Murine Placental-Fetal Phosphate Dyshomeostasis Caused by an Xpr1 Deficiency Accelerates Placental Calcification and Restricts Fetal Growth in Late Gestation
ABSTRACT
Phosphorus is a necessary component of all living organisms. This nutrient is mainly transported from the maternal blood to the fetus via the placenta, and insufficient phosphorus availability via the placenta disturbs the normal development of the fetus, especially fetal bone formation in late gestation. Key proteins (phosphate transporters and exporters) that are responsible for the maintenance of placental-fetal phosphorus homeostasis have been identified. A deficiency in the phosphate transporter Pit2 has been shown to result in placental calcification and the retardation of fetal development in mice. What roles does XPR1 (the only known phosphate exporter) play in maintaining placental-fetal phosphorus homeostasis? In this study, we found that Xpr1 expression is strong in the murine placenta and increases with age during gestation. We generated a global Xpr1 knockout mouse and found that heterozygous (Xpr1+/−) and homozygous (Xpr1−/−) fetuses have lower inorganic phosphate (Pi) levels in amniotic fluid and serum and a decreased skeletal mineral content. Xpr1-deficient placentas show abnormal Pi exchange during gestation. Therefore, Xpr1 deficiency in the placenta disrupts placental-fetal Pi homeostasis. We also discovered that the placentas of the Xpr1+/− and Xpr1−/− embryos are severely calcified. Mendelian inheritance statistics for offspring outcomes indicated that Xpr1-deficient embryos are significantly reduced in late gestation. In addition, Xpr1−/− mice die perinatally and a small proportion of Xpr1+/− mice die neonatally. RNA sequence (RNA-Seq) analysis of placental mRNA revealed that many of the transcripts are significantly differentially expressed due to Xpr1 deficiency and are linked to dysfunction of the placenta. This study is the first to reveal that XPR1 plays an important role in maintaining placental-fetal Pi homeostasis, disruption of which causes severe placental calcification, delays normal placental function, and restricts fetal growth. © 2019 American Society for Bone and Mineral Research.
Introduction
The placenta is a temporary organ with an extensive vascular network that connects the fetomaternal circulatory systems.1, 2 It plays a pivotal role in maternal-fetal nutrient transfer, and abnormal development or dysfunction of the placenta causes pathologies of pregnancy, such as fetal growth restriction, preeclampsia, and subsequent predisposition to lifelong illnesses.3-5 Physiological inorganic phosphate (Pi), which is an essential nutrient for all living organisms, plays a crucial role in the placenta for fetal growth and metabolism and skeletal growth and development.6
Dyshomeostasis of Pi in organs causes several adverse outcomes such as abnormal bone mineralization and ectopic calcification in soft tissues.7, 8 In gestation, placental-fetal Pi homeostasis involving in placental Pi transport, Pi level in amniotic fluid and fetal serum and skeletal mineral content is very important for the normal development of the fetus and health outcomes of offspring.9 However, the mechanism of transplacental Pi transfer is largely unknown. The fetal Pi concentration in the serum is greater than the maternal concentration by 0.5mM to 1.0mM, suggesting that the fetal need for Pi is met by its active transport from the maternal circulation via the placenta.10, 11 In mammals, many Na+/Pi transporters participate in regulating Pi homeostasis in the body, and three of these transporters (NaPi-IIb, PiT1 and PiT2, which are encoded by SLC34A2, SLC20A1, and SLC20A2, respectively) have been identified as being highly expressed in the placenta.12-14 NaPi-IIb expression in human/mouse placenta increases as placental development progresses, and NaPi-IIb is expressed in the fetomaternal interface of the placenta.11, 15 In addition, NaPi-IIb is highly expressed in the labyrinth zone of the mouse placenta at E12.5, and deficient mice exhibit early embryonic lethality.16 These data indicate that NaPi-IIb likely plays important roles in regulating placental-fetal Pi homeostasis. Slc20a1-deficient mice are embryonic-lethal during gestation, and Slc20a2 knockout mice show fetal growth restriction and severe placental calcification.1, 2 To the best of our knowledge, ectopic calcifications are always associated with Pi dyshomeostasis.17 Similar to most ectopic calcifications, placental calcification mainly consists of calcium phosphate deposits.18, 19 What is the relationship between Pi dyshomeostasis and severe calcification in the placenta? Moreover, placental calcification is commonly observed in the clinic, but the relationship between placental calcification and adverse pregnancy outcomes remains unclear due to the limitations of clinical studies.20, 21
XPR1 encodes a receptor for xenotropic and murine viruses and has a phosphate export function.22, 23 Xpr1 expression is most abundant in the pancreas, kidney, placenta and heart of human.23 Recently, mutations in XPR1 have been confirmed to be a cause of primary familial brain calcification (PFBC). In addition, missense mutations in the SPX domain of XPR1 alter cellular Pi export.24 Currently, XPR1 is the only known phosphate exporter in metazoans.25 Therefore, we aimed to identify the roles that XPR1 plays in maintaining placental-fetal phosphorus homeostasis. In our study, we discovered that Xpr1 is also highly expressed in the murine placenta and that its expression level rapidly increases during late gestation. To further study the function of XPR1 in the placenta, we generated a global Xpr1 knockout mouse. Xpr1 knockout resulted in severe placental calcification and fetal growth restriction by disrupting physiological placental-fetal Pi homeostasis.
