Fig. 2 J–L).
To determine if the low bone mass phenotype resulted from decreased bone formation and/or increased bone resorption, we evaluated osteoblast and osteoclast activity. Conditional YAP/TAZ deletion increased osteoclast surface per bone surface (Fig. 3 A, B) and decreased osteoblast number per bone surface (Fig. 3 C, D) in P84 distal femur metaphyseal cancellous bone. YAP/TAZ deletion decreased bone formation rate, reducing both mineralizing surface percentage and mineral apposition rate at P28 in distal femur metaphyseal cancellous bone (Fig. 3 E–H). In the cortical compartment, YAP/TAZ deletion similarly reduced osteoblast number per endosteal bone surface (Supplemental Fig. S3A, B) and decreased bone formation rate by reducing mineral apposition rate without altering mineralizing surface percentage (Supplemental Fig. S3C–F).
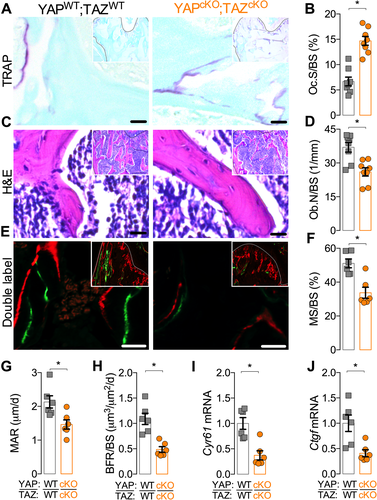
Further, YAP/TAZ deletion significantly reduced mRNA expression of YAP/TAZ-TEAD target genes, Cyr61 and Ctgf in femoral bone at P84 (Fig. 3 I, J). However, YAP/TAZ deletion did not alter sclerostin (SOST), receptor activator of nuclear factor kappa-B ligand (Rankl), or osteoprotegerin (Opg) transcript expression (Supplemental Fig. S4).
DMP1-conditional YAP/TAZ deletion impaired bone mechanical properties and matrix collagen composition
To determine whether YAP/TAZ deletion impaired functional mechanical properties, we tested P84 femurs in three-point bending to failure (Fig. 4 A). Conditional YAP/TAZ deletion did not significantly reduce maximum load to failure (Fig. 4 B; p = 0.07) but significantly reduced bending stiffness, work to maximum load, and work to failure (Fig. 4 C–E).

Intrinsic bone material properties are determined, in part, by collagen content and organization.37 To test whether osteocyte YAP/TAZ regulate the local collagen matrix, we performed polarized light and SHG microscopy (Fig. 4 F). YAP/TAZ deletion qualitatively reduced picrosirius red staining and significantly reduced SHG intensity per bone area, indicative of both collagen content and organization (Fig. 4 G). Both cross-sectional bone geometry (section modulus and moment of inertia) and collagen matrix content and organization significantly contributed to bone stiffness according to a multivariate best-subsets regression analysis using AIC, which selects the model with the lowest number of predictors with the greatest predictive power.37, 50 Neither micro-CT-measured TMD nor femur length contributed to the overall best model according to AIC for stiffness and maximum load to failure (Fig. 4 H, Supplemental Fig. S5).
DMP1-conditional YAP/TAZ deletion impaired the osteocyte canalicular network, but not lacunar morphology
We initially hypothesized that the primary function of YAP/TAZ in osteocytes was to regulate expression of genes that control osteocyte-osteoblast and -osteoclast communication, such as secreted factors CYR61 and CTGF. However, the reduced bone mechanical properties and disorganized collagen matrix in mice lacking YAP/TAZ from osteocytes phenocopied matrix metalloproteinase-13 (MMP13) knockout mice, which had defective collagen organization as a result of impaired perilacunar/canalicular remodeling.52 This led us to ask whether YAP/TAZ deletion from osteocytes impaired perilacunar/canalicular remodeling.
