EZH2 Supports Osteoclast Differentiation and Bone Resorption Via Epigenetic and Cytoplasmic Targets
ABSTRACT
Key osteoclast (OCL) regulatory gene promoters in bone marrow–derived monocytes harbor bivalent histone modifications that combine activating Histone 3 lysine 4 tri-methyl (H3K4me3) and repressive H3K27me3 marks, which upon RANKL stimulation resolve into repressive or activating architecture. Enhancer of zeste homologue 2 (EZH2) is the histone methyltransferase component of the polycomb repressive complex 2, which catalyzes H3K27me3 modifications. Immunofluorescence microscopy reveals that EZH2 localization during murine osteoclastogenesis is dynamically regulated. Using EZH2 knockdown and small molecule EZH2 inhibitor GSK126, we show that EZH2 plays a critical epigenetic role in OCL precursors (OCLp) during the first 24 hours of RANKL activation. RANKL triggers EZH2 translocation into the nucleus where it represses OCL-negative regulators MafB, Irf8, and Arg1. Consistent with its cytoplasmic localization in OCLp, EZH2 methyltransferase activity is required during early RANKL signaling for phosphorylation of AKT, resulting in downstream activation of the mTOR complex, which is essential for induction of OCL differentiation. Inhibition of RANKL-induced pmTOR-pS6RP signaling by GSK126 altered the translation ratio of the C/EBPβ-LAP and C/EBPβ-LIP isoforms and reduced nuclear translocation of the inhibitory C/EBPβ-LIP, which is necessary for transcriptional repression of the OCL negative-regulatory transcription factor MafB. EZH2 in multinucleated OCL is primarily cytoplasmic and mature OCL cultured on bone segments in the presence of GSK126 exhibit defective cytoskeletal architecture and reduced resorptive activity. Here we present new evidence that EZH2 plays epigenetic and cytoplasmic roles during OCL differentiation by suppressing MafB transcription and regulating early phases of PI3K-AKT–mTOR-mediated RANKL signaling, respectively. Consistent with its cytoplasmic localization, EZH2 is required for cytoskeletal dynamics during resorption by mature OCL. Thus, EZH2 exhibits complex roles in supporting osteoclast differentiation and function. © 2019 American Society for Bone and Mineral Research.
Introduction
In response to RANK activation, bone marrow–derived monocyte (BMM) differentiation into osteoclasts (OCL) is guided by signal transduction cascades and epigenetic events, which regulate waves of OCL gene expression. Both activation of OCL-specific genes and repression of OCL-inhibitory factors are critical to normal and inflammatory OCL differentiation. RANK activation initiates profound changes in DNA methylation and nucleosome remodeling, which affect the accessibility of transcription factors to activate or repress OCL gene promoters.1-4 Targeting either histone deacetylases (HDACs) or bromodomain and extra-terminal (BET) proteins inhibited osteoclastogenesis due to deregulation of key OCL factors c-Fos and Nfatc1.5, 6 Global chromatin changes in CBP/p300-mediated acetylation of H3 lysine 18 (H3K18ac) and monomethylation of H3K27 (H3K27me1) during OCL initiation have been shown to guide MMP-9-dependent histone H3 N-terminal tail (H3NT) proteolysis, resulting in transcriptional activation of OCL genes.7, 8 Recent evidence suggests that promoters of key OCL factors harbor bivalent chromatin architecture having both activating and repressing trimethylation of histone H3 Lysine 4 (H3K4me3) and Lysine 27 (H3K27me3) marks, respectively.9 Bivalent promoters restrict expression of important developmental genes to allow for their temporal and lineage-specific expression during cell differentiation.10 Histone demethylase Jumonji domain-containing 3 (Jmjd3), which removes repressive H3K27me3, has been implicated in global activation of RANKL-dependent genes, including Nfatc1 and protocadherin-7.9, 11 In addition to transcriptional activation, various mechanisms facilitate repression of negative OCL regulators to allow for successful osteoclastogenesis.12 Several transcription factors that inhibit OCL differentiation have been identified, including V-maf musculoaponeurotic fibrosarcoma oncogene homologue B (MafB),13 Interferon regulatory factor 8 (IRF8),14 inhibitors of differentiation (Ids),15, 16 Zinc finger protein Eos,17 and B-cell lymphoma 6 (Bcl6).18 In addition to transcription factors, OCL-inhibitory chromatin methyltransferases DOT1L19 and Jmjd520 have been recently reported to negatively regulate osteoclastogenesis. Enhancer of zeste homologue 2 (EZH2) is the methyltransferase subunit of the Polycomb repressive complex 2 (PRC2), which deposits the heterochromatic mark H3K27me3 that silences gene expression.21 EZH2 plays an important role in bone homeostasis and the use of the small molecule inhibitor GSK126 (a potent and highly selective EZH2 methyltransferase inhibitor22) exhibited promising bone protecting effects in several osteoporotic/inflammatory and malignant osteolytic bone pathologies.23-27 EZH2 was shown to epigenetically repress the OCL inhibitory factor IRF8.25 Thus, EZH2 was defined as a positive regulator of osteoclastogenesis; however, much about its role during OCL differentiation is unknown.