Materials and Methods
Animals
The Xpr1-knockout mice with a C57BL/6N background that were used for the experiments were originally produced by the CAM-SU Genomic Resource Center. All animal experiments were approved by the Ethics Committee of Animal Experiments of Huazhong University of Science and Technology and were conducted according to the standards for the care and use of laboratory animals established by the US National Institutes of Health. All mice were fed with a regular diet (1.05% calcium, 0.71% phosphate) and intercrossed between 8 and 12 weeks of age.
Genotyping
Mouse DNA was purified from the toe or tail of offspring and used for genotyping by polymerase chain reaction (PCR). Primers for the wild-type (WT) mouse genotype (forward primer: 5′-CCAGCTCAGCCTAAAGAGT-3′; reverse primer: 5′-GAATGTATTCAGGACTTCGGGTA-3′) and the knockout mouse genotype (forward primer: 5′-CCAGCTCAGCCTAAAGAGT-3′; reverse primer: 5′-GGCAAGAACATAAAGTGACC-3′) were used, and the WT and knockout products were 399 bp and 301 bp, respectively.
In situ hybridization
Fresh placentas were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) (prepared with diethyl pyrocarbonate [DEPC]-water) for 2 hours and kept in 30% sucrose solution (prepared with DEPC-water) for 8 hours before frozen sections (8 μm thickness) were cut. A digoxigenin-labeled antisense probe was synthesized by a MerMade 192E oligonucleotide synthesizer (BioAutomation, Inc., Irving, TX, USA). The probes were hybridized to frozen sections at 37°C overnight according to a standard protocol. Anti–digoxigenin–horseradish peroxidase (anti-DIG-HRP) and diaminobenzidine (DAB) were used for the color reaction.
Real-time PCR
We extracted RNA from the fresh placentas of mice using TRIzol (TaKaRa Bio, Otsu, Japan), following the manufacturer's protocol. Real-time PCR (RT-PCR) was performed with SYBR® Green Master Mix (Vazyme Biotech Co. Ltd., Nanjing, China) on a StepOnePlus™ Real-Time PCR Instrument (Life Technologies, Inc., Grand Island, NY, USA). Gapdh was used as an internal reference for each sample.
Histology
Fresh placentas and fetal femurs were harvested and imaged at different periods of gestation and then fixed in 4% paraformaldehyde for over 24 hours prior to the preparation of paraffin sections or frozen sections. Then, tissues for the paraffin sections were dehydrated by a graded series of alcohol and dimethylbenzene, immersed in paraffin wax, and embedded for over 12 hours at room temperature before sectioning. Tissues for the frozen sections were kept in 30% sucrose at 4°C until they sank and were embedded in optimal cutting temperature compound (OCT) for 20 min at −24°C before sectioning.
For Alizarin red (AR) staining, the tissue sections were dewaxed by dimethylbenzene and a series of graded alcohol, treated with Alizarin red for 20 min, washed with distilled water, and dried at 50°C for 30 min.
Silver staining of dewaxed tissue sections was performed following the same method as that used for AR staining. Component A consisted of 10% formaldehyde and 0.002% nitric acid. Component B consisted of 15% silver nitrate, 10% potassium nitrate, and 0.05% glycine. Component C consisted of 1% pyrogallol, 55% alcohol, and 0.002% nitric acid. The sections were soaked in component A for 5 min and washed with distilled water three times. Next, the sections were soaked in component B (37°C) for 5 min, taken up to remove component B, soaked in component C (45°C) for 5 to 10 s, and washed with distilled water.
Von Kossa (VK) staining of dewaxed tissue sections was performed following the same method as that used for AR staining. The sections were soaked in 5% sodium thiosulfate for 30 min and washed with distilled water three times. Then, 5% AgNO3 was added and the sections were incubated under ultraviolet radiation for 30 min. The sections were then washed with distilled water three times and stained with eosin for 5 min.
μCT analyses
Fresh E18.5 fetuses were fixed in 4% paraformaldehyde for over 48 hours. Then, the fetuses were imaged with a high-resolution in vivo μCT scanner (SkyScan 1176; Bruker, Kontich, Belgium) with a 9-μm spatial resolution (58 kV, 431 μA, 0.5-mm Al filter). For femoral and tibial diaphysis analysis, the area starting from the midshaft diaphysis and extending 1 mm (100 slices) was selected. A calcium-hydroxyapatite standard was provided for calibrations of bone mineral density (BMD).
