Control of Bone Anabolism in Response to Mechanical Loading and PTH by Distinct Mechanisms Downstream of the PTH Receptor
ABSTRACT
Osteocytes integrate the responses of bone to mechanical and hormonal stimuli by poorly understood mechanisms. We report here that mice with conditional deletion of the parathyroid hormone (PTH) receptor 1 (Pth1r) in dentin matrix protein 1 (DMP1)-8kb–expressing cells (cKO) exhibit a modest decrease in bone resorption leading to a mild increase in cancellous bone without changes in cortical bone. However, bone resorption in response to endogenous chronic elevation of PTH in growing or adult cKO mice induced by a low calcium diet remained intact, because the increased bone remodeling and bone loss was indistinguishable from that exhibited by control littermates. In contrast, the bone gain and increased bone formation in cancellous and cortical bone induced by daily injections of PTH and the periosteal bone apposition induced by axial ulna loading were markedly reduced in cKO mice compared to controls. Remarkably, however, wild-type (WT) control littermates and transgenic mice overexpressing SOST injected daily with PTH exhibit similar activation of Wnt/β-catenin signaling, increased bone formation, and cancellous and cortical bone gain. Taken together, these findings demonstrate that Pth1r in DMP1-8kb–expressing cells is required to maintain basal levels of bone resorption but is dispensable for the catabolic action of chronic PTH elevation; and it is essential for the anabolic actions of daily PTH injections and mechanical loading. However, downregulation of Sost/sclerostin, previously shown to be required for bone anabolism induced by mechanical loading, is not required for PTH-induced bone gain, showing that other mechanisms downstream of the Pth1r in DMP1-8kb–expressing cells are responsible for the hormonal effect. © 2016 American Society for Bone and Mineral Research.
Introduction
Parathyroid hormone (PTH) is secreted in response to low circulating calcium levels and has profound effects on the skeleton. PTH generates both catabolic and anabolic effects on bone depending on whether the hormone is increased chronically or intermittently as achieved by daily injections.1 Chronic increase in circulating PTH, as in severe primary hyperparathyroidism, or secondary hyperparathyroidism due to vitamin D deficiency, calcium deficiency, or chronic kidney disease, is characterized by increased remodeling that results in bone loss due to unbalanced focal bone remodeling favoring resorption within the bone remodeling unit.2 Bone loss due to chronic PTH elevation is associated with increased generation and activity of osteoclasts and coupled increase in osteoblasts.3 In contrast, intermittent PTH increase, as achieved by pharmacological daily injections, causes bone gain and is the only approved bone-forming therapy for osteoporosis in the United States. The primary effect of daily PTH injections is a rapid increase in osteoblasts and bone formation, attributed to the actions of PTH on proliferation of osteoblast precursors, inhibition of osteoblast apoptosis, reactivation of lining cells to become matrix synthesizing osteoblasts, or a combination of these effects.4 Although the effects of PTH injections on bone have been extensively reported, the target cell(s) responsible for these actions and the underlying mechanistic events downstream of PTH receptor signaling remain unclear.
In the skeleton, the PTH receptor 1 (Pth1r) is expressed in osteoblasts and osteocytes, with the latter exhibiting higher levels,5 and by other cells present in the bone marrow such as T cells.6 Osteocytes play a key role in the regulation of bone homeostasis by coordinating the activity of osteoblasts and osteoclasts.7, 8 Osteocytes are a major source of Rankl, an essential cytokine that controls the differentiation and survival of osteoclasts.9 Further, in adult animals osteocytes are the sole source of sclerostin, a potent inhibitor of Wnt signaling encoded by the SOST gene, which, by negatively regulating the differentiation and function of osteoblasts, inhibits bone formation.10
The 8-kb and 10-kb fragments of the dentin matrix protein 1(DMP1) promoter are commonly used to target genes to osteocytes in bone, as shown by green fluorescent protein (GFP) expression and Cre reporter activity.11, 12 Earlier work demonstrated that constitutive activation of PTH signaling in DMP1-8kb–expressing cells (DMP1-8kb-caPth1r) increases the rate of bone remodeling and induces bone gain,(13–15) two recognized skeletal actions of PTH. Moreover, the increase in cortical and cancellous bone formation exhibited by DMP1-8kb-caPth1r is abolished by overexpressing human SOST showing the requirement of Sost downregulation for bone gain in this mouse model.14 Likewise, load-induced periosteal bone formation in the cortical bone of the ulnae is markedly reduced in mice overexpressing human SOST under the control of the DMP1-8kb promoter (TG),16 demonstrating that reduction in sclerostin expression is an obligatory step in bone anabolism induced by mechanical stimulation. In addition, DMP1-8kb-caPth1r exhibited increased Rankl expression in osteocytes, which increases osteoclasts and bone resorption.5 Conversely, mice lacking the PTH receptor in Dmp1-10kb–expresing cells exhibit decreased bone resorption.17, 18
Previous studies showed that mice in which the Pth1r was deleted using the DMP1-10kb-Cre exhibit deficient anabolic and catabolic responses to exogenous PTH elevation.18 Nevertheless, it is unclear whether Pth1r in DMP1-expressing cells is required for bone loss induced by endogenous elevation of PTH secondary to calcium deficiency. Moreover, transient increases in circulating PTH are observed after whole-body mechanical stimulation,19 and PTHrP, the other ligand of the Pth1r, is increased by mechanical stimulation of osteocytic cells in vitro.20 However, it is not known whether signaling through the Pth1r in osteocytes modulates the response of the skeleton to local changes in mechanical force. Furthermore, Sost downregulation is indispensable for the increase in bone formation induced by mechanical loading, but it is unclear whether it also mediates bone anabolism induced by daily injections of PTH.
