Genetically Determined Later Puberty Impacts Lowered Bone Mineral Density in Childhood and Adulthood
ABSTRACT
Later puberty associates with lower areal bone mineral density (aBMD), and both are risk factors for osteoporosis. However, the association between puberty timing–associated genetic variants and aBMD during development, and the causal relationship between puberty timing and aBMD, remain uncharacterized. We constructed sex-specific polygenic risk scores (GRS) consisting of 333 genetic variants associated with later puberty in European-descent children in the Bone Mineral Density in Childhood Study (BMDCS), consisting of a longitudinal cohort with up to seven assessments (n = 933) and a cross-sectional cohort (n = 486). These GRS were tested for associations with age- and sex-specific aBMD Z-scores at the lumbar spine (LS), femoral neck (FN), total hip, and distal radius, accounting for clinical covariates using sex-stratified linear mixed models. The causal relationship between puberty timing and aBMD was tested in the BMDCS and in publicly available adult data (GEFOS consortium) using two-sample Mendelian randomization (MR). The puberty-delaying GRS was associated with later puberty and lower LS-aBMD in the BMDCS in both sexes (combined beta ± SE = –0.078 ± 0.024; p = 0.0010). In the MR framework, the puberty-delaying genetic instrument also supported a causal association with lower LS-aBMD and FN-aBMD in adults of both sexes. Our results suggest that pubertal timing is causal for diminished aBMD in a skeletal site- and sex-specific manner that tracks throughout life, potentially impacting later risk for osteoporosis, which should be tested in future studies. © 2017 American Society for Bone and Mineral Research.
Introduction
Rapid bone accrual occurs during puberty, and the process of sexual maturation is likely to impact lifelong bone health as bone mineral density (BMD) tracks throughout life.1 In particular, later age at menarche (AAM) is associated with increased osteoporosis risk in women,1-3 possibly mediated by the association between later AAM and lower peak bone mass in young adulthood.4-6 In men, later age at peak height velocity, indicating later pubertal timing, is associated with lower trabecular and cortical volumetric BMD in young adulthood,7 and there is a trend between later age at voice break (AVB), also indicative of later puberty, and osteoporosis risk.1 Heritability estimates of areal BMD (aBMD) and pubertal timing both exceed 80%,8-10 suggesting a marked genetic component to these traits. Investigating the role of genetic factors in early life is important for maximizing bone gain during development, because bone acquisition during growth appears to be more important than bone loss during adulthood for fracture risk in the elderly.11
With respect to bone health, genomewide association studies (GWASs) have implicated 65 variants with bone fragility in adults to date,12, 13 with some overlap in children and adolescents.14-16 For pubertal timing, 380 variants have been associated with AAM in females and/or AVB in males.17 Recent co-heritability studies using linkage disequilibrium score regression (LDSC) score regression found that pubertal timing and adult aBMD share a common genetic etiology; this genetic correlation suggests that some of the same genetic variants impact both traits.18, 19 In addition, in disorders of absent puberty, such as Turner and Kallmann syndromes, there are clear skeletal defects.20, 21 However, the relationships between genetic risk for later puberty and aBMD during skeletal development and later in life have not been explored.
We therefore aimed to determine if there was an association between genetic loci known to affect the timing of puberty, both individually and summarized into polygenic risk scores (GRS) that represent the “genetic load” of puberty-delaying variants for each individual, and aBMD at multiple skeletal sites in healthy children from the Bone Mineral Density in Childhood Study (BMDCS). Additionally, we applied two-sample Mendelian randomization (MR)22 to test if later pubertal timing caused lower aBMD. Using robustly associated genetic loci from large GWASs, these methods avoid confounding by environmental factors during development and across the life course.
Subjects and Methods
Study subjects
The BMDCS was a longitudinal study of healthy children that aimed to establish normal reference ranges for aBMD/bone mineral content (BMC) in children aged 5 to 20 years in the United States (n = 2014) (see Supporting Information–Study Description for a detailed cohort description). Briefly, participants were evaluated annually for up to seven visits between 2002 and 2009.23, 24 At the final study visit, blood or saliva was collected, and DNA was extracted and genomewide genotyped.16, 25 An additional, independent cross-sectional sample from two of the original study sites was enrolled in 2008/2009, completed the same protocol, and provided a DNA sample. We included genotyped participants from both the longitudinal and cross-sectional cohorts of European ancestry (n = 733 girls and 685 boys; Supporting Table 1).