Conditional YAP/TAZ deletion did not significantly alter osteocyte density but increased empty lacunae percentage in cancellous metaphyseal bone (Fig. 5 A–C). Accordingly, conditional YAP/TAZ deletion increased the number of TUNEL-positive lacunae, suggesting an increase in osteocyte apoptosis within the cancellous bone compartment (Fig. 5 D, E). Conditional YAP/TAZ deletion significantly reduced canalicular density and mean process length, measured in silver nitrate-stained, mid-cortical bone sections (Fig. 5 F–H).
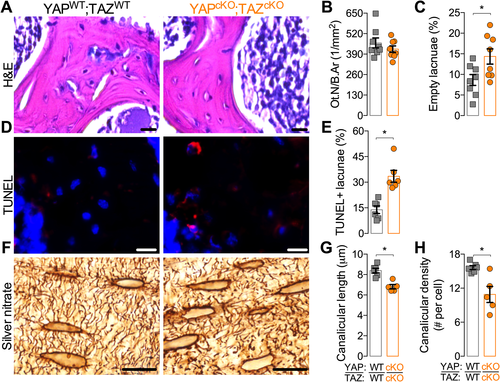
As the canalicular system is a three-dimensional network of branched cell processes that physically connect osteocytes to their neighbors,12 we next quantified the 3D canalicular network by laser scanning confocal microscopy of the osteocyte actin cytoskeleton, labeled with phalloidin (Fig. 6 A). YAP/TAZ deletion significantly reduced branch length (Fig. 6 B, C), number of branches per cell (Fig. 6 D), and number of junctions per cell (Fig. 6 E).
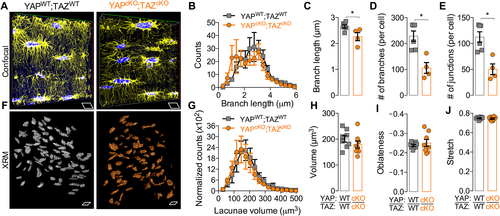
To determine the effect of YAP/TAZ deletion on osteocyte lacunar morphology, we performed high-resolution (0.6 um voxel) X-ray microscopy (XRM) imaging of both cortical and cancellous bone in the proximal tibia metaphysis (Fig. 6 F). YAP/TAZ deletion did not significantly alter lacuna volume (Fig. 6 G, H) or shape (Fig. 6 I, J), as significant interaction factors were observed between sex, bone compartment, and genotype for all lacunar morphology parameters. Using post hoc multiple comparisons, no differences in lacunar parameters were observed between genotypes within either a single sex or bone compartment.
DMP1-conditional YAP/TAZ deletion reduced osteocyte-mediated bone matrix remodeling
Perilacunar/canalicular remodeling involves both the deposition and degradation of the bone matrix directly surrounding the osteocytes. Prior studies used 3H-proline pulses and tetracycline labeling to identify osteocyte lacunae as sites of active collagen deposition and mineralization.15, 53, 54 Here, we measured collagen I gene expression and flourochrome incorporation in osteocyte lacunae to assess peri-osteocyte matrix deposition. Conditional YAP/TAZ deletion reduced the percentage of Calcein-labeled osteocyte lacunae (Fig. 7 A, B) and reduced transcript expression of Col1a1 (Fig. 7 C). However, YAP/TAZ deletion did not alter expression of either osteogenic genes, including alkaline phosphatase (Alp), osteocalcin (Ocn), and bone sialoprotein (Bsp), or osteocyte-marker genes, dentin matrix protein-1 (Dmp1) or phosphate-regulating neutral endopeptidase, X-linked (Phex) (Supplemental Fig. S6).