Morphometry of placental calcification and mineralized bone volume
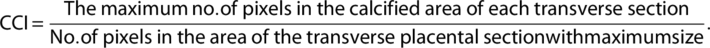 (1)
(1)The assessment of placental calcification was improved as described.26
Pi and calcium concentration measurement
The amniotic fluid was acquired by piercing the amniotic membrane with a glass capillary tube to measure the Pi concentration. Fetal serum was collected from fetal blood vessels after clipping the umbilical cord and was then quantitatively added into normal saline (blood:saline = 1:149) and centrifuged. After centrifugation, the upper portion of the liquid was retained as the fetal serum sample. The amniotic fluid and fetal serum Pi concentrations were detected by molybdate-malachite green spectrophotometry. Component A consisted of 0.045% malachite green. Component B consisted of 4.2 g ammonium molybdate dissolved in 100 mL 4 N HCl. Then, 2 μL amniotic fluid with 48 μL distilled water or a 25-μL fetal serum sample with 25 μL distilled water was added to 50 μL of standard Pi solution samples (0, 3, 6, 9, 12, 15, 18, 21, 24, 27, 30, 33, and 36μM). Next, 75 μL component A and 25 μL component B were added, and each sample was mixed for several seconds before the absorbance was measured by a microplate reader (λ = 620 nm). Calcium concentration was measured using a Calcium Colorimetric Assay Kit (BioVision Inc., Milpitas, CA, USA). A total of 24 WT and 28 Xpr1+/− female mice were used for collecting fetal serum and amniotic fluid. No adverse events appeared.
Placental Pi transfer
Pregnant mice were given 50 μCi 32P (HTA Co., Ltd., Beijing, China) via an intracardiac injection, and the 32P level of fetuses was measured with a liquid scintillation counter, as described.27 Each fetal 32P value was normalized to the mean value of WT fetuses within each litter. The WT fetuses were chosen as the baseline for comparison within each litter due to their normal viability. A total of 10 WT and 15 Xpr1+/− female mice were used for this experiment. No adverse events appeared.
Fetal ash and skeletal mineral measurement
Entire fetuses (E18.5) were placed into lidded crucibles and reduced to ash by a furnace (500°C) for 24 hours, as described.28 The Pi and calcium content of the ash was measured by the same method as that used for measurement of the mineral in amniotic fluid. Because the fetal size is influenced by the number of fetuses from each litter, the data obtained from each fetus were normalized to the mean value of WT fetuses within each litter.
Skeletal preparations
The skin and viscera were removed from E18.5 fetuses, and the fetuses were then fixed in 100% ethanol for 2 days and stained with Alcian blue 8GX and Alizarin red S, as described.29
RNA sequencing
Total RNA was extracted from E17.5 placentas. mRNA was isolated from total RNA by magnetic beads with oligo-dT (a synthetic single-stranded oligonucleotide with 5′- and 3′-hydroxyl ends) and randomly fragmented. After reverse transcription, end-repair, adaptor ligation, and PCR amplification, ligation products were purified to remove self-ligated adapters and incompletely ligated fragments. Libraries were constructed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and quantified using a Qubit 2.0 fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). The DNA was sequenced according to Illumina's standard protocol (Illumina, San Diego, CA, USA).
Immunohistochemistry
Eight-micrometer-thick (8-μm-thick) frozen placenta sections were heated in a microwave for antigen retrieval, blocked with 3% bovine serum albumin, and incubated with anti-granzyme B antibody (1/100; Abcam, Cambridge, MA, USA; ab4059). Next, the color reaction was performed with HRP-labeled antibody (Wuhan Servicebio Technology Co., Ltd., Wuhan, China; G1210) and DAB, and the sections were counterstained with hematoxylin.
Detection of alkaline phosphatase
Alkaline phosphatase (ALP) staining of frozen tissue sections was performed following the Gomori modified calcium-cobalt staining method using an ALP kit (Wuhan Servicebio Technology Co., Ltd., Wuhan, China) according to the manufacturer's instructions. The density of ALP staining was quantified by Image-Pro Plus 6.0 software.
Statistical analysis
All results are presented as the mean ± standard error of the mean (SE). Differences between two groups were determined by Mann-Whitney test. The comparisons among multiple groups were determined by ANOVA followed by Tukey's test. A value of p < 0.05 was considered significant. The above statistical analyses were performed using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA).
In the RNA sequencing (RNA-Seq) analysis, read quality was performed for all samples by using FastQC version 0.11.2 (Andrews.2010) software (Babraham Bioinformatics, Cambridge, UK; http://www.bioinformatics.babraham.ac.uk/projects/fastqc/) with the default parameters. Expression estimation of transcript was performed by using RSEM (V1.2.29).30 Raw read counts of gene expression were applied to estimate the differentially expressed genes by using edgeR package.31 The p values were obtained by edgeR using an exact test through the negative binomial distribution. The significant difference was identified with a p value <0.01 and with a fold change greater than two. The mRNA levels were calculated and presented by using fragments per kilobase of exon per million fragments mapped (FPKM) values. Volcano plot was generated by Microsoft Excel (Microsoft Corp., Redmond, WA, USA). Gene ontology (GO) analysis was performed using DAVID 6.8 (Laboratory of Human Retrovirology and Immunoinformatics [LHRI], Frederick National Laboratory for Cancer Research, Frederick, MD, USA; https://david.ncifcrf.gov/home.jsp). Heatmap was generated by MeV 4.9.0 software. Venn diagram was created by using Venny 2.1.0 (https://bioinfogp.cnb.csic.es/tools/venny/).