To address these unresolved issues, we generated a mouse model with conditional deletion of Pth1r in DMP1-8kb–expressing cells (cKO mice). Besides osteocytes, other cells including osteoblasts, chondrocytes, and perivascular mesenchymal stromal cells also show DMP1-Cre reporter activity,12, 21, 22 raising the possibility that DMP1-drivers target osteocytes as well as less differentiated cells of the osteoblastic lineage, similar to the osteocalcin (OCN) or the collagen 1 (COL1) promoters. However, different skeletal phenotypes are obtained by deleting/overexpressing a particular gene using DMP1 versus the OCN/COL1 promoters,(13,18,23–29) suggesting that indeed these promoters target osteocytes, as well as varying numbers of preosteoblasts, osteoblasts, and perivascular stromal cells, depending on which promoter is used.
We used here the DMP1-8kb-Cre mice to delete Pth1r and demonstrate that Pth1r signaling in DMP1-8kb–expressing cells is required to maintain basal physiological levels of bone resorption, but it is not required for the catabolic action of chronic PTH secretion on bone. Furthermore, Pth1r signaling in DMP1-8kb–expressing cells is essential for the anabolic action of mechanical loading as well as daily pharmacological injections of PTH, and that the latter effect is independent of Sost/sclerostin downregulation.
Materials and Methods
Mice
Mice with conditional deletion of parathyroid hormone receptor 1 (Pth1r; cKO) were generated by crossing Pth1rflox/flox mice (fl/fl), in which the E1 exon of the Pth1r is flanked by loxP sites,30 with DMP1-8kb-Cre mice.31-33 The skeletal phenotype of the DMP1-8kb-Cre mice is indistinguishable from the wild-type (WT) C57BL/6Nhsd mice or from the fl/fl mice used as littermate controls in the current study. Mice expressing GFP in osteocytes (DMP1-8kb-GFP) were provided by Dr. David W. Rowe and were maintained as homozygous.11 Generation and characterization of transgenic mice overexpressing the human SOST cDNA (TG) and littermate controls (WT) was previously published.16 Mice were fed with a regular diet (Harlan, Indianapolis, IN, USA), received water ad libitum, and were maintained on a 12-hour light/dark cycle. To chronically increase endogenous PTH secretion, 1-month-old and 4-month-old female and male mice were fed a diet containing 0.01% calcium and 0.4% phosphorous (low calcium diet, TD.95027; Harlan), and control mice received a diet containing 0.6% calcium and 0.4% phosphorous (normal calcium diet, TD.97191; Harlan), for 14 and 28 days, respectively. To intermittently increase circulating PTH levels, 4-month-old mice received daily injections of PTH (1-34) (100 ng/g/day; Bachem, Torrance, CA, USA) for 28 days.34 Control animals receive vehicle injections. Mice were randomized to the experimental groups based on total BMD prior to treatment administration. Analyses were performed in a blinded fashion.
Osteoblast and osteocyte isolation
Cells were isolated from calvaria bones of 9-day-old to 11-day-old fl/fl and cKO mice crossed with DMP1-8kb-GFP mice in which osteocytes are labeled with GFP. Bones were subjected to nine sequential 20-min digestions with a mixture of trypsin/EDTA/collagenase P as described.5, 28 The first digestion was discarded, and cells from all other digestions were pulled and immediately subjected to fluorescence-activated cell sorting (FACS).13 GFP-positive (osteocytes) and GFP-negative (osteoblasts/osteoblast progenitors, and potentially other cells) cells were sorted by FACS and total RNA was isolated as described before.5, 28
Analysis of the skeletal phenotype
BMD measurement and micro–computed tomography (μCT) analysis were performed as described.5, 28 Mice were anesthetized via inhalation of 2.5% isoflurane (Abbott Laboratories, Abbott Park, IL, USA) mixed with O2 (1.5 L/min) and BMD of the total body, excluding the head and the tail, lumbar spine (L1–L6), and femur was measured monthly by dual-energy X-ray absorptiometry (DXA) using a PIXImus II densitometer (GE Medical Systems, Madison, WI, USA) in mice up to 8 months of age. For μCT analysis, bones were dissected, cleaned of soft tissue, fixed in 10% buffered formalin, and stored in 70% ethanol until scanned at 6-μm resolution (Skyscan 1172; SkyScan, Kontich, Belgium).
Serum biochemistry
Osteocalcin, PTH, and C-telopeptide fragments of type I collagen (CTX) were measured using enzyme-linked immunosorbent assays (Biomedical Technologies, Stoughton, MA, USA; and Immunodiagnostic Systems and Immunodiagnostic Systems, Fountain Hill, AZ, USA; respectively), as published.35
Ulna strain measurements
Strain levels at the midshaft ulna were measured in 4-month-old fl/fl and cKO female and male mice as published.16, 36 Briefly, the right forearm was dissected to expose the lateral surface of the ulnar diaphysis, and a miniature (EA-015DJ-120; Vishay, Inc., Shelton, CT, USA) single-element strain gauge was bonded to the midshaft. The forearm was then placed in a computer-controlled electromagnetic mechanical actuator (Bose, Eden Prairie, MN, USA), and exposed to cyclic axial compression using a 2-Hz haversine waveform. The peak force was progressively increased with each cycle, and ranged from 1.2 to 2.5 N. During loading, conditioned voltage output from the strain gauge and output from the load cell were recorded and processed as described.37 Voltage output was converted to strain using previously described calibration procedures. From these data the force/strain relationship was derived for both genotypes individually, and low, medium, and high loads for in vivo mechanical loading were calculated for fl/fl and cKO female and male mice.