All participants aged 18 years and older gave written informed consent. Parental or guardian consent and participant assent was obtained for individuals younger than 18 years. The study was approved by the Institutional Review Board of each clinical center.
Phenotypes
Dual-energy X-ray absorptiometry (DXA) scans were used to estimate aBMD at the distal 1/3 radius (DR), femoral neck (FN), total hip (TH), and lumbar spine (LS). Age- and sex-specific aBMD Z-scores (aBMDz) were calculated as described.26 Trained pediatric endocrinology nurses or physicians estimated puberty stage based on testicular volume in males and breast development in females. Participants were categorized as prepubertal (Tanner I), pubertal (Tanner II–IV), or postpubertal (Tanner V). AAM was determined by questionnaire. Collection methods for these phenotypes as well as physical activity and dietary calcium intake have previously been described in detail.25
Genotyping
High-throughput single-nucleotide polymorphism (SNP) genotyping was performed on the Illumina Infinium II OMNI Express plus Exome BeadChip (Illumina, San Diego, CA, USA) at the Center for Applied Genomics at the Children's Hospital of Philadelphia (Philadelphia, PA, USA).16, 25 Quality control of genomewide genotype data included standard filters, including removing SNPs with poor call rate (<95%), low minor allele frequency (MAF) <1%, and Hardy-Weinberg Equilibrium p < 1 × 10–5. Imputation was performed using the Haplotype Reference Consortium panel (r1.1 2016; Michigan Imputation Server; https://imputationserver.sph.umich.edu/index.html).27 In this study, we included 333 common, well-imputed autosomal puberty-associated variants.17 To define ancestry, we used principal component analysis and ADMIXTURE (>50% European ancestry).16
GRS construction
We calculated two puberty-delaying GRS, which represent a sum of puberty-delaying alleles weighted on their effect sizes on pubertal timing derived from large-scale GWASs, using PLINK, a whole-genome association analysis toolset28: (i) female-GRS: a GRS summed across 333 AAM alleles, weighted on AAM-delaying effect estimates derived from a large-scale GWAS performed by the ReproGen Consortium (http://www.reprogen.org/; Supporting Table 2)17; and (ii) male-GRS: a GRS summing 49 alleles that were genomewide significant for AAM but also associated with AVB (p < 0.05), weighted on the AVM-delaying effect estimates (Supporting Table 3). All of the male effect estimates for AVB were directionally concordant with those for AAM.17
Statistical analyses
To verify that the GRS associated with later puberty in our sample, we tested the association of the female-GRS with AAM and earliest recorded age at entry into Tanner breast stage II, III, and IV, with and without adjustment for BMI Z-score (BMIz) using linear regression for each cross-sectional Tanner stage bin. Similarly, we tested the association between the male-GRS and earliest recorded age at Tanner genital stage II, III, and IV. Next, using linear mixed effects models taking into account correlations between multiple measurements for each individual, we tested each GRS for association with aBMDz in males and females separately. Each model was adjusted for study center, cohort (discovery or replication), and age at measurement, as well as total physical activity level29 and dietary calcium intake,25 all of which may affect aBMD during puberty. Sensitivity analyses were conducted by adding additional covariates that may impact aBMD during puberty to the base model, resulting in additional models including (i) height Z-score, (ii) height and weight Z-scores, (iii) BMIz, and (iv) pubertal status. Individual puberty-associated variants were then tested for association with aBMDz in the BMDCS at each skeletal site, adjusting for the base covariates listed in the previous sentence. Additionally, these variants were queried from publically available GWAS summary data from the GEFOS consortium (http://www.gefos.org/) on adult LS-aBMDz, FN-aBMDz, and DR-aBMDz.13 All analyses were performed in STATA v.13.1 or 14.0 (StataCorp LP, College Station, TX, USA) or R v. 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org/).
