Effects of bazedoxifene on bone mineral density, bone turnover, and safety in postmenopausal japanese women with osteoporosis†
These results were presented, in part, at the 30th Annual Meeting of the American Society for Bone and Mineral Research, Montreal, Quebec, Canada, September 12–16, 2008.
Abstract
This randomized, double-blind, placebo-controlled, dose-response late phase 2 study evaluated the efficacy and safety of bazedoxifene in postmenopausal Japanese women 85 years of age or younger with osteoporosis. Eligible subjects received daily treatment with oral doses of bazedoxifene 20 or 40 mg or placebo for 2 years. Efficacy assessments included bone mineral density (BMD) at the lumbar spine and other skeletal sites, bone turnover marker levels, lipid parameters, and incidence of new fractures. Of 429 randomized subjects, 387 were evaluable for efficacy, and 423 were included in the safety analyses (mean age, 64 years). At 2 years, the mean percent changes from baseline in lumbar spine BMD were significantly greater with bazedoxifene 20 and 40 mg (2.43% and 2.74%, respectively) than with placebo (−0.65%, p < .001 for both). Both bazedoxifene doses significantly improved BMD at the total hip, femoral neck, and greater trochanter compared with placebo (p < .001 for all). Decreases in bone turnover markers were observed with bazedoxifene 20 and 40 mg as early as 12 weeks (p < .05 for all) and were sustained throughout the study. Total and low-density lipoprotein cholesterol levels were significantly decreased from baseline with both bazedoxifene doses compared with placebo (p < .05 for all). Incidences of new vertebral and nonvertebral fractures were similar among the bazedoxifene and placebo groups. Overall, the incidence of adverse events with bazedoxifene 20 and 40 mg was similar to that with placebo. Bazedoxifene significantly improved BMD, reduced bone turnover, and was well tolerated in postmenopausal Japanese women with osteoporosis. © 2011 American Society for Bone and Mineral Research.
Introduction
Osteoporosis is a common chronic skeletal disease characterized by reduced bone strength that results in an increased susceptibility for fractures.1, 2 Osteoporosis represents an increasing global health concern, affecting an estimated 75 million people in the United States, Europe, and Japan and resulting in approximately 2.5 million fractures each year. Furthermore, osteoporotic fractures may be associated with a considerable increase in morbidity and mortality, as well as a substantial economic burden.1-3 Because this disease primarily affects postmenopausal women and elderly adults, the prevalence of osteoporosis is expected to increase as these populations expand.2, 3 This is of particular concern for some Asian countries with rapidly growing elderly populations, including Japan, where the number of affected individuals was estimated at 11 million in 2000.4
Several therapies have demonstrated efficacy for the prevention and/or treatment of postmenopausal osteoporosis, including bisphosphonates (eg, alendronate, risedronate, ibandronate, and zoledronic acid; ibandronate and zoledronic acid are not yet approved in Japan), estrogens, calcitonin, parathyroid hormone (PTH; eg, teriparatide; not yet approved in Japan), and strontium ranelate (outside the United States and Japan). The selective estrogen receptor modulator (SERM) raloxifene is also approved for the prevention and treatment of postmenopausal osteoporosis. However, these therapies may not be appropriate for all postmenopausal women primarily because of safety and/or tolerability concerns.1, 3 Therefore, the continued development of new pharmacologic agents that are safe and effective is desired.
Bazedoxifene is a novel SERM under development for the treatment and prevention of postmenopausal osteoporosis. In pivotal international phase 3 clinical trials of nearly 10,000 women, bazedoxifene was shown to prevent bone loss in postmenopausal women at risk for osteoporosis5 and to significantly reduce the risk of new vertebral fracture in postmenopausal women with osteoporosis.6 In a subgroup of osteoporotic women at higher risk for fracture, bazedoxifene was shown to significantly decrease the risk of nonvertebral fracture.6 Findings from both trials showed that bazedoxifene generally was safe and well tolerated, with no evidence of breast or endometrial stimulation.5, 6
Considering the growing impact of postmenopausal osteoporosis in Japan and the ongoing need for therapeutic alternatives, clinical trials to evaluate the safety and efficacy of bazedoxifene in this population were initiated. The results of a phase 1 trial showed that bazedoxifene was safe and well tolerated at doses of up to 60 mg/day in healthy postmenopausal Japanese women. Furthermore, single- and multiple-dose pharmacokinetic data from postmenopausal Japanese women were shown to be comparable with those from non-Japanese women, with no significant differences.
The objective of this study was to evaluate the efficacy and safety of bazedoxifene 20 and 40 mg/day in an otherwise healthy population of postmenopausal Japanese women with osteoporosis.