In vivo ulnar loading
Four-month-old mice underwent ulnar loading of the right forearm for 1 min at 120 cycles/day (2 Hz) for 3 consecutive days at low, medium, or high magnitude of strain, and euthanized 16 days after initiation of loading.16, 36 Left ulna served as non-loaded internal controls. Ulna were dissected and fixed in 10% buffered formalin and stored in 70% ethanol at 4°C until processed for dynamic bone histomorphometry. For mRNA analysis by quantitative PCR (qPCR), ulna were loaded once at high strain magnitude and mice were euthanized 24 hours later. Bones were snap-frozen in liquid nitrogen and stored at –80°C until RNA isolation.
Bone histomorphometry
Histomorphometric analysis was performed using the OsteoMeasure High Resolution Digital Video System (OsteoMetrics, Decatur, GA, USA). Mice were injected with calcein (30 mg/kg) and Alizarin Red (50 mg/kg) (Sigma Chemical Co, St. Louis, MO, USA), 11 and 4 days before sacrifice, respectively, as described,28 and dynamic histomorphometry analysis was done as published.15, 16 Briefly, thick (100 µm) cross-sections at the mid-diaphysis of ulna embedded in methyl methacrylate were prepared using a diamond-embedded wire saw (Histosaw; Delaware Diamond Knives, Wilmington, DE, USA) and ground to a final thickness of around 40 µm and total, single, and double labeled perimeter, and inter-label width were measured on periosteal bone surfaces. Thin (5 µm) longitudinal sections of lumbar vertebrae and, thick cross-sections at the mid-diaphysis of the femur were ground to a final thickness of 30 to 35 µm and labels were quantified in cancellous bone and periosteal and endosteal bone surfaces, respectively.38, 39 A value of 0.1 μm/day was used for mineral apposition rate (MAR) when double labels were not detected in sections.40 For static bone histomorphometric analysis, longitudinal sections of the lumbar vertebrae were stained for tartrate-resistant acid phosphatase (TRAPase), and counterstained with toluidine blue. Total bone volume was used when determining the effect of PTH elevation on bone volume per tissue volume (BV/TV) and trabecular parameters. The terminology and units used are those recommended by the Histomorphometry Nomenclature Committee of the American Society for Bone and Mineral Research.41
qPCR
Calvaria, vertebrae, and tibias (bone marrow cells were flushed out) were harvested and total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA) as published.5, 28 For osteocyte-enriched bone preparations, tibias were harvested, bone marrow cells were flushed out, and bones were subjected to serial digestions with collagenase and EDTA, and then treated with PTH (100 nM) or vehicle for 24 hours as published.5 RNA was isolated as described before.5, 28 cDNA was synthesized by using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA) and qPCR was performed using primer probe sets from Applied Biosystems or from Roche Applied Science (Indianapolis, IN, USA) as reported.28 Relative mRNA expression levels were normalized to the housekeeping gene ribosomal protein S2 (ChoB) by using the ΔCt method.42
Statistical analysis
Data were analyzed using SigmaStat (SPSS Science, Chicago, IL, USA). All values are reported as mean ± standard deviation (SD). Data were analyzed for normality and equivalence of variance. Basal differences between fl/fl and cKO and mice were evaluated using Student's t test. Differences between loaded (right) and non-loaded (left) ulnas for each animal were evaluated by paired t test. For other experiments, differences between group means were evaluated using two-way ANOVA, with genotype and treatment as independent variables, followed by pairwise multiple comparisons using the Tukey method. When the differences in BV/TV, Tb.Sp, and Tb.N between WT and TG mice precluded the detection of the PTH effect using two-way ANOVA, data were analyzed by Student's t test for each genotype. Values of p ≤ 0.05 were considered statistically significant.
Study approval
All animal procedures were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine, and animal care was carried out in accordance with institutional guidelines.