Two-sample MR
There is an established relationship between later puberty and lower aBMD and osteoporosis risk later in life, suggesting that puberty timing is causally related to aBMD. To test this hypothesis, we performed two-sample MR using puberty timing-associated genetic variants as an instrument for pubertal timing that avoids confounding from environmental or lifestyle factors. MR was conducted using the MR-base website (http://www.mrbase.org/)30 to test for causality between puberty-delaying variants and adult aBMD13 or the TwoSampleMR package in R (https://github.com/MRCIEU/TwoSampleMR) to test the puberty timing variants in the BMDCS.31 aBMD at the LS, FN, and DR were tested in both the adult and adolescent settings. MR-base provides an online platform for performing causal inference that utilizes published GWAS signals as genetic instruments and outcomes,30 whereas the TwoSampleMR package is a complementary suite for analyzing one's own data. The platform offers a range of methods, improving the reliability and robustness of causal inference estimation, and sensitivity analyses to assess potential violations of MR assumptions, including leave-one-out, heterogeneity, and horizontal pleiotropy tests. For the MR analyses, default parameters were used (LD clumping to ensure that instrument SNPs are independent; LD proxies were found for missing SNPs with an r2 minimum of 0.8; palindromic SNPs were allowed; MAF threshold of 0.3 for palindromic SNPs). Exposure and outcome data was harmonized to ensure that all SNPs were on the forward strand. We performed additional sensitivity analyses in MR with a smaller subset of variants, excluding those associated with adult height and BMI, potential confounders influencing aBMD. Finally, we tested the reciprocal analysis of genomewide significant BMD-associated variants13 on AAM32 using the MR-base online platform (19 variants were found in both datasets and survived default clumping and filtering as outlined here).
Results
Puberty-delaying GRS
Because the female and males GRS are aggregates of variants associated with puberty in large-scale GWASs of AAM and AVB, respectively, we would expect them to associate with these traits in our cohort. AVB was not available, but we were able to test the association of the GRS with AAM and earliest age recorded at Tanner female breast and male genital stages. As expected, in the BMDCS, the female GRS strongly associated with later AAM (beta = 0.34, p = 3.75 × 10–9; adjusted for BMIz, beta = 0.31, p = 1.56 × 10–6), and both the female and male GRS associated with later entry into Tanner II, III, and IV (Supporting Table 4). Next, we tested the association between the puberty-delaying GRS and aBMD at each skeletal site. In both sexes, the puberty-delaying GRS were associated with lower LS-aBMDz (females: beta = –0.088, p = 0.0097; males: –0.068, p = 0.039; combined: beta = –0.078, p = 0.0010) (Table 1), even when adjusting for potentially related traits (height Z-score, weight Z-score, BMIz, or pubertal stage) as covariates in a number of models (Supporting Table 5). The GRS were not associated with aBMDz at the other skeletal sites.
| Female-GRS | Male-GRS | Combined | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Phenotype | Beta | SE | p | Beta | SE | p | Beta | SE | p |
| Spine BMD Z-score | –0.0877 | 0.0339 | 0.0097 | –0.0681 | 0.033 | 0.0390 | –0.0776 | 0.024 | 0.0010* |
| Radius BMD Z-score | –0.0224 | 0.0386 | 0.5616 | –0.0349 | 0.0373 | 0.3492 | –0.0289 | 0.027 | 0.2819 |
| Femoral neck BMD Z-score | 0.0173 | 0.0368 | 0.6384 | –0.0568 | 0.0337 | 0.0925 | –0.0230 | 0.025 | 0.3547 |
| Total hip BMD Z-score | 0.0005 | 0.0352 | 0.9888 | –0.0572 | 0.0352 | 0.1036 | –0.0284 | 0.025 | 0.2547 |
- The model was adjusted for age at measurement, collection site, dietary calcium, total physical activity, and BMI Z-score. The male and female estimates were combined using a fixed-effects meta-analysis. Nominally significant results are in bold.
- * Significant at Bonferroni threshold for significance (0.05/8) = 0.00625.