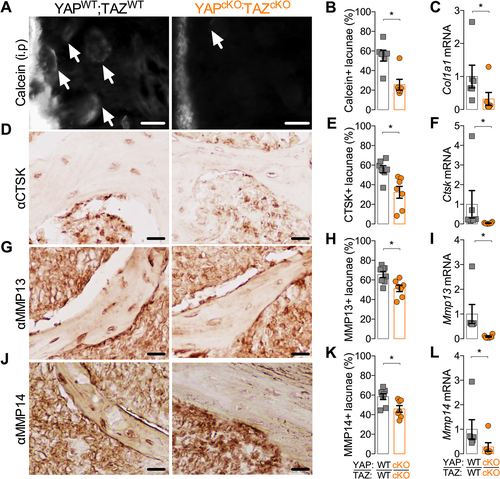
In addition to local matrix deposition, osteocytes also locally degrade their extracellular matrix. Many of the matrix metalloproteinases and other enzymes employed by osteoclasts to resorb bone are also expressed by osteocytes and are critical to perilacunar/canalicular remodeling.17, 52, 55 Therefore, to test whether YAP/TAZ regulate direct matrix remodeling by osteocytes, we measured mRNA and protein expression of the matrix proteases, cathepsin K (CTSK), matrix metalloproteinase-13 (MMP13), and matrix metalloproteinase-14 (MMP14). Conditional YAP/TAZ deletion reduced the percentage of osteocytes that stained positive for CTSK (Fig. 7 D, E), MMP13 (Fig. 7 G, H), and MMP14 (Fig. 7 J, K) and decreased mRNA expression of Ctsk (Fig. 7 F), Mmp13 (Fig. 7 I), and Mmp14 (Fig. 7 L) in vivo.
YAP/TAZ inhibition abrogated TG-β-induced gene expression in vitro
Though unexplored in osteocytes, YAP and TAZ are known to mediate TGF-β signaling in a variety of other cell types.31-34 Further, either pharmacologic inhibition or genetic ablation of TGF-β receptors from osteocytes caused defective perilacunar/canalicular remodeling and associated gene expression.30 Therefore, we next tested whether YAP/TAZ mediate TGF-β signaling, Cyr61/Ctgf expression, and perilacunar/canalicular remodeling-associated gene expression in osteocytes by treating two osteocyte-like cell lines with combinatorial TGF-β and/or verteporfin (VP) treatment. VP is a small molecule inhibitor that physically blocks the interaction of YAP and TAZ with their transcriptional co-effectors, particularly the TEAD transcription factors, preventing YAP/TAZ-TEAD transcriptional activity.37, 56
We first used mouse osteocyte-derived IDG-SW3 cells, which carry a DMP1 cis-regulatory system driving GFP expression, as a marker of living osteocytes.57, 58 As shown previously, IDG-SW3 cells exhibited robust DMP1-GFP transgene expression after 21 days of in vitro osteocytic differentiation (Fig. 8 A). Beginning at day 20, cells were treated with vehicle (DMSO) or 3 μM verteporfin (Fig. 8 A). At day 21, cells were treated with 5 ng/mL TGF-β or PBS vehicle for 6 hours and gene expression was evaluated by qPCR. Similar experiments were conducted in mouse osteocyte-derived OCY454 cells59 (Supplemental Fig. S7A).
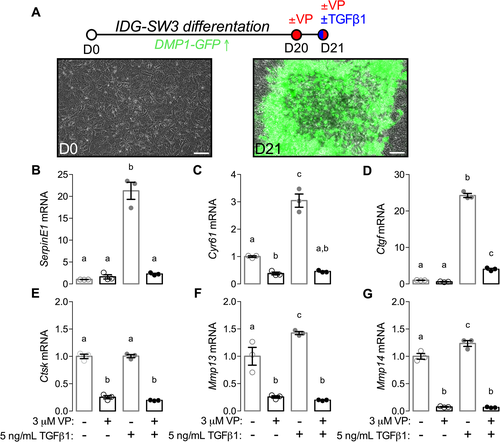
First, to test whether verteporfin blocked TGF-β-induced YAP/TAZ signaling, we evaluated expression of serpin family E member 1 (SerpinE1) and expression of known osteoblast/osteoclast paracrine signaling factors, Cyr61 and Ctgf. Each of these genes are established TGF-β-inducible, YAP/TAZ-TEAD target genes.25, 26, 60, 61 As expected, TGF-β robustly induced SerpinE1, Cyr61, and Ctgf mRNA expression, which was abrogated by verteporfin treatment (Fig. 8 B–D). Next, to test whether YAP/TAZ mediate TGF-β induction of genes associated with perilacunar/canalicular remodeling, we quantified mRNA expression of Ctsk, Mmp13, and Mmp14. TGF-β significantly induced expression of Mmp13 and Mmp14, but not Ctsk, in IDG-SW3 cells, and verteporfin treatment abrogated expression of all three genes (Fig. 8 E–G). In OCY454 cells, verteporfin similarly abrogated TGF-β-induced expression of Ctgf, Cyr61, and SerpinE1 mRNA (Supplemental Fig. S7B–D) as well as Mmp14 and