Results
Conditional deletion of the Pth1r in DMP1-8kb–expressing cells results in decreased bone resorption and a modest increase in cancellous bone mass
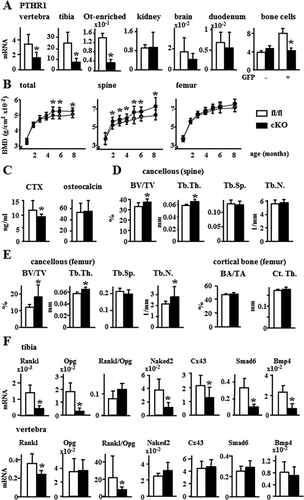
At the tissue level, conditional knockout mice (cKO) exhibited a 50% to 70% reduction in Pth1r mRNA expression in lumbar vertebrae, tibial diaphysis, and in osteocyte-enriched bones, but no changes in its expression were found in other organs, compared to control littermates (fl/fl) (Fig. 1A). At the cellular level, a 50% reduction in Pth1r mRNA was detected in GFP-positive cells but not in GFP-negative cells separated by cell sorting from calvaria cell populations of cKO and fl/fl mice crossed with DMP1-8kb-GFP, which labels osteocytes. mRNA levels of Sost, an osteocyte marker, were 240 or 137 times higher in GFP-positive cells than in GFP-negative cells derived from WT or cKO mice, respectively, whereas mRNA levels of Keratocan, a gene expressed in osteoblasts but at lower levels in osteocytes, were eight or 18 times lower in GFP-positive cells compared to GFP-negative cells derived from WT or cKO mice, respectively (Supporting Fig. 1). Female cKO mice displayed a progressive increase in BMD compared to fl/fl mice, with significant increases in spinal BMD first evidenced at 2 months, and increase in both spinal and total BMD by 5 months that was maintained up to 8 months of age (Fig. 1B). Male cKO mice exhibited a minor increase in spinal BMD at 8 months of age compared to fl/fl mice (Supporting Fig. 2). Although we could not detect changes in spinal cancellous osteoclast number/surface (not shown), the resorption marker CTX was 22% lower in plasma from cKO, whereas osteocalcin was similar to fl/fl 4-month-old female mice (Fig. 1C). Further, spinal and distal femur cancellous bone volume (BV/TV) and trabecular thickness (Tb.Th) were increased in cKO female mice compared to fl/fl mice, and trabecular number (Tb.N) was also increased in the distal femur (Fig. 1D, E). In contrast, no changes in femoral cortical bone area (BA/TA) or cortical thickness (Ct.Th) were found in mice of either gender. To investigate the mechanisms underlying the different effect of Pth1r deletion in osteocytes on cortical versus cancellous bone, gene expression was analyzed. In tibial diaphysis mainly composed of cortical bone enriched in osteocytes, the expression of Rankl and the Wnt target genes Opg, Naked2, Cx43, Smad6, and BMP4 was lower in cKO mice, whereas the Rankl/Opg ratio was unchanged (Fig. 1F). In contrast, in lumbar vertebra mostly composed of cancellous bone, only Rankl was decreased resulting in a reduced Rankl/Opg ratio. These results are consistent with the fact that osteocytes are the main source of Rankl; however, Opg is also contributed by other osteoblastic cells relatively more abundant in cancellous versus cortical bone. Thus, removal of the Pth1r from DMP1-8kb–expressing cells leads to a site-specific reduction in the Rankl/Opg ratio, resulting in increased cancellous but not cortical bone mass in the cKO mice.
Pth1r expression in DMP1-8kb–expressing cells is not needed for the increased bone resorption and the bone loss induced by chronic increase in PTH
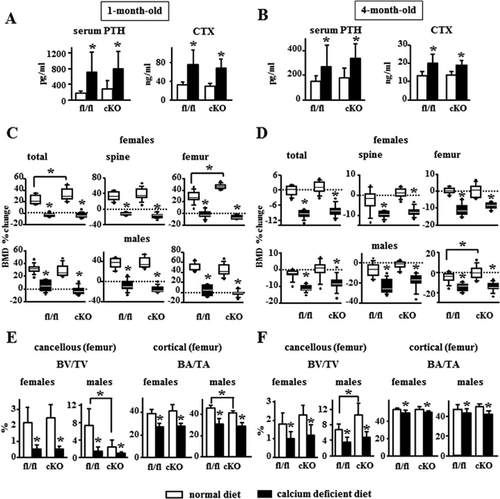
Calcium deficiency increased serum PTH similarly in fl/fl control and cKO mice, growing or adult, and circulating levels of CTX were high in mice fed the calcium-deficient diet regardless of the genotype (Fig. 2A, B). Growing (1-month-old) fl/fl and cKO female and male mice fed a normal calcium diet gained bone, whereas mice of both genotypes and gender fed the calcium-deficient diet exhibited decreased BMD (Fig. 2C). The calcium-deficient diet decreased BMD at all sites in adult mice (4-month-old), regardless of genotype or gender (Fig. 2D). μCT analysis of cancellous (distal) and cortical (mid-diaphysis) femoral bone showed that a calcium-deficient diet induced a similar decrease in BV/TV in fl/fl and cKO mice (by 75% in growing and by 15% in adult mice) and BA/TA (by 30% in growing and by 5% in adult mice) (Fig. 2E, F); decreased Tb.Th (although only in females) and Tb.N, increased trabecular separation (Tb.Sp), and decreased Ct.Th in growing fl/fl and cKO mice, as well as decreased Ct.Th in adult mice of either genotype or gender (Supporting Fig. 3).
Pth1r expression in DMP1-8kb–expressing cells is required for bone anabolism induced by daily PTH injections
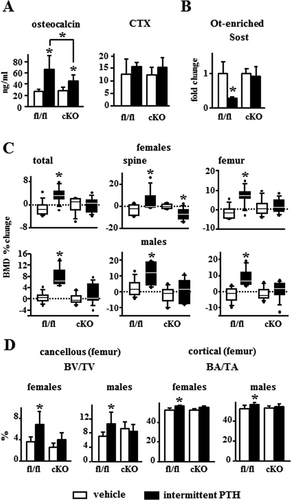
PTH increased osteocalcin levels by 200% in fl/fl mice, but only by 50% in 4-month-old cKO mice, and it did not change circulating CTX levels in mice of either genotype (Fig. 3A). Intermittent PTH administration decreased Sost expression in bones from fl/fl mice, but not from cKO mice (Fig. 3B). PTH also increased total, spinal, and femoral BMD in fl/fl control littermate mice, but not in cKO mice (Fig. 3C). Moreover, female cKO mice receiving PTH exhibited decreased BMD in the spine. μCT analyses revealed increased BV/TV in the distal femur and BA/TA in the femoral mid-diaphysis induced by PTH in fl/fl mice of both genders; whereas these responses were not observed in cKO mice (Fig. 3D, Supporting Fig. 4).