Therefore, in this study we further investigated the epigenetic role of EZH2 histone methyltransferase function during osteoclastogenesis. We found that EZH2 epigenetically represses several negative regulators of OCL formation. Unexpectedly, we also discovered that EZH2 has critical nonhistone methyltransferase functions that have cytoplasmic roles in both early RANKL signaling and mature OCL function.
Materials and Methods
Cells and reagents
Nonadherent BMM harvested from 4- to 12-week-old healthy wild-type C57BL6 mice were cultured in αMEM supplemented with 10% FCS and 1% pen/strep as previously described.28 The IACUC at the University of Pittsburgh approved all animal studies. Scrambled control (SHC002, Sigma, St. Louis, MO, USA) and mouse EZH2 shRNA (TRCN0000304506, 5'-GCACAAGTCATCCCGTTAAAG-3', Sigma) in pLKO.1-puro lentivirus particles were generated and used to transduce (with polybrene) primary BMM cells. GSK126 inhibitor (S7061) was purchased from Selleckchem (Houston, TX, USA).
OCL differentiation and TRAP staining
Primary BMM cells were expanded in αMEM with MCSF (20 ng/mL) (R&D Systems, Minneapolis, MN, USA) for 3 days (d−3 to d0) to generate OCLp. Then RANKL (10 ng/mL) (R&D Systems) and MCSF (10 ng/mL) were used at d0 to differentiate OCL for 4 to 5 days. In some cases, BMM were transduced with lentivirus particles for the first day (d−3 to d−2) of MCSF generation of OCLp. OCL were fixed with 37% formaldehyde for 15 minutes, washed with ddH2O, and stained using leukocyte acid phosphatase kit (Sigma-Aldrich, 387A-1KT) following the manufacturer's instructions. TRAP+ multinucleated cells with three or more nuclei were counted as OCL. All OCL formation experiments were scored from four to five independent wells.
Real-time quantitative PCR (qPCR) RNA expression analyses
BMM RNA was isolated using TRIzol reagent and converted to cDNA using First-Strand cDNA Synthesis System (Life Technologies, Carlsbad, CA, USA; 11904–018). qPCR was carried out using 2x Maxima SYBR Green/ROX qPCR Master Mix (K0223, Thermo Fisher Scientific, Waltham, MA, USA) in Fast 96-Well Reaction Plates (Applied Biosystems, Carlsbad, CA, USA) using a StepOnePlus (Applied Biosystems). Relative mRNA levels were calculated using the ΔΔCt method using 18srRNA for normalization. The qPCR primers are listed in Table 1.
| Gene | Forward primer (5′- > 3′) | Reverse primer (5′- > 3′) |
|---|---|---|
| mEzh2 | TACATCCCTTCCATGCAACA | TCCCTCCAGATGCTGGTAAC |
| m18srRNA | GAGCGACCAAAGGAACCATA | CGCTTCCTTACCTGGTTGAT |
| mNfatc1 | TGGAGAAGCAGAGCACAGAC | GCGGAAAGGTGGTATCTCAA |
| mDC-stamp | AAACGATCAAAGCAGCCATTGAG | ATCATCTTCATTTGCAGGGATTGTC |
| mRank | GTGGAAATAAGGAGTCCTCAG | CACCGTCTTCTGGAACCATC |
| mCatk | AATACGTGCAGCAGAACGGAGGC | CTCGTTCCCCACAGGAATCTCTCTGTAC |
| mMafB | GAGCAGGTGTGACTCACGAT | TGGCTAGTGGGTAGCTGTTG |
| mIrf8 | CTACCTGACAGCAGGGTGGT | GGCATACAGCTGCTCTACCTG |
| mArg1 | ATCCCAGCAGTTCCTTTCTG | CATCTTTTGAACAGCGTGGA |
| mBcl6 | GACGTTGTCATCGTGGTGAG | GGTTGCATTTCAACTGGTCA |
Immunoblotting
Immunoblotting was performed according to standard protocols using the following antibodies directed toward: EZH2 (Cell Signaling Technology, Danvers, MA, USA; 3147), H3K27me3 (Active Motif, Carlsbad, CA, USA; 39155), H3 (Cell Signaling Technology, 9715), H3K9ac (Active Motif, 61251), β-actin (Sigma, 85316), MafB (Santa Cruz Biotechnology, Dallas, TX, USA; sc-376387), C/EBPβ C-terminal (Santa Cruz, sc-150), C/EBPβ-LAP (Cell Signaling Technology, 3087), pPI3K-p85(Tyr458)/p55(Tyr199) (Cell Signaling Technology, 4228), pPDK1-Ser241 (Cell Signaling Technology, 3061), AKT (Cell Signaling Technology, 9272S), pAKT-Ser473 (Cell Signaling Technology, 4060), mTOR (Cell Signaling Technology, 2983), pmTOR Ser2448 (Cell Signaling Technology, 2971), and pS6RP-Ser235/236 (Cell Signaling Technology, 2211). Nuclear and cytoplasmic protein separations were performed using the Nuclear Extract Kit (Active Motif, 40010). Band signals were detected using Amersham ECL Western Blotting Detection Reagent (GE Life Sciences, Piscataway, NJ, USA; RPN2106) and analyzed and quantitated using ProteinSimple Imager and AlphaView software (ProteinSimple, San Jose, CA, USA).