Ctsk but not Mmp13 (Supplemental Fig. S7E–G). Finally, we evaluated Sost, Opg, and Rankl expression in both IDG-SW3 and OCY454 cells as potential YAP/TAZ-regulated mediators of osteocyte-osteoblast/osteoclast signaling. In IDG-SW3 cells, both verteporfin and TGF-β significantly reduced Sost transcript expression, while verteporfin abrogated TGF-β-induced Rankl and Opg expression (Supplemental Fig. S8A–C). In OCY454 cells, verteporfin abrogated TGF-β-induced Opg and reduced Sost expression but did not significantly alter Rankl expression (Supplemental Fig. S8D–F). In contrast to IDG-SW3 cells, treatment of OCY454 cells with TGF-β alone significantly reduced Rankl transcript expression (Supplemental Fig. S8E).
Discussion
This study identifies new roles of the transcriptional regulators YAP and TAZ to enhance our understanding of how osteocyte-mediated bone remodeling contributes to skeletal fragility. Here, we show that YAP and TAZ control bone matrix accrual, organization, and mechanical properties by regulating both perilacunar/canalicular remodeling and osteoblast/osteoclast activity, potentially through TGF-β signaling in osteocytes. Osteocyte-conditional YAP/TAZ deletion reduced bone mass by decreasing osteoblast number and activity and increasing osteoclast activity but also impaired osteocyte-intrinsic pericellular matrix deposition and degradation, impairing canalicular network connectivity without altering lacunar morphology. In vitro, we found that YAP/TAZ activity was required for TGF-β1 induction of matricellular growth factors involved in paracrine signaling from osteocytes to osteoblasts/osteoclasts and matrix proteases necessary for perilacunar/canalicular remodeling. Together, these data identify the transcriptional co-activators YAP and TAZ as key mediators of bone remodeling.
The roles of YAP and TAZ in skeletal-lineage cells are beginning to be clarified. Previously, we reported that dual YAP/TAZ deletion from osteoprogenitor cells and their progeny using Osterix-Cre mimicked severe cases of osteogenesis imperfecta.37 We identified a combinatorial role for YAP and TAZ in promoting osteoblast number and activity and suppressing osteoclast activity.37 Similarly, YAP deletion later in the osteoblast lineage, from committed osteoblasts using Osteocalcin-Cre, significantly reduced bone formation, further supporting a role for YAP in promoting osteogenesis in vivo.62 That study did not observe changes in osteoclast activity, potentially because of compensatory effects of TAZ or the Cre line used.62 We show here that 8 kb-DMP1-Cre-conditional YAP/TAZ deletion from osteocytes also reduced osteoblast number and activity and increased osteoclast activity. Consistent with our data,63 Xiong and colleagues found that YAP/TAZ deletion using 10 kb-DMP1-Cre reduced bone formation and increased osteoclast numbers.64 The YAP/TAZ-TEAD target CTGF was previously reported to directly bind to RANKL and OPG to induce osteoclast differentiation in vitro,65 but neither the present study nor Xiong and colleagues64 observed significant changes in transcript expression of Sost or Opg/Rankl in vivo. Our in vitro experiments in osteocyte-like IDG-SW3 and OCY454 cells did not reveal consistent regulation of Sost, Opg, or Rankl by YAP/TAZ-TEAD, warranting further mechanistic studies.