Results
Expression of Xpr1 in the placenta
XPR1 is highly expressed in the human placenta.23 We aligned the mouse XPR1 against the sequences in the human protein database and identified a human XPR1 with 95% identity to mouse XPR1 (Supplemental Fig. S1). We found that Xpr1 is also widely expressed in mouse placenta by in situ hybridization analysis. Specifically, the spongiotrophoblast layer had a higher Xpr1 expression level than the labyrinthine layer, chorionic plate, and maternal decidual tissue (Fig. 1 A). Xpr1 mRNA expression in the placenta at E12.5, E15.5, and E18.5 was assessed by RT-PCR. Xpr1 mRNA expression levels obviously increased during gestation (Fig. 1 B). These results suggested that Xpr1 might be required for normal placental function during the process of placental development. What roles does XPR1 play in the placenta and is XPR1 involved in placental-fetal Pi homeostasis? In this study, we generated Xpr1 knockout mice and performed genotyping by PCR (Supplemental Fig. S2 A-B). RT-PCR analysis showed that a significantly lower Xpr1 mRNA expression level was detected in heterozygous mouse placentas at E12.5, E15.5, and E18.5 than in the WT placentas at the same time points (Fig. 1 B).
Xpr1 knockout disturbs placental-fetal Pi homeostasis
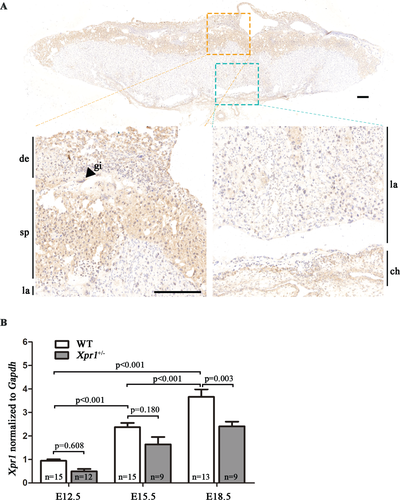
To determine whether the absence of Xpr1 affects normal placental-fetal Pi homeostasis, we measured the concentration of Pi in amniotic fluid, which is mainly composed of fetal metabolites. At the E15.5 stage, the amniotic sac contained abundant amniotic fluid. Compared with the WT fetuses, Xpr1+/− and Xpr1−/− fetuses presented significantly reduced Pi levels in amniotic fluid in Xpr1+/− mouse intercrosses (Fig. 2 A). To further verify fetal Pi homeostasis, we detected the Pi concentration in fetal serum and found that the Xpr1+/− and Xpr1−/− fetuses had a lower Pi level in serum than the WT fetuses at both E15.5 and E18.5 (Fig. 2 B,C). In male Xpr1+/− and female WT mouse intercrosses, Xpr1+/− fetuses displayed a similar reduction in the Pi level in the amniotic fluid at E15.5 and in the serum at both E15.5 and E18.5 compared with the levels in littermate controls (Supplemental Fig. S3 A-C). In addition, there were no differences in the Pi and calcium content in maternal serum and the calcium content of amniotic fluid and fetal serum between WT and Xpr1-deficient mice (Supplemental Fig. S3 D-K).
Placental Pi transport, measured by the level of 32P accumulation in fetuses, was abnormal in Xpr1-deficient placentas compared with WT placentas. Interestingly, the 32P levels in the Xpr1+/− and Xpr1−/− fetuses were significantly reduced at the E15.5 stage but increased at the E18.5 stage in Xpr1+/− mouse intercrosses (Fig. 2 D,E). In male Xpr1+/− and female WT mouse intercrosses, the 32P level in the Xpr1+/− fetuses displayed a similar reduction at the E15.5 stage and an increase at the E18.5 stage (Supplemental Fig. S4 A-B), implying these abnormal placental Pi transport have no close relationship with the genotype of the maternal portion of placenta. In addition, no significant differences were observed in 32P accumulation in placentas between WT and Xpr1-deficient placentas (Supplemental Fig. S4 C-F).