Individual puberty timing variants
We next assessed the association between each individual puberty timing-associated variant and aBMDz at each skeletal site. None of the individual variants associated with aBMDz in girls or boys in the BMDCS. However, two individual later puberty loci associated with lower LS-aBMDz in adults from the GEFOS consortium13: rs1054442-A (beta = –0.038, p = 4.85 × 10–5, MAF = 0.36) at 12q13.2 (near the gene DDN) and rs10750766-C (beta = –0.048, p = 1.10 × 10–6, MAF = 0.29) at 11q13.1 (near KAT5) (Supporting Table 6). None of the pubertal timing-associated variants were individually associated with FN-aBMDz or DR-aBMDz.
Causal inference analyses
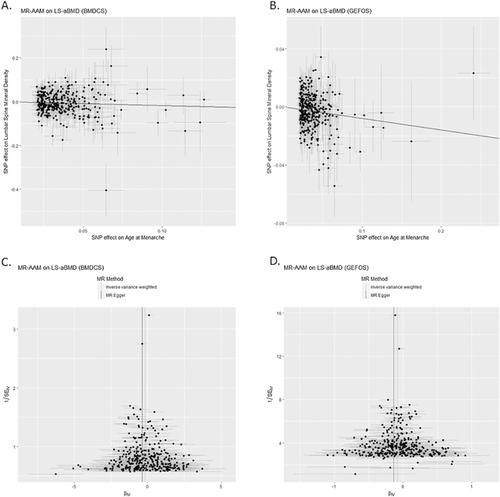
Using pubertal timing-associated variants as a genetic instrument for causal inference analysis, we performed two-sample MR to assess the effect of pubertal timing on aBMD. In a fixed-effects model, the genetic instrument revealed association with lower LS-aBMD (Table 2). In girls, genetically determined later AAM associated with decreased LS-aBMD in children in all MR models tested but was just below the significance threshold corrected for multiple testing (p = 0.004) (Fig. 1A; Supporting Table 7) (fixed effects beta = -0.18, p = 0.0046). Additionally, nearly all models supported a causal influence of genetically determined later AAM on lower LS-aBMD in adult women using genome-wide summary statistics from the GEFOS consortium13 (fixed effects beta = –0.072, p = 9.2 × 10–6) (Fig. 1B). In adult men, genetically determined later AVB also suggested a causal effect on lower LS-aBMD (fixed effects beta = –0.119, p = 0.0003); however, this was not evident in the BMDCS (Supporting Fig. 1). Furthermore, in adults, but not children (possibly due to the smaller sample size), AAM-delaying and AVB-delaying genetic instruments showed association between later puberty and lower FN-aBMD (women: fixed effects beta = –0.074, p = 9.4 × 10–8; men: beta = –0.113, p = 7.0 × 10–5) (Supporting Fig. 2). Finally, there was no association of genetically determined pubertal timing with DR-aBMD in either sex, although the beta estimates were directionally consistent (ie, negative) in childhood and adulthood.
| Lumbar spine | Femoral neck | Distal radius | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | N SNPs | Beta | SE | p | N SNPs | Beta | SE | p | N SNPs | Beta | SE | p |
| Age at menarche SNPs on aBMD in adolescent girls | 331 | −0.179 | 0.063 | 0.0046 | 331 | −0.120 | 0.063 | 0.0553 | 331 | −0.087 | 0.065 | 0.1755 |
| Age at menarche SNPs on aBMD in adult women | 309 | −0.072 | 0.016 | 9.21E–06* | 309 | −0.074 | 0.014 | 9.44E–08* | 318 | −0.038 | 0.028 | 0.1757 |
| Age at voice break SNPs on aBMD in adolescent boys | 43 | 0.052 | 0.135 | 0.7022 | 43 | −0.002 | 0.135 | 0.9860 | 43 | −0.054 | 0.138 | 0.6984 |
| Age at voice break SNPs on aBMD in adult men | 42 | −0.119 | 0.033 | 0.0003* | 42 | −0.113 | 0.028 | 7.04E–05* | 43 | −0.008 | 0.058 | 0.8861 |
- Results are shown for fixed effects models. Nominally significant results are in bold.