ChIP assay
Chromatin from BMM was analyzed using a modification of the ChIP Millipore/Upstate protocol (MCPROTO407) as described29 using Magna ChIP Protein A + G Beads (16–663, Millipore, Burlington, MA, USA). The following antibodies were used for ChIP: EZH2 (Active Motif, 39902), H3K27me3 (Active Motif, 39155), C/EBPβ C-terminal (Santa Cruz, sc-150), and C/EBPβ-LAP (Cell Signaling Technology, 3087). Aliquots for input and nonspecific IgG control samples were included with each experiment. ChIP-qPCR primers are listed in Table 2. Fold enrichment was calculated based on Ct as 2(ΔCt), where ΔCt = (CtInput – CtIP). The IgG ∆Ct was subtracted from the specific Ab ∆Ct to generate ΔΔCt = (∆Ctspecific Ab – ∆CtIgG).
| ChIP amplicons (murine) | Amplicon distance from TSS (bp) | Forward (5′- > 3′) | Reverse (5′- > 3′) |
|---|---|---|---|
| MafB 0 | −326 | CGAGGGTGGGAGTGTAAAGA | ACCCTCTGAGTCGAGGTTCC |
| MafB 1 | −1 | GCAAGCAAGAAAGCCCTAGA | GGGAACGAGTCAGGTCGAG |
| MafB 2 | +1149 | CAGCAGAAACATCACCTGGA | CCTTGTAGGCGTCTCTCTCG |
Immunofluorescence microscopy
OCLp or mature OCL were seeded on glass bottom culture dishes (D35-20-0-N, Cellvis, Mountain View, CA, USA). Cells were differentiated in MCSF/RANKL as described above. At the end of the experiment, cells were fixed with 4% paraformaldehyde for 15 minutes and permeabilized with 0.1% Triton X-100 for 10 minutes. Slides were blocked with 1% PBS/BSA for 1 hour, then incubated overnight with primary anti-EZH2 (Cell Signaling Technologies, 3147) and anti-CTR (Santa Cruz, sc-8861) antibody in PBS/BSA at 4°C. Next day, samples were incubated with secondary antibodies conjugated with Alexa Fluor 488 and/or 546 in PBS/BSA at room temperature for 1 hour. DAPI (D21490, Life Technologies) was used to visualize nuclei. F-actin was stained with rhodamine phalloidin (PHDR1, Cytoskeleton Inc., Denver, CO, USA). Fluorescent images were captured using Olympus Fluoview 1000 and Nikon AIR Inverted Research Microscope ECLIPSE Ti2-E. Image quantification was performed using Nikon General Analysis 3 to determine the cytoplasmic versus nuclear distribution of EZH2 in cell populations.
Bone resorption assay
After expansion of primary BMM in αMEM with 20 ng/mL of MCSF (R&D Systems) for 3 days to make OCLp, cells were harvested, counted, and plated onto cortical bovine bone slices (Boneslices.com, lot #12193) at 1 × 105 cells/well. Cells were treated with RANKL (10 ng/mL) + MCSF (10 ng/mL) for 7 to 9 days and media was changed every other day. At day 7 to 9 as indicated, cells were fixed with 37% formaldehyde for 15 minutes and stained for TRAP expression as described previously. After TRAP+ OCL counts were determined, bone slices were incubated in ddH2O with 1% bleach and sonicated until the cells were removed from the bone surface. Resorption pits were visualized using 0.5% toluidine blue (Sigma, 89640) staining for 5 minutes. Area of resorption pits was analyzed using Image J software (National Institutes of Health, Bethesda, MD, USA).
Statistical analyses
All experiments were repeated at least three independent times. Most data are presented as biological triplicates and results reported as means ± SEM unless otherwise stated. Statistical significance was evaluated by either the Student's t test, one-way or two-way ANOVA, or linear regression using GraphPad Prism 7 (GraphPad, La Jolla, CA, USA) as indicated. Degree of significance is represented using p values: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
Results
EZH2 plays a critical role within the first 24 hours of RANKL-activated osteoclastogenesis and mediates repression of OCL-negative regulators
To determine the role of EZH2 during OCL differentiation, we treated primary BMM (OCLp) with the highly selective EZH2 methyltransferase-domain inhibitor GSK126.22 GSK126 added to OCLp cultures at the time of RANKL induction dose-dependently inhibited OCL formation (Fig. 1 A, B). As Fig. 1 B, part 3, demonstrates, the presence of GSK126 only during OCLp MCSF-expansion phase did not alter subsequent osteoclastogenesis. Ezh2 mRNA was transiently upregulated at 12 hours after RANKL activation of OCLp (Fig. 1 C) with a concomitant increase in EZH2 protein expression levels (Fig. 1 D). GSK126 effectively blocked EZH2 methyltransferase activity as determined by the decrease in total cellular levels of H3K27me3 (Fig. 1 E), without altering Ezh2 mRNA and protein levels (Fig. 1 C, E). The acetylation mark at H3K9 (H3K9ac) remained unaffected by the inhibitor (Fig. 1 E). Consistent with decreased OCL formation, GSK126 reduced RANKL-mediated upregulation of critical early OCL regulators Nfatc1 and Rank as well as CatK30 and DC-STAMP,31 which are elevated during later stages associated with fusion and resorption by multinucleated OCL (Fig. 1 F). Further, EZH2 inhibition prevented downregulation of the OCL inhibitory factors MafB,13, 32 Irf825, 33, 34 and Arg135 (Fig. 1 G). However, to our surprise, GSK126 treatment did not affect expression of another OCL-negative regulator Bcl6 (Fig. 1 G), which argues for gene-specific repression by EZH2 in RANKL-stimulated OCLp.