Materials and Methods
Study design
This 2-year, multicenter, randomized, double-blind, placebo-controlled, phase 2 trial was conducted at 17 sites in Japan. Subjects were randomly assigned (1:1:1) to receive bazedoxifene 20 or 40 mg or placebo, taken orally once daily in the morning. Subjects also received a daily supplement (New Calci-Chew D3, Nitto Pharmaceutical Industries, Ltd., Kyoto, Japan) containing elemental calcium (610 mg), vitamin D3 (400 IU), and magnesium (30 mg). The use of drugs known to have an effect on bone metabolism, including estrogens, progestogens, androgens, SERMs, bisphosphonates, calcitonin, PTH, anabolic hormones, and vitamin K2; vitamin D preparations; additional calcium preparations; methotrexate preparations; heparin, warfarin, and anticonvulsants; systemic administration of fluorine; and other investigational drugs, was not permitted during the study.
Subjects
Subjects were enrolled according to the diagnostic criteria of the Japanese Society of Bone Mineral Metabolism.7 Eligible subjects included postmenopausal women with an intact uterus who were 85 years of age or younger at the time of consent. Postmenopause was defined as occurring more than 2 years since the last menstrual period or more than 2 years since surgical menopause or in women older than 60 years when the date of the last menstrual period was unknown. At screening, subjects without prevalent nontraumatic vertebral fracture were required to have a bone mineral density (BMD) value for the lumbar spine (L2–L4) that was less than 70% of the young-adult mean BMD of Japanese women, whereas subjects with prevalent nontraumatic vertebral fracture were required to have a BMD value of less than 80% of the young-adult mean BMD of Japanese women.
Subjects were excluded if they had complications or a previous history of conditions that may cause secondary osteoporosis, bone disorders (other than osteoporosis), disorders that affect bone metabolism, conditions that could interfere with BMD measurement, at least six vertebral fractures or a severe vertebral fracture, or at least two lumbar vertebral fractures (L1–L4). Subjects with serious conditions such as endometrial hyperplasia; endometrial or breast cancer; other malignancy within the past 10 years; abnormal vaginal bleeding; endocrine disorder requiring treatment; untreated severe malabsorption disorder; or hepatic, renal, or cardiac disorder that is difficult to control with drug therapy were excluded. In addition, subjects with complications or a previous history of venous thromboembolic events were excluded, as were those with elevated serum alanine aminotransferase, aspartate aminotransferase, creatinine, alkaline phosphatase, or total bilirubin levels and those with elevated fasting total cholesterol (≥ 310 mg/dL) or triglyceride (≥ 300 mg/dL) levels at screening. Subjects also were ineligible if they received treatment with any of the following medications: vitamin D3, vitamin K2, ipriflavone, and calcitonin preparations within 2 months of screening; estrogens, progestogens, androgens, thyroid hormone or PTH, protein anabolic hormone, heparin and/or warfarin, anticonvulsive drugs, and SERM preparations within 6 months of screening; and bisphosphonates within 12 months of screening.
In accordance with the ethical principles included in the Declaration of Helsinki, all subjects provided written informed consent before enrollment in the study. The study protocol (including any amendments) and an informed consent form were approved by the institutional review board at each institution.
Study endpoints and measurements
The primary efficacy endpoint was the percent change from baseline in BMD of the lumbar spine (L1–L4) after 104 weeks (2 years) of treatment. Secondary efficacy endpoints included the percent change from baseline in BMD of the lumbar spine (L2–L4), total hip, femoral neck, and greater trochanter; percent change from baseline in levels of bone turnover markers [serum C-telopeptide (CTX), serum N-telopeptide (NTX), serum osteocalcin, and urinary NTX]; percent change from baseline in lipid parameters [total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, lipoprotein(a), and triglycerides]; and incidence of new vertebral and nonvertebral fractures. BMD was measured by dual-energy X-ray absorptiometry (DXA) at screening and at weeks 24, 52, 76, and 104 (or at early treatment discontinuation), and two measurements of the lumbar spine and hip BMD were obtained at screening and at week 104 (or at early treatment discontinuation). BMD measurements were analyzed at a centralized institution under blinded conditions. Radiography of the thoracic and lumbar spine was used to identify the number and sites of vertebral fractures. Radiograms were obtained at screening and at weeks 24, 52, 76, and 104 (or at early treatment discontinuation) and were interpreted as reported previously.8-10
Safety and tolerability were assessed through adverse event (AE) monitoring, routine physical examination, clinical laboratory determination (ie, hematologic, serum chemistry, coagulation, endocrinologic, and urinary examinations), and gynecologic examination [ie, breast examination, transvaginal ultrasonography (TVU; evaluation of endometrial thickness, ovarian volume, and presence of ovarian cysts), and cytology of the uterine cervix and endometrium]. In this study, AEs were defined as any undesired medical event occurring after administration of study medication regardless of any correlation with study medication and including symptoms and signs caused by deterioration of the primary disorder.