To investigate the basis for the defective anabolic response to PTH injections in cKO mice, bone formation in different bone envelopes was quantified (Fig. 4A). PTH induced greater increases in MAR and bone formation rate (BFR/BS) in cancellous and periosteal surfaces in fl/fl mice compared to cKO mice. Further, fl/fl mice given PTH injections displayed increased endocortical MAR and BFR, whereas this response was absent in cKO mice. PTH only increased the mineralizing surface per bone surface (MS/BS) in the periosteum, an effect that remained intact in cKO mice. These results demonstrate that Pth1r in DMP1-8kb–expressing cells is partially responsible for the actions of PTH on cancellous bone, whereas the anabolic response to PTH in cortical bone, in particular on the endocortical surface, is fully mediated by PTH signaling in DMP1-8kb–expressing cells. Furthermore, PTH markedly increased mRNA expression of the osteoblastic markers Bglap, Alp, and Runx2, and the Wnt target genes Cyclin D1, Smad6, and Wisp2 in both cancellous bone and cortical bone from fl/fl control mice (Fig. 4B). In contrast, PTH had no effect on any of these genes in cKO mice, consistent with a defective response to PTH in cKO mice.
Sost/sclerostin downregulation is dispensable for the bone anabolic effects of daily PTH injections
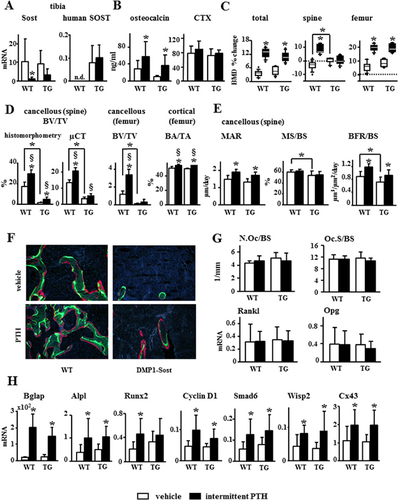
To determine whether the defective response to daily PTH injections exhibited by cKO mice was due to the inability of PTH to downregulate Sost/sclerostin, the response of transgenic mice overexpressing human SOST (TG) to the hormone was studied. WT control and TG mice express similar levels of murine, endogenous Sost mRNA, and human SOST mRNA was only detected in TG mice (Fig. 5A). Consistent with SOST overexpression, TG mice exhibited a 20% decrease in cancellous BV/TV but no changes in cortical bone area (Fig. 5D); decreased cancellous MS/BS and BFR/BS (Fig. 5E, F), but no differences in serum CTX or osteoclast number/surface compared to WT mice (Fig. 5B, G). Endogenous Sost expression was reduced in response to PTH by 50% in both control and TG mice (although differences were not statistically significant in the latter). In contrast, the human SOST expressed in TG mice remained unchanged by the hormone (Fig. 5A). WT and TG mice receiving PTH injections exhibited increased serum osteocalcin (Fig. 5B). In addition, WT and TG mice exhibited similar PTH-induced increase in total and femoral BMD, whereas PTH only increased spinal BMD in control mice, but not in TG mice (Fig. 5C). However, μCT and histomorphometric analysis of cancellous bone in the spine revealed that the TG mice responded to PTH similarly or even better than the WT mice. Despite the lower bone volume in the vertebral cancellous bone exhibited by the TG mice (Fig. 5D, white bars), daily PTH injections increased BV/TV by threefold versus 1.7-fold in TG and WT mice, respectively, quantified by histomorphometry, and 1.46-fold versus 1.42-fold, respectively, quantified by μCT (Fig. 5D). Further, PTH increased Tb.Th in TG mice, and it decreased Tb.Sp and increased Tb.N in both WT and TG mice, as revealed by histomorphometry (Supporting Fig. 5A). μCT analysis also showed that in TG and WT mice PTH increased femoral cancellous BV/TV (3.4-fold versus 2.8-fold, respectively, although did not reach statistical significance in TG mice) and similarly increased cortical BA/TA and Ct.Th (Fig. 5D, Supporting Fig. 5B). In addition, intermittent administration of PTH increased MAR and BFR (22% and 24%, respectively) similarly in both WT and TG mice, with no changes in MS/BS or osteoclast number/surface, regardless of the genotype (Fig. 5E–G; Supporting Fig. 5C, D). Furthermore, WT and TG mice exhibited similar gene expression profiles in response to PTH, with elevated mRNA levels Alpl, Runx2, Bglap, and several Wnt target genes, including CyclinD1, Smad6, Wisp2, and Cx43 (Fig. 5H).