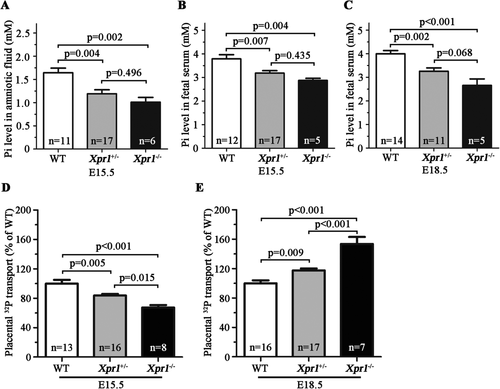
Histological analysis of fetal femurs by VK staining and quantification revealed a significant reduction in the mineral content in the Xpr1−/− fetuses compared with that in littermate WT fetuses at E18.5 (Fig. 3 A,B). Reduced bone mineralization in Xpr1-deficient fetuses was also confirmed by μCT analysis of fetal skeletons, which exhibited decreased femoral and tibial BMD (~10% and >40% loss in Xpr1+/− and Xpr1−/− fetuses, respectively) and length (~5% and ~25% loss in Xpr1+/− and Xpr1−/− fetuses, respectively) (Fig. 3 C,D), and by the decreased number of Alizarin red S–stained caudal vertebra (Fig. 3 E,F). The Pi and calcium content in fetal skeletons were assayed by reducing entire fetuses to ash and then measuring the Pi and calcium content of the ash. In both the Xpr1+/− mouse intercrosses and male Xpr1+/− and female WT mouse intercrosses, Xpr1−/− fetuses showed significantly reduced ash weight and Pi and calcium content compared with WT fetuses, and Xpr1+/− fetuses showed a similarly reduced trend (Fig. 3 G,H and Supplemental Fig. S4 G-H). The reduction in the Pi and calcium content of the ash was disproportionate to the reduced fetal weight in the Xpr1-deficient fetuses, as shown by the significantly reduced ratios of the ash Pi and calcium content to fetal weight in Xpr1−/− fetuses and by a similarly reduced trend in Xpr1+/− fetuses (Fig. 3 I and Supplemental Fig. S4 I), which might suggest a reduced overall growth rate with decreased net skeletal mineralization in the Xpr1-deficient fetuses.
Loss of XPR1 expression results in severe placental calcification
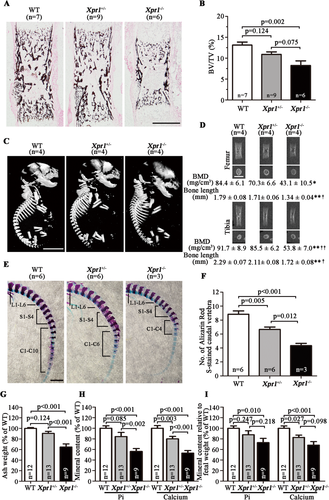
Considering that XPR1 plays an important role in placental-fetal Pi homeostasis and is expressed at high levels in the placenta, we carried out a pathological examination to investigate whether XPR1 deficiency results in placental dysfunction and/or abnormal development of the placenta. In comparison to WT placentas, the Xpr1+/− and Xpr1−/− placentas showed severe calcification in AR-stained transverse and horizontal sections at E15.5 and E18.5, and the severe calcification was mainly concentrated in the labyrinthine layer (Fig. 4 A–F, A′–F′ and Supplemental Fig. S5 A-F, A′-F′). A comparison of the degree of calcification among the WT, Xpr1+/−, and Xpr1−/− placentas from E15.5 to E18.5 showed the following average CCI values: 0.05%, 0.26%, and 0.83% for WT, Xpr1+/−, and Xpr1−/−, respectively, at E15.5; and 0.23%, 2.52%, and 6.51% for WT, Xpr1+/−, and Xpr1−/−, respectively, at E18.5. The degree of Xpr1+/− and Xpr1−/− placental calcification was increased with age and greater than that in the WT placentas, and the placental calcification was more severe for the Xpr1−/− placentas than for the Xpr1+/− placentas (Fig. 4 G,H). Similarly, in male Xpr1+/− and female WT mouse intercrosses, compared with the WT placentas, the Xpr1+/− placentas also showed an increasing degree of calcification with age (Supplemental Fig. S6 A-F, A′-D′). To further discern the patterns of initial calcification formation, horizontal sections of E15.5 Xpr1+/− placentas with slight Xpr1-associated calcification were stained with silver stain. The mineral crystal deposits were positively stained by black staining. Crystals were small in size and widely distributed in the labyrinthine layer. Amounts of crystals were observed in the trophoblasts and endothelial cells, indicating that Xpr1-associated calcification initially occurred inside the cells (Fig. 4 I).
