A Rare Mutation in SMAD9 Associated With High Bone Mass Identifies the SMAD-Dependent BMP Signaling Pathway as a Potential Anabolic Target for Osteoporosis
ABSTRACT
Novel anabolic drug targets are needed to treat osteoporosis. Having established a large national cohort with unexplained high bone mass (HBM), we aimed to identify a novel monogenic cause of HBM and provide insight into a regulatory pathway potentially amenable to therapeutic intervention. We investigated a pedigree with unexplained HBM in whom previous sequencing had excluded known causes of monogenic HBM. Whole exome sequencing identified a rare (minor allele frequency 0.0023), highly evolutionarily conserved missense mutation in SMAD9 (c.65T>C, p.Leu22Pro) segregating with HBM in this autosomal dominant family. The same mutation was identified in another two unrelated individuals both with HBM. In silico protein modeling predicts the mutation severely disrupts the MH1 DNA-binding domain of SMAD9. Affected individuals have bone mineral density (BMD) Z-scores +3 to +5, mandible enlargement, a broad frame, torus palatinus/mandibularis, pes planus, increased shoe size, and a tendency to sink when swimming. Peripheral quantitative computed tomography (pQCT) measurement demonstrates increased trabecular volumetric BMD and increased cortical thickness conferring greater predicted bone strength; bone turnover markers are low/normal. Notably, fractures and nerve compression are not found. Both genome-wide and gene-based association testing involving estimated BMD measured at the heel in 362,924 white British subjects from the UK Biobank Study showed strong associations with SMAD9 (PGWAS = 6 × 10−16; PGENE = 8 × 10−17). Furthermore, we found Smad9 to be highly expressed in both murine cortical bone–derived osteocytes and skeletal elements of zebrafish larvae. Our findings support SMAD9 as a novel HBM gene and a potential novel osteoanabolic target for osteoporosis therapeutics. SMAD9 is thought to inhibit bone morphogenetic protein (BMP)-dependent target gene transcription to reduce osteoblast activity. Thus, we hypothesize SMAD9 c.65T>C is a loss-of-function mutation reducing BMP inhibition. Lowering SMAD9 as a potential novel anabolic mechanism for osteoporosis therapeutics warrants further investigation. © 2019 The Authors. Journal of Bone and Mineral Research published by American Society for Bone and Mineral Research.
Introduction
Age-related bone loss with deterioration of skeletal architecture leads to osteoporosis, affecting 8.2 million women and 2.0 million men aged 50 years and older in the United States (US).1 Worldwide, osteoporosis causes more than 8.9 million fractures annually.1 Osteoporotic fractures and their treatment are a major cause of morbidity and mortality, with annual US health care costs exceeding $20 billion.2 Most osteoporosis treatment approaches, including all oral medications, reduce bone resorption and prevent further bone loss, rather than enhance bone formation. Affordable anabolic treatments, which can restore bone mass and skeletal architecture, are much needed.
Romosozumab, a monoclonal antibody against sclerostin, represents a new class of anti-osteoporosis drug, recently approved by the FDA.3, 4 Sclerostin, a key inhibitor of bone formation, was discovered through study of two rare syndromes of extreme high bone mass (HBM) due to mutations in SOST.5, 6 SOST encodes Sclerostin, which binds to low-density lipoprotein receptor-related proteins 5 and 6 (LRP5 and LRP6) to prevent activation of canonical WNT signaling in bone, resulting in decreased bone formation. Gain-of-function mutations in LRP5 and LRP6 can also cause extreme HBM.7, 8 Together these sclerosing bone dysplasias are characterized by mandible enlargement with tori of the palate and mandible, bone overgrowth leading to nerve compression, a tendency to sink when swimming, and, importantly, resistance to fracture.5, 7, 9 These important gene discoveries validate the study of rare monogenic HBM as an approach to identify novel therapeutic targets for drug development toward osteoporosis treatments.
We have previously shown that HBM (defined as a total hip and/or first lumbar vertebral bone mineral density [BMD] Z-score of ≥ +3.2) is observed in 0.18% of dual-energy X-ray absorptiometry (DXA) scans in the UK.10 Most cases are unexplained; ie, they do not carry mutations in established HBM genes.9 Although such HBM populations do show enrichment for common variant associations with established BMD-associated loci,11 we hypothesized that novel causes of monogenic HBM remain to be determined. Thus, we aimed to identify novel monogenic causes of HBM to provide insight into regulatory pathways amenable to therapeutic intervention.
Materials and Methods
The UK HBM cohort
The HBM study is a UK-based multicenter observational study of adults with unexplained HBM, identified incidentally on routine clinical DXA scanning. Full details of DXA database screening and participant recruitment have been reported10 (Supplemental Methods in File S1). In brief, DXA databases containing 335,115 DXA scans across 13 UK centers were searched; all scans explained by artefact or known causes of high BMD were excluded. Unexplained HBM was defined as 1) first lumbar vertebra (L1) Z-score of ≥ +3.2 plus total hip (TH) Z-score of ≥ +1.2 and/or 2) TH Z-score ≥ +3.2 plus L1 Z-score of ≥ +1.2. A total of 533 unexplained HBM cases were invited to participate; 248 (47%) were recruited. They passed on study invitations to first-degree relatives and spouse/partner(s). Finally, 236 of 893 (26.4%) invited relatives were recruited, as were 61 of 217 (28.1%) invited spouses/partners.10 All participants underwent structured clinical assessment and DXA scanning (Supplemental Methods in File S1). Peripheral quantitative computed tomography (pQCT) scans were performed at the distal and mid-shaft of the tibia (4% and 66% from distal endplate) in the nondominant lower limb using a Stratec XCT2000L (Stratec Medizintechnik, Pforzheim, Germany) as published previously12 (Supplemental Methods in File S1). Two non-fasted EDTA samples were collected and serum separated and frozen within 4 hours to −80°C. Bone formation (Procollagen type 1 amino-terminal propeptide [P1NP], total osteocalcin) and resorption (β-C-telopeptides of type I collagen [βCTX]) markers and sclerostin were measured (Supplemental Methods in File S1). DNA was extracted from peripheral venous blood using standard phenol/ chloroform extraction. Sanger sequencing of all HBM index cases for exons 2, 3, and 4 of LRP5, SOST (including the van Buchem disease deletion), and LRP4 (exons 25 and 26) excluded seven individuals with LRP5 mutations and one with a SOST mutation, leaving 240 unexplained HBM individuals.9
Anglo-Australasian Osteoporosis Genetics Consortium (AOGC) HBM and LBM cases
The original AOGC extreme truncate population included 1128 Australian, 74 New Zealand, and 753 British women, aged 55 to 85 years, ≥5 years postmenopausal, with either HBM (age- and sex-adjusted TH BMD Z-scores +1.5 to +4.0, n = 1055) or low bone mass (LBM) (Z-scores −4.0 to −1.5, n = 900).13 LBM cases were excluded if they had secondary causes of osteoporosis (as previously described13). Unrelated samples of white ancestry with complete height and weight data and enough high-quality genomic DNA were available in 947 individuals (426 AOGC high and 521 AOGC low BMD), from which (computation capacity limited sample size) the most extreme HBM cases were selected using a threshold TH or LS Z-score ≥ +2.5, and the most extreme LBM cases using a LS Z-score ≤ −0.5, so 126 HBM and 493 LBM samples were chosen to undergo whole exome sequencing (WES).