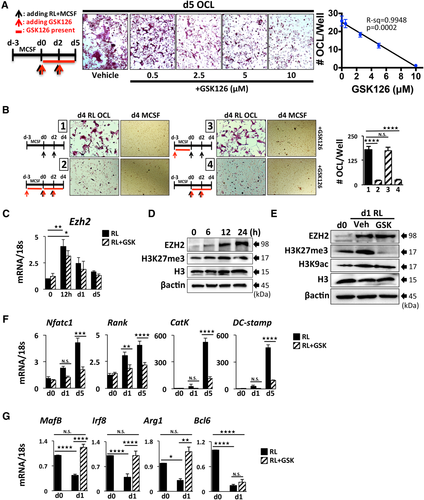
To further demonstrate the specificity of GSK126 inhibition of EZH2 on OCL differentiation, we employed lentiviral transduction of shEzh2 in primary OCLp to knock-down (KD) EZH2, which resulted in reduced Ezh2 mRNA (Fig. 2 A) and protein (Fig. 2 B) together with decreased total levels of H3K27me3 in cells (Fig. 2 B) at d1 RANKL. Consistent with the GSK126 inhibition results, the Ezh2 KD significantly inhibited formation of TRAP+ multinucleated OCL (Fig. 2 C) and reduced expression of OCL-specific genes (Supplemental Fig. S1 A). In contrast, the OCL-negative regulator MafB mRNA and protein were significantly upregulated in Ezh2-KD OCLp (Fig. 2 A, B). Next, we overexpressed EZH2 (OE) using lentivirus transduction in primary OCLp as confirmed by both mRNA and protein levels (Fig. 2 D, E). EZH2-OE enhanced the RANKL-driven decrease in MafB expression (Fig. 2 D) and elevated the cellular H3K27me3 levels (Fig. 2 E). Although we did not detect an increase in Rank and CatK mRNA (Supplemental Fig. S1 B), EZH2-OE cultures exhibited significantly higher formation of multinucleated TRAP+ OCL (Fig. 2 F, G) with increased bone resorption (Fig. 2 G) but did not alter the resorption/OCL. Furthermore, EZH2-OE OCL formation was more resistant to GSK126 treatment compared with control cells (Supplemental Fig. S1 C).
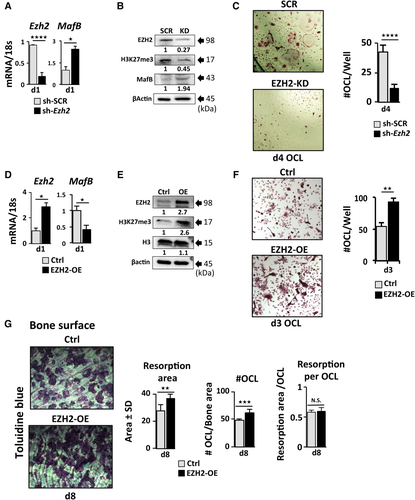
To further delineate the timing of EZH2 activity, we applied GSK126 within selective windows throughout the course of OCL differentiation. In the first set of experiments, GSK126 was applied to differentiating OCL for the period of 24, 48, and 72 hours after RANKL stimulation. Inhibition of EZH2 within the first 24 hours of primary RANKL treatment was sufficient to inhibit OCL formation as shown by the reduced number of TRAP+ multinucleated OCL (Fig. 3 A). The inhibition of OCL differentiation by GSK126 was not due to decreased cell viability (Supplemental Fig. S1 D). The inhibitory effect of the 24- and 48-hour GSK126 treatment windows was reduced when RANKL was added at day 2 of the OCL differentiation (Fig. 3 B). To further analyze the importance of the 24-hour EZH2 activity window, we delayed the addition of GSK126 by 24, 48, and 72 hours after initial RANKL treatment to differentiating OCL cultures (Fig. 3 C). Addition of GSK126 at 24 hours after the initial RANKL treatment instead of at day 0 significantly reduced the inhibition of osteoclastogenesis, but the number of nuclei per OCL was fewer (predominantly 3 to 5 nuclei/OCL) compared with the vehicle-treated control group, which was predominantly ≥10 nuclei/OCL (Fig. 3 C). Increased delay of GSK126 addition to differentiating cultures resulted in increased OCL numbers and #nuclei/OCL (Fig. 3 C). Subsequent addition of RANKL at day 2 did not significantly alter the results of the GSK126 delay experiment (Supplemental Fig. S1 E). Next, we asked whether increased dosage of MCSF or RANKL during activation of OCL differentiation would rescue the GSK126-mediated OCL inhibition. Although high MCSF doses increased OCLp proliferation, neither increased RANKL nor increased MCSF rescued formation of TRAP+ OCL in the presence of GSK126 (Supplemental Fig. S2 A).