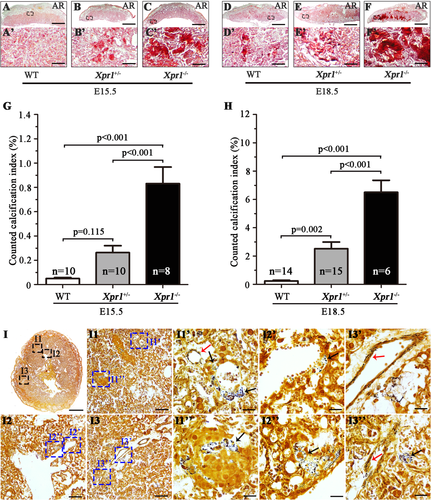
Growth restriction occurred in Xpr1 knockout fetuses
As Xpr1 deficiency disturbs placental-fetal Pi homeostasis and results in severe placental calcification in late gestation, these might increase the risk of adverse outcomes in the offspring. Mendelian inheritance statistics was performed for offspring at the time of birth. On rare occasions Xpr1−/− pups were born, but all of them were found dead within 1 hour after birth (P0 [1 hour]) (100% loss), indicating that the rare Xpr1−/− pups died in utero or immediately after birth. Xpr1+/− pups were born at lower rates (~36% loss), and a small proportion (~20%) of those pups died prior to postnatal day 7 (P7) (Supplemental Tables S1 and S2). These results indicate that Xpr1 deficiency likely results in severe fetal growth restriction during gestation. Therefore, different stages of gestation were examined to characterize the embryonic phenotypes. In Xpr1+/− mouse intercrosses, the number of genotypes (WT:Xpr1+/−:Xpr1−/− = 1:2:1) was in accordance with Mendelian inheritance at E15.5 (Supplemental Table S1), but the Xpr1−/− fetuses were smaller and had a lower body weight than the WT fetuses, whereas no significant differences were observed between the WT and Xpr1+/− fetuses (Fig. 5 A,C and Supplemental Fig. S7 A,C). At the E18.5 stage, the number of Xpr1−/− embryos was largely reduced (75% loss) (Supplemental Table S1), and the Xpr1−/− fetuses were smaller and had lower body weights than their WT littermates (Fig. 5 B,D). Both Xpr1+/− mouse intercrosses and male Xpr1+/− and female WT mouse intercrosses generated Xpr1+/− embryos, and these were always accompanied by dead fetuses and reduced embryo numbers at E18.5 (~34% loss). At that stage, Xpr1−/− fetuses exhibited more severe abnormal features than Xpr1+/− fetuses (Fig. 5 B,D, Supplemental Fig. S7 B,D and Supplemental Tables S1 and S2).

Identification of potential factors related to the loss of XPR1 expression
To determine the potential factors associated with placental dysfunction resulting from Xpr1 deficiency, we performed RNA-Seq with four WT and four Xpr1+/− littermate placentas at E17.5 that were derived from male Xpr1+/− and female WT mouse intercrosses. A total of 425 transcripts showed changes (p < 0.01) in expression in the Xpr1+/− placentas compared with their expression in the WT placentas, including 250 (58.8%) upregulated and 175 (41.2%) downregulated transcripts (Fig. 6 A). GO analysis showed that the upregulated transcripts were enriched for cytolysis, proteolysis, collagen fibril organization, cartilage development, angiogenesis, blood vessel development, immune response, and other biological processes (Fig. 6 B).
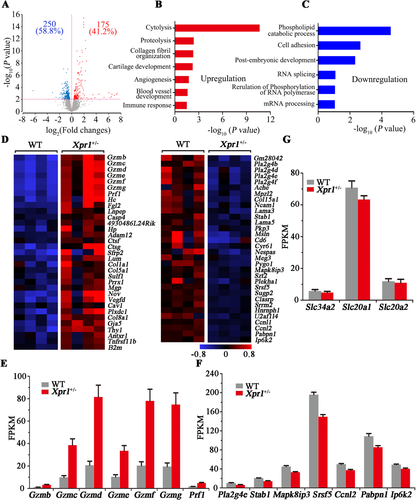
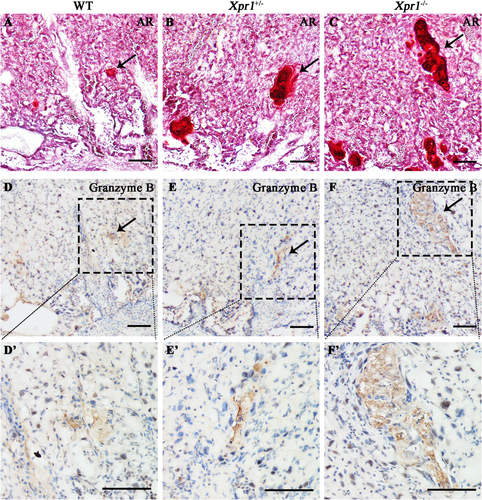
Notably, a set of granzymes (Gzmb, Gzmc, Gzmd, Gzme, Gzmf, and Gzmg), which play crucial roles in the biological processes of cytolysis, proteolysis, and immune response, showed significantly increased mRNA level (p < 0.01, fold change greater than two) in the Xpr1+/− placentas. In addition, the expression level (p < 0.01, fold change greater than two) of the perforin Prf1, which is functionally related to granzymes, was also significantly higher (Fig. 6 D,E). In addition, granzymes and perforin showed a similarly increased trend in the Xpr1-deficient placentas at E18.5 but no differences between WT and Xpr1-deficient placentas at E15.5 (Supplemental Fig. S8 C-D), implying that granzymes and perforin might be associated with the placental calcification in late gestation. To assess the roles that granzymes play in the Xpr1-deficient placentas, immunohistochemical analysis of granzyme B was performed with WT, Xpr1+/−, and Xpr1−/− placentas at E18.5. We found significantly increased granzyme B accumulation at the sites of placental calcification, suggesting that the granzymes were associated with the formation of placental calcification (Fig. 7 A–F, D′–F′).