Whole exome sequencing
Sequencing libraries for 859 samples (240 UK HBM, 126 AOGC HBM, and 493 AOGC LBM) were constructed. Base calling, sequence alignment and variant calling were performed as previously described14 (details in Supplemental Methods in File S1).
Filtering pipeline applied to unexplained HBM pedigrees
After quality-filtering, data were analyzed for carriage of at least one rare (either novel or maximum population-based minor allele frequency [MAF] <0.005) nonsynonymous single-nucleotide variant (SNV) or indel in a highly conserved region (GERP score < 1.5) of a gene, carried by the affected individuals and not carried by unaffected individuals (ie, autosomal dominant carriage model). Data were then filtered based on functional prediction of SNVs using Polyphen15 to identify “probably damaging” and SIFT16 “deleterious” SNVs. Compound heterozygous and homozygous inheritance were also assessed.
Sanger sequencing validation of pedigree-based HBM mutation
Polymerase chain reaction (PCR) amplification of identified exons was performed on 50 ng genomic DNA (see Supplemental Methods 4 in File S1). Electropherograms were aligned and analysed using sequence analysis software Genalys (Version 2.0 ß, Masazumi Takahashi).
Multi-marker analysis of GenoMic annotation (MAGMA) in UK Biobank
Gene-based tests of association were performed on 362,924 unrelated white British subjects (54% female) from the UK Biobank study with ultrasound-derived heel estimated BMD (eBMD) and high-quality genomewide HRC and 1000G/UK10K imputed data (Supplemental Methods in File S1). Detailed methodology has been published.17 Gene-based tests of association were implemented in MAGMA v1.0618 using a multimodel approach combining association results from three separate gene analysis models: principal components regression, single-nucleotide polymorphism (SNP)-wise mean chi-square model (ie, test statistic derived as sum of -log(SNP p value)) and SNP-wise top chi-square model (test statistic derived as sum of -log(SNP p value) for top SNPs) to produce an aggregate p value corresponding to the association between each of the 19,361 protein coding genes (±20 kb) and BMD, adjusting for age, sex, genotyping array, assessment center, and 20 ancestry informative principal components, with gene-based significance threshold (p < 2.87 × 10−6).17
Phenomewide association study (PheWAS)
PheWAS was conducted using GWASATLAS (https://atlas.ctglab.nl/), an online database of publicly available summary results statistics from 4,155 GWAS from 295 unique studies across 2960 unique traits and 27 domains.19 Significance for pleiotropic associations used a traditional genome-wide significance threshold for SNP-trait PheWAS (p < 5 × 10−8).17
Gene expression in murine osteocytes
Whole transcriptome sequencing data from the primary osteocytes of four different bone types (tibia, femur, humerus, and calvaria) from mice (marrow removed, n = 8 per bone) were analyzed. A threshold of expression was determined based on the distribution of normalized gene expression for each sample.20 “Expressed” genes were those exceeding this threshold for all 8 of 8 replicates in any bone type. Osteocyte-enriched expression of these genes in the skeleton was determined by comparing transcriptome-sequencing data from bone samples with osteocytes isolated versus those samples with marrow left intact (n = 5 per group).21
Replication in high BMD populations
WES data from AOGC were analyzed to identify any individual who carried the same rare (MAF < 0.025) mutation as identified from analysis of the HBM pedigree. Polyphen15 and SIFT,16 PMut22 and MutationTaster23 were used for in silico functional prediction. When the same point mutation was identified in more than one individual, haplotypes were compared between index case samples genotyped using an Infinium OmniExpress-12v1.0 GWAS chip read using an Illumina iScan (San Diego, CA, USA), with genotype clustering performed using Illumina BeadStudio software.