Here, we found that YAP/TAZ deletion in vivo and inhibition in vitro reduced osteocyte expression of the matricellular growth factors Cyr61 and Ctgf. Both CYR61 and CTGF are induced by TGF-β60, 61 and are transcriptionally regulated directly by the YAP/TAZ-TEAD complex.25, 26 CYR61 and CTGF are expressed by osteocytes18 and have been implicated in both osteoblastogenesis22, 23 and osteoclastogenesis.24, 66 During osteoblastogenesis in vitro, CYR61 enhances mesenchymal stem cell migration and regulates WNT3A-induced osteogenic differentiation.22 CTGF also enhances osteoblastogenesis in vitro, in part by inhibiting Notch signaling and inducing HES-1 transcription and NFAT transactivation.23 In vivo deletion of CTGF in Osteocalcin-expressing cells led to a mild low bone mass phenotype in male mice without alterations in osteoblast or osteoclast numbers.67 Similarly, deletion of CYR61 at various stages of the osteoblast lineage (Osterix-Cre, Collagen1(2.3 kb)-Cre, and Osteocalcin-Cre) resulted in low bone mass phenotypes of similar severity, suggesting mature osteoblasts/osteocytes are the primary source of CYR61 in bone.19 Deletion of CYR61 from Osteocalcin-expressing mature osteoblasts also increased osteoclast numbers in vivo,19 consistent with the effects of osteocyte-conditional YAP/TAZ deletion here. CYR61 inhibits osteoclastogenesis in vitro through a RANKL-independent mechanism,24 while CTGF has been observed to promote osteoclast-precursor fusion through interaction with dendritic cell-specific transmembrane protein (DC-STAMP).66 Future studies will be required to dissect the varied mechanisms by which osteocytes direct osteoblast/osteoclast-mediated remodeling, but these data identify a role for YAP/TAZ in osteocyte-mediated promotion of osteoblast activity and suppression of osteoclast activity and implicate a TGF-β–YAP/TAZ-TEAD signaling axis in CYR61 and CTGF expression by osteocytes.
In addition to regulating osteocyte-mediated coordination of osteoblasts and osteoclasts, we found that YAP/TAZ deletion also impaired perilacunar/canalicular remodeling of the bone matrix directly by osteocytes. TGF-β signaling is an osteocyte-intrinsic regulator of perilacunar/canalicular remodeling.30 Here, we found that DMP1-conditional YAP/TAZ deletion phenocopied the effects of both DMP1-conditional TGF-β receptor deletion and pharmacologic inhibition, which impaired canalicular network length and reduced expression of matrix-degrading enzymes required for perilacunar/canalicular remodeling (ie, MMP13, MMP14, and CTSK) without altering lacunar morphology,30 as observed here. Confirming the function of these regulated genes, DMP1-conditional YAP/TAZ deletion also partially phenocopied knockout mouse models of these important matrix proteases. First, global knockout of MMP13 in mice osteocyte inhibited perilacunar/canalicular remodeling by reducing collagen organization, flourochrome-labeled mineral deposition around osteocytes, and canalicular network connectivity with similar effect sizes to DMP1-conditional YAP/TAZ deletion.52 Second, global knockout of MMP14 in mice significantly reduced canalicular network development and maintenance, reducing osteocyte processes' density and length.55 Notably, targeted deletion of MMP14 in skeletal lineage cells using Dermo1-Cre reduced YAP/TAZ activity in osteocytes,68 suggesting a potential feedback loop between matrix protease activity and matrix mechanotransduction. Lastly, global knockout of CTSK in mice impaired bone matrix collagen organization that increased bone fragility in spite of high bone mass and increased osteoclast activity.69 We therefore pose the working hypothesis that YAP and TAZ act downstream of TGF-β to mediate perilacunar/canalicular remodeling in osteocytes; further investigation will be required to test this in vivo.