In contrast, a series of transcripts with decreased expression levels (p < 0.01) were markedly enriched for phospholipid catabolic process (Pla2g4d), cell adhesion (Stab1), postembryonic development (Mapk8ip3), RNA splicing (Srsf5), regulation of phosphorylation of RNA polymerase (Ccnl2), and mRNA processing (Pabpn1), which implied a delay in placental development and function (Fig. 6 C,D,F). Interestingly, the expression level (p < 0.01) of Ip6k2, which encodes the mammalian enzyme inositol pyrophosphate that is associated with eukaryotic phosphate homeostasis, was also decreased in the Xpr1+/− placentas (Fig. 6 D,F).
Although previous studies have shown that the Na+/Pi transporters Slc34a2, Slc20a1, and Slc20a2 are highly expressed in the placenta and might play important roles in maintaining maternal-fetal Pi homeostasis, RNA levels of these three genes showed no marked differences between the WT and Xpr1-deficient placentas at E15.5, E17.5, and E18.5 (Fig. 6 G and Supplemental Fig. S8 E-F). In addition, no significant differences were found in RNA levels of genes known to be involved in physiological or pathological calcification, including the osteoblastic and chondrocytic differentiation molecules (Runx2, Bmp2, Msx2, and Sox9), smooth muscle cells markers (Tagln and Acta2), osteochondrogenic markers (Runx1, Spp1, Bglap2, and Alpl), and tissue calcification inhibitor (Mgp) (Supplemental Fig. S9 A-C). However, ALP staining for E18.5 placentas showed that ALP was mainly concentrated in the labyrinthine layer and significantly increased in Xpr1-deficient placentas (Supplemental Fig. S10 A-D).
Discussion
In this study, Xpr1 knockout mice were established to explore the function of XPR1 in the placenta. Xpr1 was found to be widely expressed in the mouse placenta, with particularly high expression levels in the spongiotrophoblast layer. Furthermore, clear increases in the Xpr1 expression level were observed during gestation, suggesting that XPR1 likely plays an important role in maintaining placental-fetal Pi homeostasis. The Pi level in amniotic fluid and serum and the skeletal mineral content were significantly decreased in the Xpr1-deficient fetuses and Pi was abnormally transported in Xpr1-deficient placentas, indicating that Xpr1 deficiency disrupted placental-fetal Pi homeostasis. Histopathologic examination of the Xpr1+/− and Xpr1−/− placentas demonstrated placental-fetal Pi dyshomeostasis resulting in severe calcification in the labyrinthine layer, which might disrupt the normal development and function of the placenta for maternal-fetal nutrient transfer. RNA-Seq analysis provided clues for the potential factors associated with the placental calcification and abnormal placental development resulting from Xpr1 deficiency. Mendelian inheritance statistics for the offspring outcomes showed that the number of Xpr1-deficient embryos was significantly reduced in late gestation. In addition, Xpr1 knockout resulted in perinatal lethality of the Xpr1−/− pups and neonatal death of a small proportion of Xpr1+/− pups. Further morphological analysis showed abnormalities in the Xpr1+/− and Xpr1−/− embryos compared with the WT embryos at E15.5 and E18.5, suggesting that the Xpr1 deficiency resulted in severe fetal growth restriction during late gestation. These data showed that XPR1 plays an essential role in maintaining placental-fetal Pi homeostasis in the mouse placenta, disruption of which causes severe placental calcification and fetal growth restriction.
At the stage of E15.5, Xpr1+/− fetuses showed a normal size and unaltered weight whereas 32P accumulation was significantly decreased compared with WT fetuses (Fig. 2 D, Fig. 5 A,C, Supplemental Fig. S4 A and Supplemental Fig. S7 A,C), indicating that Xpr1 deficiency resulted in a decreased placental Pi transport which was not caused by reduced weight or size. On the other hand, Xpr1−/− fetuses showed reduced size and ~20% reduction in weight compared with WT fetuses whereas 32P accumulation was reduced by ~33% (Fig. 2 D and Fig. 5 A,C). Therefore, we believe in Xpr1−/− fetuses the reduction in weight cannot fully account for the reduction in Pi transport. Taken together, our results further support a role for XPR1 in promoting placental Pi transport from maternal blood to fetal blood at E15.5.
The labyrinthine layer functions as the site of fetal-maternal exchange of nutrients, wastes, and gases. Within the labyrinthine layer, the fetal blood vessels lined by endothelial cells are separated from the maternal blood sinusoids by a trilaminar barrier, which is composed of two layers of syncytiotrophoblasts and a layer of mononuclear trophoblasts. The syncytiotrophoblasts are adjacent to the fetal endothelial cells, and the mononuclear trophoblasts are in direct contact with maternal blood (Supplemental Fig. S11).32, 33 In the present study, the reduced 32P accumulation in the Xpr1-deficient fetuses at E15.5 suggested that XPR1 deficiency in labyrinthine cells might reduce the forward flow of Pi toward the fetal circulation. However, 32P accumulation in the Xpr1-deficient fetuses at E18.5 was significantly increased, indicating that the fetal-maternal Pi exchange was significantly increased. Compared with E15.5 placentas, the Xpr1-deficient E18.5 placentas showed more severe placental calcification in the labyrinthine layer, indicating that a large area of the structures necessary for separation of maternal and fetal blood had been disrupted, which might destroy normal active Pi transport or even result in direct contact between maternal and fetal blood, thus contributing to an increase in the rate of maternal-fetal Pi exchange. Hence, we speculated that XPR1 deficiency likely reduces the labyrinthine Pi export capacity from maternal blood to fetal blood in early gestation, but the severe placental calcification caused by XPR1 deficiency in late gestation may largely increase Pi exchange between maternal and fetal blood (Supplemental Fig. S11).