GSK126 blocks RANKL-induced mTOR activation thereby resulting in a decreased C/EBPβ LIP to LAP ratio leading to increased binding of C/EBPβ LAP at the MafB gene promoter
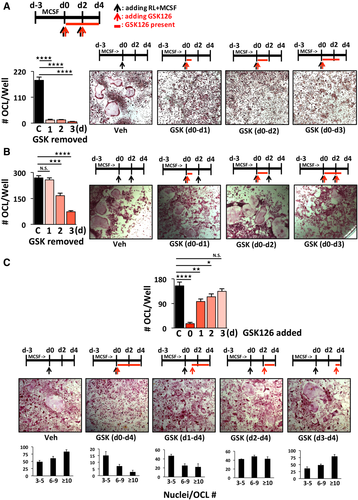
RANKL-induced switching of the translational regulation of C/EBPβ from the LAP isoform toward enhanced production of the dominant-negative LIP isoform (increasing the LIP:LAP ratio) plays a critical role during progression of osteoclastogenesis by inducing transcriptional repression of the OCL inhibitory factor MafB.32 In search of the mechanism controlling MafB expression, we asked whether EZH2 methyltransferase activity is required for the RANKL-induced translational switching of the C/EBPβ-LIP:LAP isoform ratio in primary OCLp. GSK126 exposure downregulated total cellular C/EBPβ-LIP levels in d1 RANKL-treated OCLp (Fig. 4 A). Using cytoplasmic and nuclear separation, we confirmed that RANKL treatment decreased the levels of cytoplasmic LAP and increased the nuclear C/EBPβ-LIP isoform (Fig. 4 B). This translocation ratio was reversed by GSK126 treatment with significant reduction of C/EBPβ-LIP in the nuclear fraction (Fig. 4 B). We used α-tubulin as cytoplasmic and H3K27me3 and total H3 as nuclear markers to demonstrate the purity of the separated cellular fractions (Fig. 4 B). The alteration in C/EBPβ-LIP:LAP isoform ratio was also confirmed in the EZH2-KD OCLp vs scrambled (sh-SCR) controls (Fig. 4C). Further, a time course (0, 1, 12, and 24 h) revealed that the RANKL-induced translational switch from LAP to LIP starts within an hour, and this is prevented by GSK126 (Supplemental Fig. S3 A). On the other hand, it takes more than 12 hours for GSK126 to begin to decrease the global level of H3K27me3 (Supplemental Fig. S3 B). Chromatin IP (ChIP) confirmed that RANKL activation increased recruitment of EZH2 together with increased enrichment of H3K27me3 at MafB in OCLp (Fig. 4 D). Although EZH2 binding at the MafB promoter was not significantly affected by GSK126 treatment, inhibition of EZH2 enzymatic activity prevented RANKL-induced H3K27 trimethylation of the MafB promoter. We also examined the direct involvement of endogenous C/EBPβ-LIP/LAP isoform switching at the MafB promoter through ChIP analyses with antibodies directed toward either the N- (LAP) or the C-terminus (LAP+LIP) of C/EBPβ (Fig. 4 C, E). Here we show that GSK126 inhibits the recruitment of RANKL-induced inhibitory LIP while increasing the binding of the LAP isoform (Fig. 4 E), which results in increased MafB expression (Fig. 1 G).
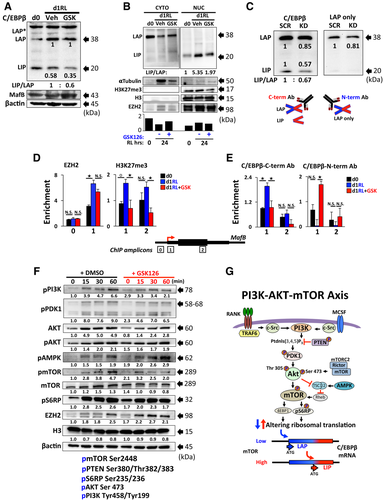
A critical pathway regulating OCL differentiation downstream of RANK activation is the PI3K-AKT–mTOR signaling axis.36, 37 Smink and colleagues reported that mTOR pathway activation directly affects the C/EBPβ-LIP:LAP translation ratio by favoring production of the LIP isoform translation to allow for progression of osteoclastogenesis.38 Since GSK126 treatment downregulated total cellular C/EBPβ-LIP levels, we analyzed the effects of GSK126 on the RANKL activation of mTOR signaling within the first 60 minutes. GSK126 treatment significantly reduced RANKL-mediated activation of PI3K and the subsequent phosphorylation of its downstream cytoplasmic effectors PDK1 and AKT (pSer473 and pThr 308) leading to reduced cellular levels of pmTOR together with pS6RP (Fig. 4 F,G). Pathways regulating ERK activation, p38, and STAT3 phosphorylation were not affected by GSK126 treatment (Supplemental Fig. S2 B). These data suggest that EZH2 methyltransferase activity has a cytoplasmic role that supports rapid events in RANKL signaling.