Pth1r expression in DMP1-8kb–expressing cells is necessary for the anabolic effect of mechanical loading in mice
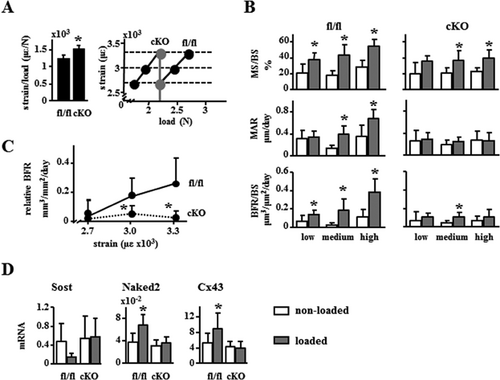
Exercise transiently increases circulating PTH,19 and in vitro stimulation increases PTHrP expression in osteocytic cells.20 To examine the potential role of Pth1r signaling in DMP1-8kb–expressing cells, we examined the osteogenic response to axial loading of the ulna of 4-month-old fl/fl and cKO mice. Female cKO mice exhibited no significant changes in ulna geometry or in material density as measured by μCT (not shown). However, ex vivo loading induced 20% higher strains in cKO mice compared to fl/fl control mice, indicating increased compliance in cKO bones (Fig. 6A, left panel). Based on these measurements the right ulna from female fl/fl and cKO mice were loaded at equal strains of low, medium, and high magnitude (Fig. 6A, right panel). Dynamic histomorphometric analysis revealed that basal periosteal BFR was similar in fl/fl and cKO mice (Fig. 6B). Loading caused a strain-dependent increase in BFR in fl/fl, resulting from an increase in both MS/BS and MAR. In contrast, loading-induced BFR was markedly reduced in cKO mice, with significant, but minimal changes induced only by medium strains (Fig. 6B, C). This effect resulted mainly from lack of stimulation of MAR by loading at any strain magnitude. Consistent with earlier findings,16 loaded bones from fl/fl mice exhibited a decrease in Sost mRNA gene expression, although in this experiment the difference did not reach statistical significance, and increased expression of Wnt targets including Naked2 and Cx43 (Fig. 6D). None of these changes were observed in cKO mice. In contrast to the effect in female cKO mice, ex vivo loading induced 15% lower strains in cKO compared to male fl/fl mice (Supporting Fig. 6A). Further, male cKO mice still responded significantly to mechanical loading, in particular to high strains, with similar increases in BFR, MAR, and MS/BS compared to fl/fl mice (Supporting Fig. 6B, C).
Discussion
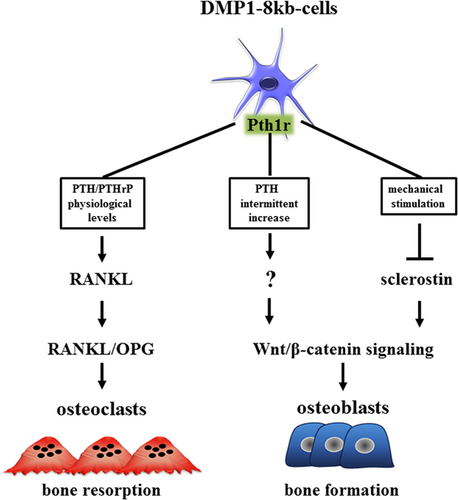
In this study, using mice lacking the Pth1r in DMP1-8kb–expressing cells (cKO), we examined the contribution of Pth1r signaling to physiological bone homeostasis, and dissected its role in the bone catabolic and anabolic signaling resulting from sustained and transient elevation of PTH (Fig. 7). We show that under physiological levels of PTH cKO mice exhibited a modest increase in cancellous bone compared to WT littermates, suggesting that endogenous PTH/PTHrP signaling in DMP1-8kb–expressing cells is required to maintain basal levels of bone resorption in cancellous bone. In contrast, the bone loss induced by chronic elevation of PTH in the circulation secondary to low dietary calcium was similar in cKO and WT mice, demonstrating that Pth1r expression in DMP1-8kb–expressing cells is dispensable for bone resorption induced by chronic elevation of the hormone, thereby suggesting that this effect must be mediated by actions of PTH on other bone cells. Further, we found that cKO mice exhibit a deficient response to loading or to daily injections of PTH, showing that the Pth1r expressed in DMP1-8kb–expressing cells is required for bone anabolism induced by both stimuli. Remarkably, however, the mechanisms downstream of Pth1r signaling appeared to differ between mechanical loading and intermittent PTH administration. Thus, we previously showed that the bone gain induced by mechanical loading requires a reduction in Sost/sclerostin expression.16 We now show that, in contrast, the anabolic effect of intermittent PTH administration is intact in mice overexpressing SOST and therefore independent of Sost/sclerostin downregulation, indicating that PTH activates alternative mechanisms leading to bone anabolism in DMP1-8kb–expressing cells. Collectively, these results demonstrate that Pth1r signaling in DMP1-8kb–expressing cells plays a central role in transducing physiological PTH/PTHrP catabolic actions as well as the anabolic actions of PTH and mechanical force.
In our study, deletion of Pth1r signaling in DMP1-8kb–expressing cells resulted in a mild increase in cancellous bone mass, only detected when a large cohort of animals was used. Although BMD data suggested the existence of sex-specific responses in cKO mice, further analysis using μCT revealed that male cKO mice also exhibit increased femoral cancellous bone at 5 months of age. Our findings showing that cKO mice exhibit a reduction in the expression of Rankl, but not Opg, which decreased the Rankl/Opg ratio specifically in cancellous bone, and could potentially explain the decrease in CTX and the increase in bone mass/volume in this site without changes in cortical bone. Reduced bone resorption in cKO mice is consistent with earlier evidence showing that, conversely, activation of Pth1r signaling in the same bone cell population increases osteoclasts and bone resorption.13 All together, these findings suggest that under physiological levels of PTH, Pth1r in DMP1-8kb–expressing cells is required to maintain normal levels of bone resorption in cancellous bone by regulating the levels of Rankl.