Perilacunar/canalicular remodeling and osteoblast/osteoclast-mediated remodeling coordinately determine skeletal strength.5, 6 Compromised bone strength caused by defects in bone mass and/or quality results in fragility fracture.4-6 Bone quantity is primarily regulated by the balance of bone turnover rate, while quality includes matrix composition and microarchitectural geometry.4, 5 Both of these factors contribute to the pathogenesis of skeletal fragility diseases such as osteoporosis, Paget's disease, and osteogenesis imperfecta.5 Here, we found that osteocyte-conditional YAP/TAZ deletion decreased bone mass and altered collagen matrix content and organization, producing moderate defects in bone mechanical behavior. Osteocyte-specific YAP/TAZ knockouts did not exhibit the spontaneous bone fractures prevalent in mice lacking YAP/TAZ in the full osteoblast lineage,37 but their defects correspond with the increased bone fragility described in other models of defective perilacunar/canalicular remodeling.30, 52 Age-related decreases in canalicular network connectivity are associated with microdamage accumulation that contributes to age-associated skeletal fragility,70 further suggesting a contribution of the canalicular network to bone strength.
This study has several limitations. First, small sample sizes (n = 4–8) produced underpowered analyses for some outcome measures. Post hoc power analyses for indicated perilacunar/canalicular phenotypic results are shown in Supplemental Table S2. Power ranged from 0.74 to 0.99. Thus, not all the studies performed had enough power to compare sex as a variable, though our initial assessment of skeletal phenotype did not show a significant effect of sex. Second, two different age of mice were used (P28 and P84). Both time points represent young mice with robust osteocyte canalicular networks, avoiding the potentially confounding effects of age-related canalicular network deterioration.70 However, significant changes in development and bone growth occur in mice between P28 and P84; future studies will characterize the extent of canalicular development during postnatal growth. Third, recent reports suggest that both the 8-kb and 10-kb DMP1 promoter fragments used to drive Cre recombinase expression for conditional ablation in osteocytes can also induce recombination in mature osteoblasts and other cell types, depending on the sensitivity of the floxed alleles.35, 71-73 Although we observed minimal recombination in osteoblasts and growth plate chondrocytes, the 8-kb DMP1-Cre model used here is not an inducible model, and potential targeting of earlier stage osteogenic cells could still contribute to the developmental changes observed. Fourth, our data suggest that YAP/TAZ act downstream of TGF-β in osteocytes, but continued study will be required to determine whether YAP and TAZ act downstream of TGF-β in vivo and to define the transcriptional mechanisms by which YAP and TAZ regulate perilacunar/canalicular remodeling genes. Finally, osteocytes are the key mechanosensing cells in bone,74 and YAP and TAZ are important mediators of mechanotransduction in many cell types75; however, the putative roles of YAP and TAZ in osteocyte mechanotransduction are unknown. Because mechanical loading also induces perilacunar/canalicular remodeling,76 continued investigation is warranted.
In conclusion, this study identifies the transcriptional co-activators YAP and TAZ as regulators of bone quantity and quality that mediate osteocyte regulation of osteoblast/osteoclast activity and perilacunar/canalicular remodeling. Specifically, osteocyte YAP and TAZ are required for the expression of paracrine growth factors that regulate osteoblast/osteoclast-coupled remodeling (ie, Cyr61, Ctgf) and the effector enzymes that enable perilacunar/canalicular remodeling. Further elucidation of the mechanisms by which the TGF-β and YAP/TAZ signaling pathways interact to regulate osteocyte function may help guide the development of targeted therapies for the treatment of skeletal fragility diseases.
Disclosures
The authors have nothing to disclose.
Acknowledgments
YAPfl/fl;TAZfl/fl mice were provided by Dr Eric Olson (University of Texas Southwestern Medical Center). Theresa Sikorski (University of Notre Dame) provided initial mouse husbandry and maintenance.
This work was supported by the National Institute of Arthritis, Musculoskeletal, and Skin Diseases (NIAMS) of the National Institutes of Health through grants T32 AR007132 (to CDK), P30 AR069619 (to JDB), and R21 AR071559 (to JDB, AGR, and TMB).
Authors' roles: CDK and JDB designed research; CDK, JCC, KMJ, AGR, VLF, TMB, and JDB analyzed data; CDK, JCC, KMJ, and DJH performed research; JCC, DJH, AGR, LQ, VLF, and TMB contributed new reagents/analytic tools; CDK and JDB wrote the paper; all authors reviewed and approved the paper. CDK and JDB take responsibility for integrity of data analysis.