Protein structural modeling
The amino-acid sequence of human SMAD9 was passed to the HHPred server.24 This located the best template structures in the Protein Databank for the MH1 domain, 5×6G (mouse SMAD5; 92% identity), and the MH2 domain, 3GMJ (Drosophila melanogaster MAD; 75% identity). Modeler was used to build the domain models according to the HHPred alignments.25 Chimera was used to introduce point mutations and remodel the domain swapping in the SMAD9-MH1 model.26
Zebrafish studies
BMPre:GFP (Tg(5xBMPRE-Xla.Id3:GFP))27 and sp7:GFP (Tg(Ola.sp7:NLS-GFP))28 transgenic fish (in London AB background) were housed and maintained in standard conditions.29, 30 Experiments were approved by the University of Bristol Animal Welfare and Ethical Review Body (AWERB) and performed in accordance with a UK Home Office project license. Developmentally staged larvae (after euthanization in MS222) were fixed in 4% paraformaldehyde (1 hour), dehydrated to 100% methanol, and stored at −20°C before staining. Immunolabeling was as previously described.31 Primary antibodies were anti-Smad9 (rabbit polyclonal, Abcam, Cambridge, MA, USA, ab96698) used at a 1/100 dilution and anti-GFP (chicken polyclonal, Abcam, ab13970) used at a 1/200 dilution in blocking buffer (5% horse serum). Secondary antibodies were used (A21206 and A11041, Invitrogen, Carlsbad, CA, USA) in a 1/400 dilution and samples incubated with DAPI (Sigma-Aldrich, St. Louis, MO, USA, 1/1000 dilution) to visualize nuclei. Samples were mounted in 1% low melting point agarose and imaged with a confocal laser scanning microscope (Leica, Buffalo Grove, IL, USA, SP5II AOBS attached to a Leica DM I6000 inverted epifluorescence microscope) using a 40× PL APO CS (1.3 numerical aperture) lens. Images were processed and color balanced in Fiji.32
Results
HBM pedigree with a segregating SMAD9 c.65T>C p.Leu22Pro variant
We investigated a pedigree with unexplained and apparently autosomal dominant HBM (Fig. 1),9 identified from our large UK HBM cohort10 (Fig. S2).
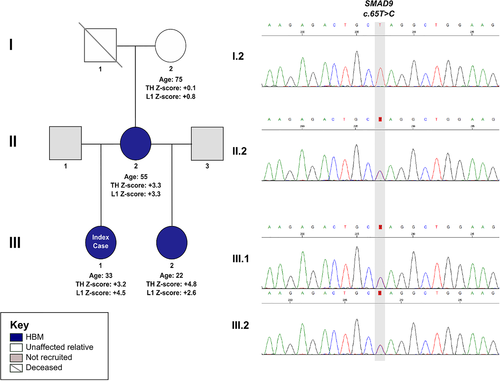
Clinical phenotype
Clinical phenotypes of the UK family individuals are shown in Table 1; extended clinical histories are provided in Supplemental Results. In summary, affected individuals had high BMD Z-scores and very high body mass index (BMI) and had not had any adult fractures, nerve compression, or dental problems; however, bone pain was common, without a clear cause. There was no clinical history of intellectual impairment, pulmonary hypertension, vascular hypertension, hematological abnormalities, pubertal delay, or other clinical conditions. None had been exposed to anabolic or antiresorptive medications.
| HBM pedigree | Additional isolated HBM cases | ||||||
|---|---|---|---|---|---|---|---|
| UK proband III.1 | UK half-sister III.2 | UK mother II.2 | UK grandmother I.2 (unaffected) | UK case | Australian case | ||
| SMAD9 mutation | Leu22Pro | Leu22Pro | Leu22Pro | WT | Leu22Pro | Leu22Pro | |
| Age (years) at assessment | 33 | 22 | 55 | 75 | 55 | 57 | |
| Sex | Female | Female | Female | Female | Female | Female | |
| Ethnicity | White | White | White | White | White | White | |
| Anthropometry | |||||||
| Height (cm) | 178.0 | 173.3 | 175.0 | 160.6 | 160.0 | 162.2 | |
| Weight (kg) | 138.0 | 133.8 | 127.8 | 72.6 | 89.7 | 69.9 | |
| Body mass index (kg/m2) | 43.6 | 43.3 | 41.4 | 28.1 | 35.0 | 26.6 | |
| DXA measurements | |||||||
| Total hip BMD Z-score | +3.2 | +4.8 | +3.3 | +0.1 | +4.3 | +2.7 | |
| L1 BMD Z-score | +4.5 | +2.6 | +3.3 | +0.8 | +5.0 | +3.4* | |
| Bone mineral content (kg) | 3.49 | 3.77 | 3.65 | 2.12 | 3.24 | - | |
| Fat mass (kg) | 73.2 | 64.5 | 64.8 | 25.4 | 34.0 | - | |
| Lean mass (kg) | 61.3 | 65.6 | 59.5 | 45 | 52.4 | - | |
| Clinical phenotype | |||||||
| Adult fracture | No | No | No | No | No | No | |
| Sinks/floats | Floats | Sinks | Floats | Floats | Sinks | - | |
| Bone pain | Yes | Yes | Yes | No | No | - | |
| Visual/auditory impairment | Myopia | No | No | Impaired hearing | Astigmatism | - | |
| Dentition | Normal | Normal | Normal | Normal | Retained cuspid tooth | - | |
| Shoe sizea | 10 | 10 | 9 | 6 | 5.5 | - | |
| Broad frame | Yes | Yes | Yes | No | Yes | - | |
| Enlarged mandible | Yes | Yes | Yes | No | Yes | - | |
| Torus | Yes | Yes | No | No | No | - | |
| Nerve compression | No | No | No | No | No | - | |
| Pes planus | No | No | Yes | No | Yes | - | |
| Blood tests | |||||||
| ALP (IU/L) | 99 | 83 | 102 | 202 | 61 | - | |
| Adjusted calcium (mmol/L) | 2.50 | 2.46 | 2.40 | 2.46 | 2.33 | - | |
| P1NP (ug/L) | 58 | 36 | 22 | 95 | 35 | - | |
| CTX (ug/L) | 0.15 | 0.18 | 0.19 | 0.10 | 0.16 | - | |
| Osteocalcin (ug/L) | 12.4 | 17.1 | 13.5 | 14.8 | 11.5 | - | |
| Sclerostin (pmol/L) | 71.0 | - | 56.1 | 44.4 | 50.4 | - | |
- WT = wild type; DXA = dual-energy X-ray absorptiometry; BMD = bone mineral density.
- a UK measurements. Reference ranges: ALP 20–120; adjusted calcium 2.25–2.70; P1NP: premenopausal 30–78 ug/L, postmenopausal 26–110 ug/L, male 20–76 ug/L; serum CTX 0.1–0.5 ug/L; osteocalcin 6.8–32.2 ug/L; sclerostin <80 pmol/L.