Our results show that deletion of the Pth1r in DMP1-8kb–expressing cells does not change cortical bone. In contrast, deletion of the Pth1r in DMP1-10kb–expressing cells or in OCN-expressing cells increases cortical bone.18, 23 This evidence suggests that the 8-kb and the 10-kb DMp1 promoters potentially target different numbers of osteoblasts. In spite of this difference, using R26R reporter mice, it was shown that both DMP1 promoter fragments drive the expression of Cre recombinase to osteocytes. However, Cre activity was also found in matrix-synthesizing osteoblasts and other bone cells.12, 21, 22 Based on these reporter studies, it is possible that some of the effects observed in our cKO mice, as well as in mice in which the Pth1r was deleted with DMP1-10kb-Cre,18 are due to deletion of Pth1r from osteocytes, osteoblasts, and potentially other bone cells. However, the Cre reporter studies conflict with the different skeletal phenotypes of mice in which the same gene(s) is deleted using DMP1-Cre drivers versus Cre promoters targeting osteoblasts and their progeny (OCN and COL1).(13,18,23–29) Consistent with the differences in the skeletal phenotypes observed when the Pth1r is deleted using DMP1 versus OCN/COL1 Cre drivers, overexpression of a constitutively active Pth1r driven by the 2.3-kb COL1 promoter24 or the DMP1-8kb promoter13 reduces or increases cortical bone, respectively. Based on these functional studies, we interpret that osteocytes are targeted by the DMP1-8kb promoter and thus are major contributors to these phenotypes. However, Cre activity is also detected in other cells, thus it is possible that deletion of Pth1r from preosteoblasts, osteoblasts, and perivascular stromal cells could also contribute to the observations reported here.
Our results showing increased bone resorption and bone loss in cKO and fl/fl mice induced by chronic elevation of systemic PTH levels secondary to calcium deficiency indicate that direct actions of PTH on DMP1-8kb–expressing cells are not required and point toward other PTH-responsive cells as mediators of these effects. Recent findings showed that mice with conditional deletion of Rankl in DMP1-10kb–expressing cells are only partially protected from bone loss induced by calcium deficiency,43 suggesting that other Rankl-producing cells can contribute this effect. Consistent with this notion, PTH stimulates Rankl production in stromal/osteoblastic cells,44, 45 and also stimulates T cells to produce interleukin-17A, which in turn increases Rankl expression in osteoblasts and osteocytes.46 In addition, we cannot exclude the possibility that systemic elevation of PTH upregulates Rankl indirectly by increasing the production of 1,25D3, which in turn could upregulate Rankl gene transcription in osteocytes and other bone cells.47
In contrast to our study, Saini and colleagues18 reported that mice lacking Pth1r in DMP1-10kb–expressing cells exhibit partial protection against the bone loss induced by continuous administration of exogenous PTH for 2 weeks. Besides the differences derived from the use of a different Cre-driver to knockout the Pth1r, other factors might contribute. One possibility is that the mechanisms responsible for the bone loss induced by primary hyperparathyroidism in Saini and colleagues18 differ from those activated by secondary hyperparathyroidism in our study. Further, only partial protection from PTH-induced bone loss in their study was observed after 2 weeks of chronic elevation of exogenous PTH, raising the possibility that after 4 weeks this protection would disappear, similar to our findings. In fact, in the aforementioned report,18 2 weeks of chronic PTH elevation did not increase osteoclasts in mice lacking Pth1r in DMP1-10kb–expressing cells. However, similar to our findings, chronic PTH elevation markedly increased circulating CTX to a similar extent than in WT control mice. Finally, the age of the mice could also influence the response to the hormone. We found that both 1-month-old and 4-month-old WT and cKO mice exhibited similar bone loss in response to endogenous elevation of PTH. Unfortunately, the age of the mice used in Saini and colleagues's study18 was not reported.
It is recognized that mechanical loading stimulates new bone formation on surfaces adjacent to areas to which strain is applied, and alters the expression of molecules that lie downstream or upstream of the Pth1r, such as Sost/sclerostin48 and PTHrP,49 the other ligand of the Pth1r, respectively. Indeed, PTHrP expression is stimulated in osteocytic cells in vitro,20 and transient increases in circulating PTH have been reported in both human and animals after exercise.19, 50, 51 Although we were unable to detect differences at the time point studied in PTHrP mRNA levels between loaded and non-loaded bones, from WT or cKO mice (Supporting Fig. 6D), we cannot exclude the possibility that PTHrP expression had increased at earlier time points. In the current experiment, we detected a decrease in Sost mRNA gene expression in the loaded ulna of WT mice, although it did not reach statistical significance, whereas Sost mRNA expression in cKO mice remained unchanged. Our inability to detect statistical significant reduction in Sost mRNA expression in whole bone might be explained by fact that downregulation of Sost/Sclerostin by loading in this model is confined to osteocytes located in specific areas of the ulna.16, 48 Moreover, the current studies showing that cKO mice did not exhibit the expected reduction in Sost/sclerostin expression, the increase in Wnt/β-catenin target gene expression nor the increase in bone formation in response to mechanical loading, are consistent with previous observations showing that mice overexpressing SOST or lacking the Wnt co-receptor LRP5 also exhibit a defective response to mechanical loading.16, 36 Taken together, these findings support the notion that Pth1r in DMP1-8kb–expressing cells is required to downregulate Sost/sclerostin and activate Wnt/β-catenin signaling for mechanostransduction.