EZH2 changes cellular localization at distinct stages of OCL differentiation
Increasing evidence suggests the involvement of EZH2 methyltransferase activity in regulating actin dynamics, cytoplasmic signaling, and cytoskeletal rearrangements.39 Immunofluorescent microscopy and quantitation revealed that EZH2 in MCSF expanded OCLp is largely cytoplasmic in punctate structures (Fig. 5 A, B), with only 3% of cells having >50% nuclear fluorescence. Consistent with its nuclear role as an epigenetic repressor, RANKL treatment upregulated and induced most of EZH2 to translocate to the nucleus such that 85% of cells had >50% nuclear fluorescence (Fig. 5 A, B, Supplemental Fig. S4). Surprisingly, the RANKL-induced EZH2 nuclear translocation was partially blocked by GSK126 such that EZH2 was distributed throughout the cell with a pronounced cytoplasmic localization and only 28% of cells had >50% nuclear fluorescence (Fig. 5 A, B). This indicates that EZH2 activity is required for the RANKL signal that triggers EZH2 nuclear translocation. Western blot analyses of EZH2 cellular localization further supports this with the % nuclear EZH2 about twofold higher in RANKL-treated cells versus d0 cells. (Fig. 4 B). To our further surprise, we observed that during fusion of multinucleated OCL, EZH2 was again largely diffused throughout the cytoplasm (Fig. 5 A). EZH2 localization changes in OCLp on the bone surface resembled the changes in OCLp on glass (Fig. 5 C), but there was a more mixed nuclear/cytoplasmic distribution in mature OCL on bone (Fig. 5 D).
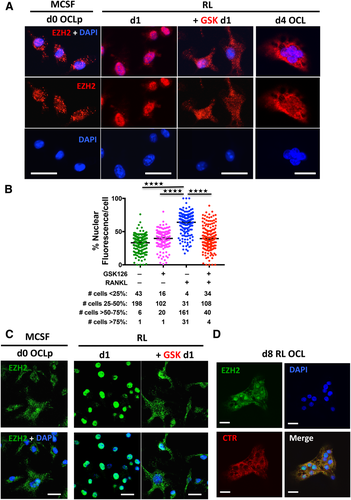
Cytoplasmic EZH2 is required for cytoskeletal organization and resorption of mature OCL
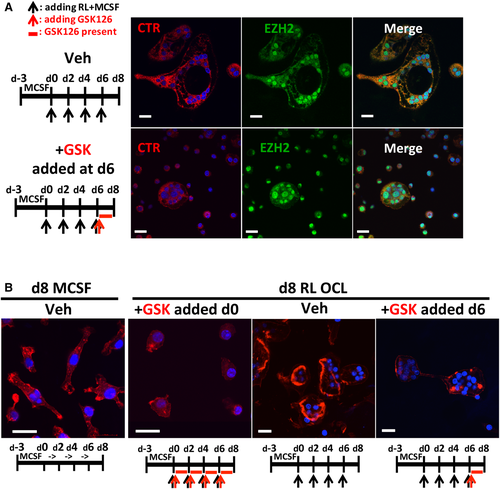
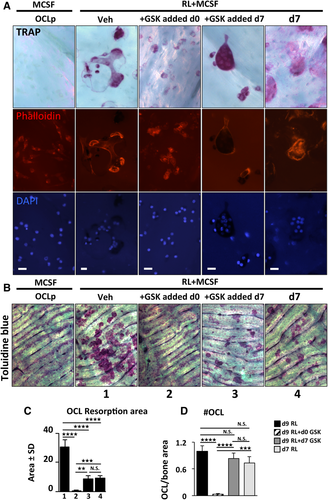
Our results demonstrated that EZH2 methyltransferase plays a critical cytoplasmic role in promoting RANKL-mediated PI3K signaling to activate the mTOR complex to regulate protein translation in OCLp. We next explored the function of cytoplasmic EZH2 in mature OCL. Because inhibition of EZH2 during the initial phase of OCLp RANKL activation totally blocks OCL fusion, we asked whether blocking EZH2 methyltransferase after formation of TRAP+ multinucleated OCL affects their ability to resorb bone. To address this, OCLp were subjected to RANKL differentiation for 8 days on bone chips and GSK126 was added to OCL cultures only during the last 48-hour window. This allowed us to assess the effect of GSK126 on OCL function. Compared with the vehicle-treated controls, GSK126-treated OCL exhibited condensed cytoskeletal architecture (Fig. 6 A, Supplemental Fig. S5). Of note, EZH2 was clearly localized in both the cytoplasm and the nuclei in both untreated and GSK-treated OCL. Further analyses using phalloidin staining demonstrated that GSK126-treated OCL lack well-defined F-actin ring/sealing zones (Fig. 6 B, Supplemental Fig. S6 A, B). Mononuclear cells that remain in GSK126-treated cultures are largely TRAP+ and also exhibit impaired adhesive shape and actin morphology (Figs. 6 B and 7, Supplemental Fig. S6). To specifically assess the effect of GSK126 on resorption, OCL were cultured for 9 days on the bone surface and GSK126 was applied either throughout the entire course or only during the last 48 hours of the differentiation protocol from day 7 to day 9. We included an additional control group (last panel day 7), in which cells were fixed at day 7. This enabled us to compare the amount of resorption that occurred before GSK126 addition. As expected, GSK126 added with the initial RANKL treatment prevented OCL fusion (Fig. 7 A). We were able to visualize TRAP with nuclei together with the F-actin of multinuclear cells to show that GSK126 treatment d7–d9 inhibited proper sealing zone formation compared with vehicle-treated OCL at either d7 or d9. Mature OCL cultured on bone in the presence of GSK126 d7–d9 exhibited dense TRAP staining and condensed cytoskeletal architecture (Fig. 7 A, Supplemental Fig. S6 C). Toluidine blue staining showed that OCL in the absence of GSK126 went from forming smaller individual pits at d7 to well-defined resorption trails by d9. However, the OCL treated with GSK126 from d7–d9 essentially stopped resorption (Fig. 7 B–D, Supplemental Fig. S7). This suggests that the methylation activity of EZH2 is required for adhesion and resorption by mature OCL.