- * measured from L1-4.
III.1: Index case (c.65T>C, p.Leu22Pro)
The 33-year-old index case, with BMD Z-scores +3.2 at the total hip and +4.5 at first lumbar vertebra (L1), had only sustained one traumatic fracture at age 20 months. She reported lower leg and ankle pain. She was tall (>97th centile) and obese, with increased shoe size, a broad frame, enlarged mandible, and a 4 mm torus mandibularis. She had normal joints. Radiographs showed increased cortical thickness and new bone formation at the anterior inferior iliac spines bilaterally (Supplemental Fig. S1 in File S1).
II.2: Mother of the index case (c.65T>C, p.Leu22Pro)
The 55-year-old affected mother, with BMD Z-scores +3.3 at the total hip and at L1, had never sustained a fracture. Six years earlier she had had a right calcaneal spur surgically removed. She had widespread pain with a diagnosis of fibromyalgia. She was tall (97th centile) and obese, with above average shoe size, a broad frame, enlarged mandible, but no tori. She had a full range of movement in all joints, bilateral knee crepitus, and bilateral pes planus.
III.2: Half-sister to index case (c.65T>C, p.Leu22Pro)
The 22-year-old affected half-sister, with BMD Z-scores +4.8 at the total hip and +2.6 at L1, had not fractured. She had had sciatica for 5 years, lumbar back pain and fronto-temporal headaches for 11 years, with a diagnosis of migraine. She was tall (93rd centile) and obese, with above average shoe size, a broad frame, enlarged mandible, a torus palatinus in the midline of her hard palate (3 cm × 7 mm), and normal joint movement.
I.2: Grandmother of index case (wild type)
The 75-year-old grandmother, who did not have HBM, had also never sustained a fracture. She had widespread osteoarthritis and on examination had reduced extension of the right elbow and left knee, and bilateral knee crepitus. However, in contrast to other family members, she was less overweight with normal shoe size, frame, mandible, and no tori.
Sequencing of pedigree
WES identified a heterozygous missense variant in SMAD9 (SMAD family Member 9 referring to homologies to the Caenorhabditis elegans SMA (small worm phenotype) and Drosophila MAD (“Mothers Against Decapentaplegic”)) (NM 001127217: exon2: c.65T>C, p.Leu22Pro), segregating with HBM (ie, present in all three individuals with HBM (III.1, II.2, III.2) but absent from I.2 (Fig. S2). This variant (rs111748421) is rare (Exome Aggregation Consortium [ExAC] minor allele frequency [MAF] 0.0023 in European non-Finnish populations), affects a highly evolutionarily conserved base (genomic evolutionary rate profiling [GERP] 5.53), and is predicted to be pathogenic by multiple protein-prediction algorithms (deleterious by SIFT,16 probably damaging by Polyphen,15 and disease causing by MutationTaster23 and PMut22).
A novel variant in CHRNA1 (cholinergic receptor, nicotinic, alpha 1) (c.560T>C, p.Leu187Pro) was also identified (GERP 5.29). Mutations in CHRNA1 have been associated with congenital myasthenic syndromes (OMIM#100690), not present in this pedigree. No variants were identified when applying a compound heterozygous or an autosomal recessive inheritance model.
Sequencing of other HBM cases identifies two further isolated HBM cases harboring a c.65T>C, p.Leu22Pro variant
WES of a further 366 HBM cases (240 isolated cases from the UK cohort with a total hip (TH) or L1 Z-score ≥+3.2 and 126 individuals from the Anglo-Australasian Osteoporosis Genetics Consortium (AOGC)33 with either a total hip and/or lumbar spine (LS) Z-score between +2.5 and +4.0) (Supplemental Fig. S2 in File S1) identified two individuals with the same SMAD9 c.65T>C, p.Leu22Pro variant. Haplotypic analysis confirmed these women were neither related to each other nor to the pedigree described above.
Clinical phenotype
Isolated HBM case (c.65T>C, p.Leu22Pro) from the UK (Table 1; Supplemental Fig. S3 and Results in File S1). This 55-year-old female, with BMD Z-scores +5.0 at the total hip and +4.7 at L1, had never fractured and had no symptoms of nerve compression. Her adult left upper cuspid tooth had never erupted; wisdom teeth had been extracted for overcrowding. She had noticed her own mandible enlargement. She had a congenital astigmatism of her left eye with poor vision and congenital bilateral pes planus. Height was normal (30th centile). She was obese with a broad frame, mandible enlargement, but no tori. She had normal joints.
Isolated HBM case (c.65T>C, p.Leu22Pro) from Australia
This 57-year-old female, with BMD Z-scores +3.0. at the total hip and +2.7 at L1, reported a nose fracture as a child. Height was on the 45th centile and she was overweight. She did not have any history of conditions affecting bone health and had not received antiresorptive or anabolic medications. No further clinical details were available.
Tibial pQCT evaluation
All members of the HBM pedigree, plus the additional isolated HBM case from the UK underwent pQCT scanning of the tibia (Table 2; Supplemental Table S1 and Supplemental Fig. S4 in File S1). To set these findings in context, the mean (SD) values from the four c.65T>C, p.Leu22Pro SMAD9 HBM cases were compared against values from 76 unrelated female HBM cases (without SMAD9, LRP5, LRP4, or SOST mutations) and 32 female family controls with normal DXA-measured BMD who had had pQCT scans following the same protocol.12 The four SMAD9 HBM cases had greater trabecular density, cortical area and thickness, and predicted bone strength (strength stain index [SSI]) than other HBM cases and, to a greater extent, than unaffected family controls. Muscle size (cross-sectional area) was also notably larger in the SMAD9 HBM group (Table 2).