Similar to mechanical loading, intermittent elevation of PTH achieved by daily injections failed to increase cancellous and cortical bone mass and to downregulate Sost/sclerostin or stimulate Wnt/β-catenin signaling in cKO mice, showing the requirement of the Pth1r in DMP1-8kb–expressing cells for the full anabolic response to daily injections of PTH. However, in contrast to mechanical loading, PTH induced equivalent increases in total and femoral BMD, osteocalcin, cortical and cancellous bone mass in femur and spine measured by μCT, Wnt signaling target genes, and bone formation rate in WT control littermates and TG mice overexpressing SOST/sclerostin. Moreover, the surface covered by single or double labels (sL.S and dL.S) as well as the number of double labels were increased in WT or TG mice treated with PTH. However, because PTH also increased bone surface (BS), when sL.S and dL.S are corrected by BS or when MS/BS is calculated, the difference between vehicle and PTH-treated mice disappear. One of the limitations of this study is our inability to detect increases in spinal BMD or statistically significant increases in femoral cancellous BV/TV induced by PTH in the TG mice. This could be explained by the very low bone mass exhibited by TG mice in this compartment together with the low resolution of DXA. In addition, similar to previous observations from our laboratory,(14–16) at baseline the cortical bone and the expression of various Wnt target genes appear to be unchanged in TG when compared to WT mice, thus questioning the activity/potency of the human SOST transgene. Although not shown in this study, we have showed before that the human SOST transgene is indeed expressed in both cortical and cancellous bone, and is potent enough to inhibit the increase in bone formation as well as the activation of Wnt signaling induced by two different strong anabolic stimuli.(14–16) Taken together, these data suggest that daily PTH injections induced anabolic responses in the cancellous and cortical bone of both WT and TG mice. These results are in sharp contrast to the deficient response of TG mice to mechanical stimulation,16 and show that, although the Pth1r signaling in DMP1-8kb–expressing cells is central to the bone forming ability of both anabolic stimuli, the molecular mediator(s) appear to be different, and also suggest that PTH must induce in osteocytes other responses besides downregulating Sost/sclerostin. Future studies are warranted to unravel alternative pathways activated by PTH in osteocytes that lead to bone anabolism.
The lack of requirement of Sost/sclerostin downregulation for PTH anabolic actions is in line with earlier studies showing normal or even enhanced response to anabolic regimens of PTH in SOST knockout mice.52 In contrast to the current study, Kramer and colleagues53 reported that PTH-induced bone gain is reduced in mice overexpressing SOST. This discrepancy could be explained by the use of different transgenic mouse models. Whereas in our TG mice the expression of the human SOST gene is driven by the DMP1-8kb promoter, Kramer and colleagues53 used a mouse harboring the entire human SOST gene (BAC-SOST), including its regulatory elements, to systemically overexpress the gene.54 As a consequence, although in our study the levels of the human SOST transgene remained unchanged by PTH administration, in the study by Kramer and colleagues53 a 50% reduction in SOST expression was observed in the BAC-SOST mouse model treated with PTH, thus hindering the interpretation of the results. In addition, the BAC-SOST mice exhibit a modest bone phenotype compared to our DMP1-SOST TG mice, with bone formation indexes indistinguishable from control mice at 6 months of age. Further, although the doses of PTH administered are similar between the two studies, the length of the treatment differs (4 versus 8 weeks). In fact, opposite to our observations, both control and SOST-BAC mice exhibited an increase in bone resorption induced by PTH, suggesting that some of the bone effects observed in the Kramer and colleagues53 study are not merely due to differences in bone formation. Based on these considerations, we believe that our results using TG mice provide clear evidence that Sost/sclerostin downregulation is dispensable for the anabolic effects of PTH.
In conclusion, Pth1r in DMP1-8kb–expressing cells is required to stimulate Wnt/β-catenin signaling and to elicit bone gain resulting from daily injections of PTH and mechanical loading; however, it is not necessary for the catabolic actions promoted by chronic elevation of the endogenous levels of the hormone. Further, our mechanistic studies demonstrate that Pth1r-mediated downregulation of Sost/sclerostin expression is dispensable for the bone gain induced by daily injections of PTH, suggesting that other mechanisms lying downstream of the Pth1r in osteocytes are responsible for the anabolic effects of the hormone in the skeleton. Understanding the mechanistic bases of Pth1r signaling could result in the identification of new osteoanabolic therapeutic targets, thus providing new avenues for the treatment of bone diseases.
Disclosures
All authors state that they have no conflicts of interest.
Acknowledgments
Research reported in this publication was supported by the National Institutes of Health (NIH) (National Institute of Arthritis and Musculoskeletal and Skin Diseases [NIAMS] R01-AR059357 to TB; National Heart, Lung, and Blood Institute [NHLBI] T35 HL110854-01 to RE and KK) and the Veterans Administration (1 I01 BX002104-01 to TB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. RPC received a scholarship from Coordination of Improvement of Higher Level Personnel (CAPES), Brazil (PDE: #232636/2014-1). We thank Galli C., Ben-Awadh A., Richardson D., Dr. Condon K., Acton A., and Kim W. for their assistance in tissue collection; and Dr. David Burr for insightful discussions.
Author contributions: JDC, XT, and TB designed the research; JDC, XT, RPC, KWC, GP, ER, and NO performed the research; JDC, XT, LIP, AR, MP, and TB analyzed and interpreted the data; JDC and TB wrote the manuscript. TB takes responsibility for the integrity of the data analysis.