Discussion
It is increasingly evident that epigenetic-based mechanisms play an instrumental role in orchestrating gene activation and repression programs in OCL.40 In particular, histone methylation of bivalent promoters is central to progression of RANKL-induced OCL differentiation by both activating9, 11, 41 and repressing25 selected important gene promoters in OCLp. EZH2 directed H3K27me3-dependent chromatin repression of developmental genes is central to cellular differentiation programs.42 A recent report by Qiao and colleagues demonstrated the importance of H3K27me3-based heterochromatin silencing of a subset of anti-inflammatory and pro-osteoclastogenic genes during INF-γ-mediated macrophage activation.43 The role of epigenetic-based gene suppression by EZH2 in combination with DNMT3a has only been reported for the IRF8 gene in a human OCL differentiation system.4, 25 The realization that EZH2 is a multifunctional histone methyltransferase, subjected to various regulatory post-translational modifications with both nuclear and cytoplasmic functions,44 prompted us to better define the role of EZH2 during early osteoclastogenesis and in mature bone-resorbing cells. EZH2 activity and total cellular H3K27me3 levels are upregulated during the initial 12 to 24 hours of RANKL-induced pre-OCL, and blocking its methyltransferase activity using the selective inhibitor GSK126 during the first 24 hours of RANKL treatment was necessary and sufficient to inhibit formation of mature OCL. In contrast, treatment of primary BMM during the 3-day MCSF expansion phase to form OCLp did not alter subsequent OCL formation (Figs. 1 A, B and 3). We found both GSK126 inhibition and EZH2-KD upregulated expression of the OCL inhibitory factors MafB and Arg1 as well as the previously reported Irf825 (Figs. 1 G and 2 A). With the focus on epigenetic regulation of the MafB gene, we confirmed that GSK126 treatment prevented RANKL-induced H3K27 trimethylation of the MafB promoter in OCLp during the first 24 hours (Fig. 4 D).
A collection of reports demonstrates that translational regulation of C/EBPβ-LIP:LAP isoform ratio influences various cell processes including proliferation and tumorigenesis of certain B-cell malignancies.45, 46 As a result of mTOR-dependent alternative initiation of translation, a single intronless C/EBPβ mRNA gives rise to two long transcriptional activator (LAP* and LAP) isoforms and one truncated repressor (LIP) isoform.47 Smink and colleagues showed that mTOR-dependent activation of the eukaryotic translation initiation factor 4E (eIF4E) regulates the relative C/EBPβ isoform levels during OCL differentiation.38 Rapamycin inhibition of mTOR decreased the C/EBPβ-LIP:LAP ratio, which resulted in enhanced transcriptional activation of MafB and inhibition of osteoclastogenesis.32, 48 We observed that both GSK126 and EZH2-KD decreased the C/EBPβ-LIP:LAP isoform ratio in RANKL-stimulated OCLp with a reduction in the levels of nuclear LIP (Fig. 4 A–C). To demonstrate the direct involvement of endogenous C/EBPβ-LIP:LAP isoform switching at the MafB promoter, we performed ChIP analyses with antibodies directed toward either the N- (LAP) or the C-terminus (LAP+LIP) of C/EBPβ (Fig. 4 C). We showed that GSK126 inhibits the recruitment of RANKL-induced inhibitory LIP, while increasing the binding of the LAP isoform to result in increased MafB expression (Fig. 4 E). Although LIP and EZH2 exhibit similar RANKL-induced enrichment at the Mafb promoter, it is not clear whether LIP is responsible for direct recruitment of EZH2 to MafB. Several cytokine signaling pathways converge on the mTOR/pS6RP-signaling axis to regulate protein translation and OCL differentiation.36 Therefore, we hypothesized that the switch in C/EBPβ-LIP:LAP isoform ratio induced by GSK126 treatment of OCLp may be due to upstream changes in this mTOR signaling pathway. Here we show that EZH2 inhibition reduces RANKL rapid activation of the pPI3K (tyr458/tyr199) signal transduction pathway including downstream effector proteins pAKT (ser 473), and pmTOR (ser 2448) resulting in downregulation of ribosomal protein pS6RP (ser235/236) (Fig. 4 F). We argue that GSK126 acts predominantly by targeting the mTOR-signaling pathway as p38 and pERK signaling is not affected by the inhibitor treatment (Supplemental Fig. S2B). Although the precise mechanisms of GSK126-mediated inhibition (and EZH2 activation) of mTOR is unknown, Zhang and colleagues showed that inhibition of EZH2 suppressed the PI3K-AKT–mTOR signaling pathway in malignant gliomas49 and Wei and colleagues reported that EZH2 can influence autophagy by transcriptional regulation of mTOR pathway-related genes.50 Furthermore, elevated EZH2 levels are associated with upregulated pAKT1 levels at Ser473 and enhanced PI3K/AKT signaling in breast cancer cells.51 Although we cannot exclude transcriptional changes in mTOR pathway genes 24 hours post RANKL treatment, the fact that GSK126 effects are also observed minutes after OCLp activation (Fig. 4 F) suggests that cytoplasmic EZH2 methyltransferase activity is required in the vicinity of the RANKL signaling path to permit rapid RANKL signal transduction in OCLp.