| SMAD9 HBM cases Leu22Pro n = 4 Mean (SD) | WT female HBM casesa n = 76 Mean (SD) | p Valueb | Female family controls with normal BMD n = 32 Mean (SD) | p Valuec | ||
|---|---|---|---|---|---|---|
| Age (years) | 41.3 (16.5) | 60.8 (12.3) | 54.8 (13.5) | |||
| Total hip BMD Z-score | +3.8 | +2.9 | +0.39 | |||
| 4% distal tibia | Total bone area (mm2) | 1038 (160.6) | 1052 (122.6) | 0.820 | 817.1 (223.5) | 0.066 |
| Trabecular BMD (mg/cm3) | 342.3 (13.3) | 324.3 (22.5) | 0.118 | 308.0 (24.6) | 0.010 | |
| Cortical thickness (mm) | 2.12 (0.79) | 1.04 (0.81) | 0.011 | 0.87 (0.83) | 0.007 | |
| 66% mid-shaft tibiad | Total bone area (mm2) | 608.3 (4.7) | 601.5 (81.9) | 0.886 | 572.7 (73.9) | 0.416 |
| Cortical BMD (mg/cm3) | 1150 (10.1) | 1126 (35.9) | 0.255 | 1111 (65.7) | 0.319 | |
| Cortical thickness (mm) | 4.96 (0.13) | 4.37 (0.62) | 0.104 | 3.80 (0.71) | 0.008 | |
| Cortical bone area (mm2) | 356.3 (9.1) | 316.6 (36.6) | 0.065 | 274.1 (42.4) | 0.002 | |
| Cortical/total bone area (%) | 58.6 (1.1) | 53.3 (7.57) | 0.236 | 48.3 (8.34) | 0.043 | |
| SSI (mm3) | 1680 (21.1) | 1506 (236.6) | 0.211 | 1298 (248.2) | 0.013 | |
| Muscle area (mm2) | 8334 (536.5) | 6939 (980.8) | 0.017 | 6542 (1033) | 0.006 | |
| Muscle density (mg/cm3) | 42.1 (1.5) | 40.1 (4.0) | 0.392 | 40.2 (3.1) | 0.323 |
- BMD = bone mineral density; SD = standard deviation; SSI = strength strain index; WT = wild type.
- a Female subgroup (without SMAD9, LRP5, LRP4, SOST mutations) analyzed using data previously published, collected, and analyzed with the same protocols as SMAD9 HBM cases.9
- b Analysis of SMAD9 HBM cases versus WT HBM cases.
- c Family controls with normal BMD.
- d n = 3.
Sequencing of low bone mass (LBM) cases
WES data from 473 women with LBM from the AOGC consortium with TH Z-scores between −1.5 and −4.0 and a LS Z-score ≤−0.5, obtained using similar methodology to the AOGC HBM cases, was interrogated (Supplemental Fig. S2 in File S1). The c.65T>C, p.Leu22Pro SMAD9 variant was not observed.
Common SMAD9-associated genetic variants and BMD
Publicly available data from a recent population-based genomewide association study (GWAS) of eBMD (estimated BMD by heel ultrasound in the UK Biobank study)17 were used to investigate variants surrounding both SMAD9 and CHRNA1. Regional association plots suggested that SNPs intersecting SMAD9 are strongly associated with eBMD (lead SNP rs12427846 [MAF 0.25], β 0.02, SE 0.002, p = 5.5 × 10−16; Fig. 2). In contrast, SNPs surrounding CHRNA1 were not robustly associated with eBMD (Supplemental Fig. S5 in File S1). These observations were further supported by gene-based tests of association performed in-house using 362,924 unrelated white British subjects from the UK Biobank Study. Specifically, SMAD9 was more strongly associated with eBMD (PJOINT = 7.94 × 10−17), when compared with neighboring genes within ±800 kb (p > 2.4 × 10−2) (Supplemental Table S2 in File S1). No such enrichment was found for CHRNA1 (Supplemental Table S3 in File S1). Further investigation of rs12427846 in the UK Biobank Study identified weak associations with body weight (β −0.14, SE 0.04, p = 1.6 × 10−3) and with height (β −0.07, SE 0.03, p = 3.5 × 10−3) with effects in the opposite direction from that found with eBMD; however, adjustment for weight and height did not attenuate the strong association between rs12427846 and eBMD reported above. Interrogating the GWAS Catalog (https://www.ebi.ac.uk/gwas/) did not identify associations of the rare (MAF 0.0014) variant rs111748421 with any trait (neither bone-related nor any other).
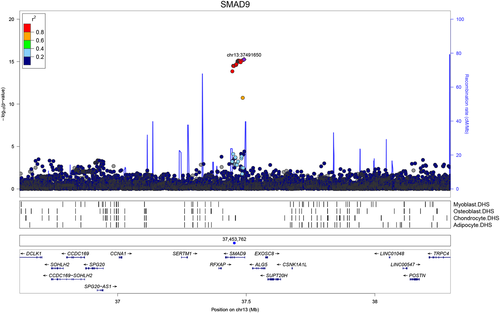
Phenomewide association study
PheWAS involving nearly 3000 traits19 identified no clear evidence for pleiotropy for the c.65T>C, p.Leu22Pro SMAD9 variant (rs111748421) (Supplemental Fig. S6 and Supplemental Table S4 in File S1). Analysis involving the common SMAD9 variant (rs12427846) revealed robust pleiotropic associations with BMD traits. Similarly, a gene-based PheWAS of SMAD9 identified robust evidence of gene-level pleiotropy with BMD. To investigate further possible pleiotropic associations with metabolic phenotypes, we looked up rs111748421 and rs12427846 in the Myocardial Infarction Genetics and CARDIoGRAM Exome meta-analysis34, 35 and the subsequent meta-analysis to which results from UK Biobank SOFT CAD GWAS and CARDIoGRAMplusC4D 1000 Genomes-based GWAS were added.36 No association was found for rs111748421 in either. Although rs12427846 was not present in the first meta-analysis, the eBMD-increasing allele was only nominally associated with the composite cardiovascular disease outcome in the second (log OR 0.03 [SE 0.01], p = 9 × 10−4; significance threshold p < 5 × 10−8).36
Smad9 expression in murine osteocytes
We next determined whether Smad9 and Chrna1 are expressed in osteocytes, the master cell regulators in the skeleton and key regulators of bone mass,37 and enriched in osteocytes compared with other cells in bone.21 Smad9 mRNA was highly expressed in murine osteocytes, whereas Chrna1 was not (Supplemental Table S5 in File S1).