- * Significant at Bonferroni threshold for significance (0.05/12) = 0.00417.
Sensitivity analyses
To test whether any individual variant biased the fixed effect estimates, we performed leave-one-out analyses, which showed that no single SNP was a driver for these associations (Supporting Table 8). rs1054442 had the strongest individual SNP effect (Supporting Table 9), but excluding this SNP did not drastically change the overall results. Next, a range of sensitivity analyses were carried out. First, we tested whether there is heterogeneity, or variability, in the causal estimates across SNPs (Fig. 1C, D; Supporting Table 10). The results showed that this is unlikely, as the Q-values for all models tested were not statistically significant. Figure 1C, D also shows little directional bias among SNPs. There was little evidence for horizontal pleiotropy (Supporting Table 11; Supporting Figs. 3 and 4), suggesting a low likelihood that these variants influence the outcome through a pathway other than the exposure (ie, pubertal timing), except for LS-BMD in males. Finally, removing SNPs associated with height and BMI (p < 0.05) and those without association data for these traits showed the persistence of an association with lower pediatric aBMD in the LS in adults (Supporting Table 12).
Genetic instrument for BMD
Lower aBMD has been observed prior to puberty in girls who subsequently have later-than-average puberty.33 Thus, we tested if genetic variants for lower aBMD,13 previously shown to collectively associate with pediatric aBMD,25 also associate with pubertal timing by performing reciprocal two-sample MR of aBMD-associated variants on puberty timing based on the AAM GWAS by Perry and colleagues,32 which was available on MR-base.org. We found that an instrument built on 19 LS-BMD-associated genetic variants available on MR-base.org was negatively associated with AAM32 in some of the models assessed (Supporting Table 13).
Discussion
In this study, we examined the relationship between pubertal timing-associated genetic variants and aBMD using complementary methods: (i) association analysis of GRS built on puberty-delaying effect estimates, and (ii) causal inference analysis using two-sample MR. Our results show that genetic risk for later-than-average puberty associated with lower LS-aBMDz. This association, although stronger in girls in childhood, was evident in adulthood in both sexes. The spine is rich in trabecular bone, and may be particularly responsive during puberty.34 Additionally, we found some evidence that genetically determined later puberty associated with lower FN-aBMD, a site that also has a high amount of trabecular bone. Although aBMD is correlated at various skeletal sites, previous studies have seen both shared and unique genetic influences on aBMD at different sites, which may partly be due to different genetic regulatory mechanisms, bone composition, or factors such as load bearing.
The epidemiological link between later puberty and higher osteoporosis risk is stronger in women than men.1 Importantly, our results suggest that boys who are genetically predisposed to later-than-average puberty are also more likely to have reduced bone density. Consistent with these results, in a study of 19-year-old men, later age at attainment of peak height velocity was associated with lower aBMD, volumetric BMD, bone size, and higher rates of previous fractures.35 Another study of the 19-year-old men described in the previous sentence with later-normal puberty showed deficits in aBMD at all skeletal sites, but at a 5-year follow-up visit these deficits did not persist, except at the radius.7 Despite this apparent “catch-up” in aBMD in young adulthood, lower aBMD during adolescence remains clinically relevant, because it is a period of high fracture risk.36, 37 Additionally, the trend observed in another study toward increased osteoporosis risk in men with later AVB,1 and the results of our MR analyses, suggest that these early life deficits may not all remit and may therefore be important for later life bone health.
The association between later puberty and osteoporosis risk in girls is partially attributed to a shorter duration of estrogen exposure.4 Our data show that part of the link between puberty timing and osteoporosis is due to shared genetic factors that influence both pubertal timing and bone mass in both sexes, although sex-specific mechanisms are likely involved. However, genetic variants affecting puberty timing may exert their effects on bone health via mechanisms other than pubertal timing itself. Shared genetic factors may impact bone mass even before puberty begins.33, 38 In this study, when we adjusted for pubertal timing by including pubertal stage in the GRS models (Supporting Table 5), we found that the association at the LS remained, although the magnitude of the effect was slightly attenuated, supporting the idea that these genetic factors influence bone acquisition partly independently of pubertal timing itself.