As the only known phosphate exporter in metazoans,25 XPR1 is expressed in all human tissues.23 Conditional inactivation of Xpr1 in renal tubules in mice will impair renal Pi reabsorption, which results in a reduced serum Pi level and bone mineralization.34 Hence, in the Xpr1-deficient fetuses, although the abnormal placental Pi exchange might be a contributing factor, the XPR1 deficiency throughout all fetal tissues, especially in the kidneys and intestines, might be the main factor underlying the reduced Pi level in fetal serum and amniotic fluid and the reduction in fetal skeletal mineralization.
The mechanism of placental calcification is poorly understood. Three known pathways of tissue calcification including metastatic calcification associated with a supersaturated environment of calcium and phosphate, dystrophic calcification such as that occurring in injured or necrotic tissues and physiologic calcification such as that occurring in the formation of bone and teeth, were considered to be possibly involved.35-38 The fetal serum Pi level being above the maternal value suggested that Pi was enriched in the placenta, which might promote the accumulation of metastatic calcification.10, 11 XPR1 has been confirmed as a Pi exporter in mammals. Disruption of XPR1 function through mutations or the use of targeted siRNA decreases intracellular Pi efflux, and mutations in the SPX domain of XPR1 result in brain calcification.24 In this study, the XPR1 deficiency in the labyrinthine cells likely resulted in an excessive intracellular Pi concentration with resultant toxic effects, which could accelerate the formation of hydroxyapatite. Supporting this is the observation that the initial calcification sites were located in the trophoblasts and endothelial cells of the Xpr1-deficient placentas.
In the RNA-Seq analysis, the comparisons of Xpr1-deficient versus WT placentas on E18.5 showed more of the transcripts that were significantly differentially expressed, and many that were associated with the immune response compared with the comparisons of Xpr1-deficient versus WT placentas on E15.5 (Supplemental Fig. S8 A-B). The histological analysis showed that the Xpr1-deficient placentas on E18.5 presented more severe placental calcification compared with the Xpr1-deficient placentas on E15.5. The severity of these global transcriptome changes correlate with the severity of placental calcification, which implied that severe placental calcification might have a great impact on placental function. In the immune response, a set of granzymes (B, C, D, E, F, and G) with high expression levels caught our attention; they showed no change at the stage of the occurrence of initial calcification (E15.5) but largely increased at the stage of severe calcification (E17.5 and E18.5) in the Xpr1-deficient placentas. Granzymes are involved in cytolysis, proteolysis and immune response. When functioning as serine proteases, granzymes are expressed in cytoplasmic granules of natural killer and cytotoxic T cells39, 40 and participate in cell death.41, 42 In addition, the expression level of perforin (Prf1), which has been shown to induce cell death in synergy with the granzymes,43 also showed a notable increase in the Xpr1+/− placentas. Immunohistochemistry showed significantly increased granzyme B accumulation at the sites of placental calcification in labyrinthine layer, supporting the notion that granzymes are closely related to the observed placental calcification. Hence, we speculate that the increased expression levels of granzymes and perforin were likely caused by Xpr1-associated placental calcification, which could disrupt the normal function of the immune system in the placenta. In addition, although the osteochondrogenic marker ALP showed no significant difference at the mRNA level by RNA-Seq analysis between WT and Xpr1-deficient placentas, the protein level of ALP was significantly increased in Xpr1-deficient labyrinthine layer at E18.5, suggesting that ALP likely had participated in the process of Xpr1-associated placental calcification.
In summary, we showed that Xpr1 deficiency disturbs placental-fetal Pi homeostasis, including lower Pi level in amniotic fluid and fetal serum, a reduced fetal skeletal mineral content, and abnormal placental Pi exchange. The placental-fetal Pi dyshomeostasis accelerates severe placental calcification and fetal growth restriction. These findings are crucial for understanding placental-fetal Pi homeostasis and placental calcification.
Disclosures
All authors state that they have no conflicts of interest.
Acknowledgments
This study was primarily supported by the National Natural Science Foundation of China (grants 31671301, 31871262, and 31230045 [to JYL]; and 31501019 [to CW]) and by the National Key Research and Development Program of China (2016YFC1306000 to JYL).
Authors' roles: Study design: JYL and XX. Study conduct: XX, XL, HS, ZC, RG, TN, YW, TM, RC, and CW. Data collection: XX, XL, and HS. Data analysis and interpretation: XX, XL, HS, and CW. Drafting manuscript: JYL and XX. Revising manuscript content: JYL and ZY. Approving final version of manuscript: JYL, XX, XL, and HS.