Smad9 expression in zebrafish skeletal tissue
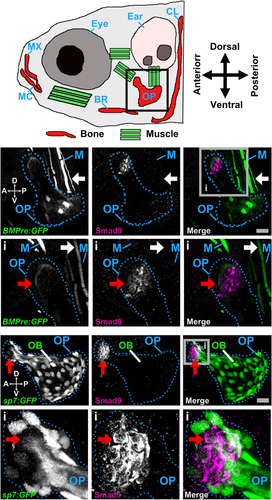
We also examined Smad9 protein expression in the developing zebrafish skeleton38 at 6 and 7 days post fertilization (dpf) (Fig. 3 A). A focus of Smad9 expression was observed at the dorsal tip of the opercle, an intramembranous bone overlying the gills, adjacent to but distinct from a region of bone morphogenetic protein (BMP) reporter activity (Fig. 3 B). The opercula muscle group also showed evidence of BMP reporter activity, whereas Smad9 expression at this site was absent. Smad9-expressing cells in the opercle were negative for the osteoblast marker, sp7 (osterix), suggesting they are likely to represent pre-osteoblasts (Fig. 4 C and Supplemental Movie S1 in File S1). Equivalent findings were observed in the branchiostegal ray bones and in the notochord at 6 and 7 dpf (Supplemental Fig. S7 in File S1).
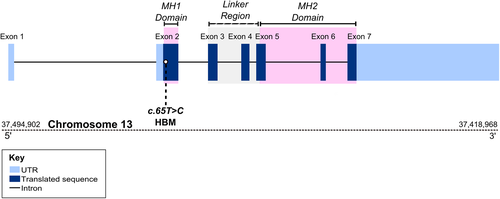
SMAD9 protein structural modeling
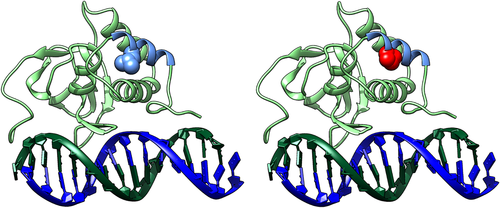
SMAD9 is a TGF-beta family member DNA binding transcription factor. Phosphorylation by BMP-ligand-bound type 1 receptor kinase activates SMAD9, which translocates from the cytoplasm to the nucleus to regulate target gene expression.39 The seven exons of human SMAD9 encode a protein of 467 amino acids that contains two MAD-homology (MH) domains (MAD: Mother against Dpp) separated by a linker region (Fig. 4). The p.Leu22Pro SMAD9 mutation is located within the MH1 domain responsible for DNA binding (Fig. 4) and lies in the hydrophobic face of the N-terminal alpha helix (helix-1) (Fig. 5, Supplemental Movie S2 in File S1). Helix-1 packs against a groove made by helix-2 and -3 within MH1, forming part of the hydrophobic core of this domain. Substitution of leucine by proline will: 1) introduce a less hydrophobic residue into this position; and 2) compromise the α-helical fold by disrupting the canonical hydrogen bonding of helix-1. Thus, modeling suggests that this mutation will disrupt the MH1 domain so severely that SMAD9 can no longer bind DNA and/or will be unstable, leading to protein degradation.
Discussion
We report the first HBM pedigree with a segregating SMAD9 mutation, with replication in two further unrelated individuals with HBM. SMAD9 (also known as SMAD8, MADH6, and MADH9) encodes a downstream modulator of the BMP signaling pathway. BMPs, members of the TGF-β superfamily, induce the formation of bone and cartilage.40 SMADs, activated by ligand-binding of cell surface BMP receptors, mediate downstream intracellular signaling and biological responses induced by BMPs.41 Smad6 and Smad7 both inhibit BMP receptor activation and downstream signaling, as does Smad9 by more direct transcriptional repression.39 Our in silico protein modeling predicts that the p.Leu22Pro mutation severely disrupts the structure of the MH1 DNA binding domain of SMAD9, leading to loss of function.
Few previous studies have examined sites of Smad9 tissue expression. We have confirmed that Smad9 is expressed in mouse cortical bone-derived osteocytes and the Smad9 protein in skeletal elements of zebrafish larvae. Moreover, we observed that BMP reporter activity in zebrafish was absent at sites of Smad9 expression, consistent with a functional role in BMP repression.39 Mutant mouse models with a LacZ insertion causing Smad9 truncation have not undergone BMD phenotyping; however, they have shown strong LacZ expression (under control of an endogenous Smad9 promoter) within developing skeletal sites (eg, ribs, maxilla, mandible), gut, and lungs.42, 43 Taken together, our findings suggest that SMAD9 c.65T>C is a loss-of-function mutation, causing HBM through a novel mechanism of enhanced bone formation due to reduced BMP inhibition.
Further, we have shown that the region containing SMAD9 is strongly associated with BMD within the general population. Common variants intersecting SMAD9 associate with population-based measures of eBMD, as evidenced recently by fine-mapping of target genes17, 44 and from our gene-based tests of association presented here. Furthermore, rs12427846 (the lead SNP from these eBMD results) is associated with DXA-measured total body BMD45 and fracture risk,17 also consistent with associations with BMD identified in our PheWAS. These findings provide further evidence of the importance of SMAD9 in bone biology and are equivalent to reported associations for common variants annotated to LRP5 and SOST genes, both similarly implicated in monogenic HBM disorders.46, 47
We have previously estimated unexplained HBM to have a prevalence of 0.181% amongst a DXA-scanned adult population in the UK.10 As two of 248 cases fulfilling our stringent HBM phenotype definition (Supplemental Fig. S2 in File S1) were found to harbor the c.65T>C p.Leu22Pro SMAD9 variant (rs111748421), we would estimate prevalence of SMAD9 HBM as approximately 1 in 100,000 (1.46 × 10−5), less common than LRP5 HBM.9 As rs111748421 has a reported MAF of 0.0028, this raises the possibility of incomplete penetrance, variable expressivity, gene–gene or gene–environment interaction with a currently unknown factor; it is also possible that rs111748421 might be in linkage disequilibrium (LD) with an intronic regulatory variant not captured by WES.