Although various signaling molecules such as AKT,52 CDK1,53 STAT3,54 TRAF6,55 and p3856 have been shown to directly interact with and modulate EZH2 activity and cellular localization, methylation of these molecules by EZH2 is not completely understood. We showed that EZH2 changes its cellular partitioning and plays distinct roles during OCL differentiation. Consistent with the hypothesis that EZH2 is involved in cytoplasmic signaling, its localization in OCLp is largely cytoplasmic in large punctate structures (Fig. 5 A, B). RANKL treatment induced a majority of the EZH2 to translocate to the nucleus, which was largely prevented by GSK126 (Fig. 5 A, B). This result suggests that EZH2 function on cytoplasmic targets is required for the RANKL signal transduction that induces EZH2 nuclear translocation. Interestingly, in a recent report by Kamikawa and colleagues, regulation of endogenous Jmjd3 involves Exportin-1-mediated nucleo-cytoplasmic shuttling in murine fibroblasts.57 A similar mechanism may regulate the EZH2 shuttling observed during osteoclastogenesis. In multinucleated OCL, EZH2 was again largely diffused throughout the cytoplasm (Figs. 5 A, C and 6 A). Although EZH2 in multinucleated OCL on glass surface was primarily cytoplasmic, EZH2 distribution in OCL differentiated on bone surface was localized to both nucleus and cytoplasm (Figs5 A, C and 6 A). EZH2 was still nuclear in mononuclear OCL in the same cultures. Inhibition of EZH2 during the initial phase of RANKL activation of OCLp, prevents OCL fusion and formation, but we noticed that mononuclear cells that remain in GSK126 treated cultures are largely TRAP+ and exhibit impaired adhesive shape and actin morphology (Figs. 6 B and 7 A). Because the majority of the GSK126 exposed cells are TRAP+, this suggests that some of the early activation events of RANK/MCSF receptor signaling are intact. Further, addition of GSK126 to already fused and mature OCL cultures condensed their cytoskeletal architecture, impaired sealing zone formation, and ultimately blocked their bone resorption (Figs. 6 B and 7). Cytoplasmic non-histone lysine methylation is becoming a widely recognized post-translational modification known to affect a plethora of signaling events including receptor-activated actin polymerization.39, 58, 59 The long non-coding RNA taurine upregulated gene (TUG1) was reported to be essential to couple EZH2 to α-actin, resulting in methylation of α-actin and polymerization of F-actin in vascular smooth muscle cells.60 By complexing with Vav1, EZH2 was shown to directly methylate extranuclear protein Talin, thereby controlling cell adhesion dynamics and migration of neutrophils and dendritic cells.61 Talin plays a critical role in inside-out activation of β1 and β3-integrin in osteoclasts and is critical for resorptive function.62 Furthermore, cytoplasmic EZH2 has been reported to bind LIM domain kinase 1 (LIMK1), which in turn regulates F-actin polymerization and migration.63 These data suggest that EZH2 methylation of cytoplasmic molecules such as α-actin, Talin, or LIMK1 may regulate the cytoskeletal dynamics during mature OCL bone resorption.
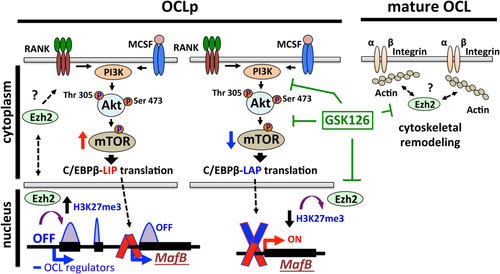
In conclusion, our data demonstrate that EZH2 has dynamic subcellular localization and plays different functions in distinct phases of osteoclastogenesis (Fig. 8). In addition to heterochromatin silencing of MafB expression, EZH2 activity has non-chromatin-related cytoplasmic role during early RANKL activation of the PI3K-AKT–mTOR-pS6RP signaling axis as well as in regulating cytoskeletal organization of mature bone-resorbing OCL (Fig. 8). Since small molecule inhibitors targeting various epigenetic regulators are becoming a new frontier of medical intervention, it is critical to understand their potential cytoplasmic non-histone-related functions in the context of cellular physiology. Therefore, targeting chromatin regulators such as EZH2 in the context of OCL biology may have more widespread effects than previously recognized as well as new potential therapeutic uses.
Disclosures
All authors state that they have no conflicts of interest.
Acknowledgments
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases awards R01AR057310 (DLG) and R01AR059679 (DLG), and the Duquesne University Charles Henry Leach II Fund (PEA). In addition, this project used the Cell and Tissue Imaging Facilities at the University of Pittsburgh that are supported in part by National Cancer Institute award P30CA047904 and the Duquesne University Biological Sciences microscopy facility supported by National Science Foundation grant DBI1726368. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the National Science Foundation.
Authors' roles: Study design: JA and DLG. Study conduct: JA, SHP, PZ, QS, KL, and DAM. Data interpretation: JA, PEA, and DLG. Drafted the manuscript: JA. Revised the manuscript: JA, PEA, and DLG. All authors approved the final version of manuscript. DLG takes responsibility for the integrity of the data analysis.