On the other hand, physiological pathways that operate in tandem with puberty, such as height growth and body mass, may also impact bone acquisition in adolescence.11 In this study, sensitivity analyses for horizontal pleiotropy in the MR framework did not show evidence that puberty-associated variants influence aBMD through a biological mechanism other than puberty and related, but unmeasured, pathways (eg, height growth, BMI, or steroid hormone levels). Given that a large number of puberty-associated variants also associate with body mass and/or adult stature, the key exposure for reduced BMD may not be puberty timing itself. However, we removed SNPs at least nominally associated with height and BMI, and using this more stringent puberty-delaying genetic instrument showed the persistence of an association with lower pediatric aBMD in some of the methods used, but only at the LS in adults (Supporting Table 12). This is consistent with the results of the GRS association models, in which the association remained at the spine even when height, weight, or BMI Z-scores were adjusted for as covariates. Thus, although part of the link between puberty-associated genetic variants and aBMD occurs prior to puberty, during puberty skeletal development may also be influenced by the pubertal development process itself.
Clearly, during adolescence pubertal maturation and bone acquisition develop in tandem, and a complex relationship likely exists between genetic variation, puberty timing, and BMD, as evidenced by reciprocal MR analyses that showed a negative relationship between LS-BMD-associated genetic variants12 and AAM32 in some models (Supporting Table 13). Additionally, one genetic locus at chromosome 12q13 is genomewide associated with both AAM17 and adult BMD,13 although the lead variants differ and are in partial LD (rs1054442 [AAM] and rs12821008 [BMD], r2 = 0.57), and the causal gene(s) is unknown. Further work is needed to identify the key genes at these loci that mediate links between pubertal timing and bone acquisition.
The strengths of this study include the ability to address the association between puberty timing-associated variants and aBMD using several complementary approaches, assessing these genetic variants both individually and in combination, and addressing the association in both childhood/adolescence and adulthood in both sexes. However, although we show important relationships between bone health and puberty-delaying GRS, the use of GRS as an investigative tool is a relatively new area, and much remains to be learned about its optimal application. Furthermore, some of the observed puberty-adult BMD associations may in fact be present in children, but were not detected in our study due to the limited sample sizes available. Additionally, in this study we were unable to directly link the puberty-delaying, BMD-lowering GRS to osteoporosis risk, which should be the focus of further studies in elderly populations.
In conclusion, our results provide evidence delineating the genetic links between puberty timing and bone mineral accrual during critical years of skeletal development. Future studies should investigate specific functional mechanisms linking these two traits at various points to optimize bone health across the life-course.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by the National Institutes of Health (R01 HD58886 to BZ and SG; K01 HL123612 to JM); the Eunice Kennedy Shriver National Institute of Child Health and Human Development (N01-HD-1-3228, N01-HD-1-3329, N01-HD-1-3330, N01-HD-1-3331, N01-HD-1-3332, N01-HD-1-3333); the Clinical and Translational Science Awards Program (8 UL1 TR000077); American Diabetes Association Grant 1-17-PDF-077 (to DC); and the Institute for Translational Medicine and Therapeutics (ITMAT) Transdisciplinary Program in Translational Medicine and Therapeutics (to DC, BV, and SG). The project described was supported by the National Center for Research Resources (UL1RR024134), and is now at the National Center for Advancing Translational Sciences (UL1TR000003). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. We appreciate the dedication of the study participants and their families, and the support of Dr. Karen Winer, Scientific Director of the Bone Mineral Density in Childhood Study.
Authors' roles: Study conception and design: DC, JM, BZ, and SG. Acquisition of data: HK, JL, VG, SO, JS, BZ, and SG. Data analysis: DC and JM. Interpretation of data: DC, JM, AC, SR, HK, JL, VG, SO, JS, AK, SM, BV, BZ, and SG. Drafting manuscript: DC, JM, BZ, and SG. Revising manuscript content: DC, JM, BZ, and SG. Approving final version of manuscript: DC, JM, BZ, and SG. DC, SG, and BZ take full responsibility for the integrity of the data analysis.