The clinical phenotype of c.65T>C, p.Leu22Pro SMAD9 HBM includes mandible enlargement, a broad frame, torus palatinus, pes planus, increased shoe size, and, in 2 of 5 subjects, a tendency to sink when swimming. Adult fractures were not reported, raising the possibility of increased skeletal strength, supported by evidence of greater cortical bone and an increased strength-strain index (SSI) quantified by pQCT (discussed further below), both of which promote fracture resistance. Mandible enlargement, torus palatinus, a tendency to sink when swimming, and an absence of adult fractures are reminiscent of LRP5 HBM.7, 48 Encouragingly, unlike sclerosteosis (due to anabolic SOST mutations) and some LRP5 HBM cases,9, 49 nerve compression was not a feature of SMAD9 HBM.
The musculoskeletal phenotype of p.Leu22Pro SMAD9 HBM includes high BMD Z-scores (+3 to +5), with increased fat and lean mass. Although morbid obesity with increased stature was found in 3 of 5 SMAD9 HBM cases, this was not ubiquitous to all. The increases in BMD of +3.3 to 5 SDs above normal exceeded increases in fat-mass index of +0.9 to 3.4, supporting HBM as the primary phenotype, rather than high BMD occurring as a consequence of high fat mass.50 Furthermore, fat-associated phenotypes were not identified in our PheWAS. pQCT revealed increased volumetric trabecular bone density with greater cortical thickness and area, suggesting reduced bone remodeling to reduce endosteal expansion. In support, bone turnover markers are at the lower end of the normal range. This phenotype mimics that previously described for human LRP5 HBM51 although plasma sclerostin is not elevated, in contrast to LRP5 HBM,52 suggesting that a negative feedback loop downregulating WNT signaling is not present. pQCT further identified larger muscle size in SMAD9 HBM cases, including in the independent UK case with normal stature. This contrasts with findings from our zebrafish studies that Smad9 expression is absent from skeletal muscle tissue, as is also observed in murine models.43 Given the well-recognized cross-talk between muscle and bone53 and the large BMI of these individuals, it is conceivable that the increase in muscle size is secondary to a need to carry the substantial weight of both fat and bone mass. However, similar increases in muscle size have not been reported in other monogenic HBM conditions (ie, LRP5 or SOST HBM) with equivalent BMD.
A clinical report of 13q13.3-q21.3 deletion, leading to haploinsufficiency of SMAD9 amongst other genes, identified a phenotype of skeletal overgrowth with infant height >95th percentile, consistent with the adult phenotype we describe, implicating SMAD9 in the regulation of linear growth.54 We found limited, but consistent, evidence that SMAD9 HBM may affect longitudinal growth. Although differences in height can artefactually affect DXA-measured BMD, pQCT measures of increased trabecular bone density and cortical thickness are usually more independent of body size. Heterozygous truncating SMAD9 mutations are associated with primary pulmonary hypertension (OMIM#615342),55 a phenotype not apparent in our HBM cases. Reported mutations affect a different domain from the mutation observed here, with p.Cys202X55 and p.Arg294X56 truncating the SMAD9 protein in the linker region between MH1 and MH2. A truncating mutation (p.Arg247X) has been associated with cerebral arteriovenous malformations in childhood.57 An activating heterozygous p.Val90Met germline mutation, affecting the 4th α-helix of MH1 and close to the DNA binding interface, has been described in one pedigree with hamartomatous polyposis.58 In contrast to p.Leu22Pro, p.Val90Met appears to be a gain-of-function mutation, thought to arise from a steric clash, prompting a His104 residue to enhance DNA binding.58 Such examples of diverse phenotypes arising from mutations in differing exons of the same gene are well recognized, eg, differing mutations in FBN1 (Fibrillin 1) can cause Marfan syndrome (with associated tall stature) (OMIM#154700), acromicric dysplasias (with short stature) (OMIM#102370), or stiff skin syndrome (OMIM#184900).59-61
We are only aware of one other skeletal dysplasia reported in association with an inhibitory SMAD (which include SMAD6 and SMAD7). A rare SMAD6 mutation has been associated with susceptibility to nonsyndromic midline craniosynostosis 7 (OMIM#617439), but only in the context of co-inheritance of a common variant in BMP2 strongly associated with this condition, a rare example of two locus inheritance.62 Interestingly, amongst the 1103 conditionally independent SNPs reaching genomewide significance in the UK Biobank eBMD GWAS (population n = 426,824), as well as identifying the SMAD9 locus, four novel SNPs annotating to SMAD7 were reported (plus three established SNPs associated with SMAD3), all suggesting variation in inhibitory SMADs is likely of functional importance in human bone biology.17
The phenotype we describe here contrasts with that of activating mutations of the BMP receptor, ACVR1, which increase BMP signaling. However, in contrast to p.Leu22Pro SMAD9 HBM, ACVR1 mutations lead to a fatal condition, fibrous ossificans progressiva (FOP, OMIM#135100).63 In FOP, muscle tissue differentiates into bone after trivial injury, resulting in the formation of mature bone at multiple extraskeletal sites. ACVR1 mutations may produce a more severe phenotype, compared with loss-of-function mutations in SMAD9 reported here, since ACVR1 also activates non-SMAD-dependent BMP signaling cascades, such as the NF-κB and p38 MAP kinase (p38MAPK) pathways, which are upregulated in FOP ACVR1 R206H monocytes.64
Given the benign phenotype observed in c.65T>C, p.Leu22Pro SMAD9 carriers, our findings suggest that SMAD9 is worth consideration as a drug target for osteoporosis. Our zebrafish studies suggest that Smad9 is expressed in pre-osteoblasts, consistent with the profile of an anabolic target capable of stimulating new bone formation through recruitment of early osteoblast progenitors. Given the pathological consequence of excess BMP activation in FOP, this pathway has not been prioritized as a possible therapeutic target in osteoporosis, despite the profound bone anabolic potential. Interestingly, phosphorylation of Smad9, as part of the Smad1/5/9 heterotrimer, has been researched in relation to fracture healing and bone regeneration: G-protein-coupled receptor kinase 2-interacting protein-1 (GIT1), a shuttle protein in osteoblasts, regulates Smad1/5/9 phosphorylation, which in turn mediates BMP2 regulation of Runx2 expression and thus endochondral bone formation at fracture sites.65, 66 Moreover, BMP has been administered locally to promote bone repair after surgery.67 Based on our findings, it is tempting to speculate that treatments suppressing SMAD9 activity might prove useful in treating osteoporosis, fractures, and possibly also sarcopenia. The potential pleiotropic association between one SMAD9 variant and a composite cardiovascular phenotype represents the results of lifelong exposure to a variant rather than any potential short-term perturbations in a gene pathway as might be exploited therapeutically.
Our study has limitations. All individuals with c.65T>C, p.Leu22Pro SMAD9 HBM were female, reflecting the study design that favored those with a historical DXA scan who are more likely to be female. Whether findings will be similar in men is unknown, although no sex-gene interaction has been described for the LRP4, LRP5, LRP6, or SOST sclerosing bone dysplasias. In the recent UK Biobank eBMD GWAS, LD score regression analyses suggested that the genetic architecture influencing male and female eBMD was largely shared with some significant differences between the sexes (rG = 0.91, SE = 0.012, p < 0.001),17 consistent with earlier epidemiological studies.68 The small sample of SMAD9 HBM cases (n = 4 with pQCT) limited our ability to robustly evaluate associations statistically. The c.65T>C, p.Leu22Pro mutation is a reported SNP carried within the general population (eg, in the UK, an estimated 92,428 people might be expected to carry this mutation). This may be the case, given there is no indication that the phenotype affects reproductive fitness and HBM will not be overt unless a DXA scan is performed. Our GWAS was based on estimated heel BMD quantified by ultrasound rather than DXA-measured BMD. Estimated heel BMD is not used routinely in clinical practice. However, we have previously demonstrated a strong overlap between genetic loci identified by eBMD GWAS and by DXA-measured BMD GWAS.44
We report SMAD9 as a novel HBM-causing gene. The clinical phenotype of c.65T>C, p.Leu22Pro SMAD9 HBM has many features in common with that of LRP5 HBM but lacks the deleterious features that characterize SOST HBM (sclerosteosis). As reported for both LRP5 and SOST, we demonstrate that a rare mutation in SMAD9 is associated with an extreme bone phenotype and that common variation in SMAD9 affects bone density within the general population. The role of SMAD9 in bone biology is supported by our finding of high levels of Smad9 expression in murine osteocytes and in skeletal elements of zebrafish larvae. Smad9 is thought to inhibit BMP signaling to reduce osteoblast activity; thus, we hypothesize SMAD9 c.65T>C is a loss-of-function mutation reducing BMP inhibition, ultimately leading to enhanced bone formation. Our findings support SMAD9, and its role within the SMAD9-dependent BMP signaling pathway, as a potential novel anabolic target for osteoporosis therapeutics that warrants further investigation.
Disclosures
The authors declare that no competing interests exist.
Acknowledgments
Full acknowledgements are listed in the Supplemental Material, including details of the AOGC Consortium.
CLG was funded by the Wellcome Trust (080280/Z/06/Z), the EU 7th Framework Programme ref 247642 (GEoCoDE), a British Geriatric Society travel grant, and Versus Arthritis (formerly Arthritis Research UK) (grant ref 20000). This study was supported by the NIHR CRN (no. 5163). DB received travel grants from The Harold Hyam Wingate Foundation (DMMTF-180208) and an Elizabeth Blackwell Institute for Health Research (University of Bristol) discipline hopping fellowship via a Wellcome Trust Institutional Strategic Support Grant (204813/Z/16/Z). AH is funded by the Wellcome Trust (20378/Z/16/Z). LP, AH, and GDS work in a unit that receives UK Medical Research Council funding (MC_UU_12013/4, MC_UU_00011/1). CH and DB were funded by Versus Arthritis (21211, 21937, 19476). AML is supported by an NHMRC Early Career Fellowship. JPK is funded by a University of Queensland Development Fellowship (UQFEL1718945); his contribution is supported by a National Health and Medical Research Council (Australia) project grant (GNT1158758). MAB is supported by an NHMRC Senior Principal Research Fellowship. PIC is funded by a Wellcome Trust Strategic Award (101123). The AOGC was funded by the National Health and Medical Research Council (Australia) (511132 and 1032571). Funders had no role in study design, analysis, or manuscript preparation.
Authors' roles: Conception: CLG, GDS, MAB, JHT, and ELD. Design: CLG, JPK, GDS, MAB, PL, JHT, and ELD. Data acquisition: CLG, DB, LW, PC, SY, WF, JCYT, CH, MAB, and ELD. Analysis: CLG, DB, RBS, LW, AH, SY, PC, AML, CH, JPK, PL, and ELD. Interpretation: CLG, DB, RBS, AH, SY, PC, AML, MAB, CH, JPK, PL, JHT, and ELD. Manuscript draft: CLG, DB, LW, AH, JPK, LP, CH, JPK, PL, JHT, and ELD. Manuscript revision: CLG, DB, RBS, AH, AML, JCYT, LP, MAB, CH, JPK, PL, JHT, and ELD. Approval of final manuscript: all authors. All authors take responsibility for their contributions as outlined above.