Statistical analysis
Efficacy analyses were performed on the full analysis set population, which included all randomized subjects who received at least one dose of study medication, had no critical violation of the major criteria for inclusion or exclusion, and had a BMD assessment both at screening and during at least one on-therapy visit. All subjects who received at least one dose of study medication and underwent a safety observation or examination were included in the safety analyses.
Sample size calculation was based on the assumption that a clinically significant minimum difference of at least +3% (common standard deviation [SD], 4.0%) would be observed between the bazedoxifene 40-mg group and the placebo group. Thirty-eight subjects per group would be required to provide 90% power to detect this difference at the .05 significance level (two-sided test). The discontinuation rate was expected to be approximately 20%. Because at least 100 subjects per group are desirable for long-term safety evaluation, the goal was to enroll 375 subjects (three groups with 125 subjects each).
Between-group differences in the mean percent change from baseline in BMD were analyzed using a t test, with percent change from baseline as the dependent variable; treatment, body mass index (BMI), and years since menopause as main effects; and the type of DXA machine and baseline BMD as covariates. If the difference between bazedoxifene 40 mg and placebo was significant, the difference between bazedoxifene 20 mg and placebo was analyzed. Secondary analyses of data for BMD, bone turnover markers, and lipid parameters were compared between treatment groups using an analysis of covariance (ANCOVA) based on percent change from baseline, with treatment as a factor and baseline as a covariate. The incidence of new fractures was compared between treatment groups using the Fisher exact test. The last observation carried forward method was used for imputation of missing data (eg, from early study discontinuation or missed visits). Data were evaluated at the .05 significance level (two-sided).
Overall differences in the incidence of AEs and study discontinuations among treatment groups were evaluated using a chi-square test. Each vital sign and laboratory parameter with at least one potentially clinically important value was compared across groups using the Fisher exact test. Data on changes from baseline in endometrial thickness and ovarian volume were analyzed using an ANCOVA, with treatment as a factor and baseline as a covariate.
Results
Study population
Of the 429 women who were randomly assigned to a treatment group, 423 took at least one dose of study medication and were included in the safety analysis (Fig. 1). Of these, 387 women were evaluable for the efficacy analysis. The majority of subjects (n = 311) completed the trial; the most common reasons for study discontinuation were AEs and subject request, which occurred with comparable frequency across treatment groups. Subject demographic and baseline characteristics generally were similar among the bazedoxifene and placebo groups (Table 1), with no differences in age, BMI, or years since menopause. The rate of compliance through week 104 (or at early treatment discontinuation) was high among all three treatment groups (96.9%, 97.1%, and 96.1% for bazedoxifene 20 and 40 mg and placebo, respectively).
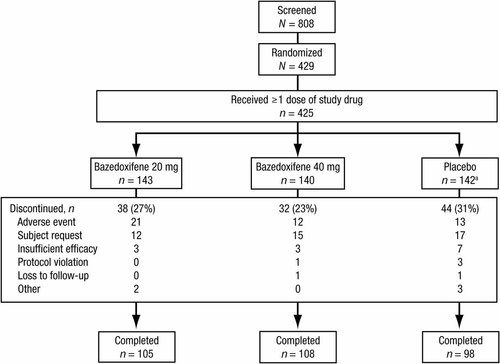
Disposition of subjects. aIncludes two subjects who received but never took the study medication.
| Characteristic | Bazedoxifene 20 mg (n = 132) | Bazedoxifene 40 mg (n = 127) | Placebo (n = 128) |
|---|---|---|---|
| Age, years, mean (SD) | 63.0 (6.4) | 63.2 (6.3) | 64.1 (6.6) |
| BMI, kg/m2, mean (SD) | 21.5 (2.5) | 21.2 (2.6) | 21.5 (2.6) |
| Years since menopause, mean (SD) | 13.4 (6.8) | 13.9 (6.7) | 14.9 (7.0) |
| Smoking, n (%) | 12 (9.1) | 13 (10.2) | 12 (9.4) |
| Alcohol use, n (%) | |||
| Rarely | 88 (66.7) | 86 (67.7) | 80 (62.5) |
| Occasionally | 37 (28.0) | 34 (26.8) | 41 (32.0) |
| Daily | 7 (5.3) | 7 (5.5) | 7 (5.5) |
| BMD, g/cm3, mean (SD) | |||
| Lumbar spine (L1–L4) | 0.63 (0.07) | 0.63 (0.07) | 0.63 (0.07) |
| Total hip | 0.66 (0.08) | 0.65 (0.08) | 0.66 (0.08) |
| Femoral neck | 0.56 (0.08) | 0.56 (0.07) | 0.56 (0.08) |
| Greater trochanter | 0.49 (0.07) | 0.49 (0.07) | 0.49 (0.07) |
- SD = standard deviation; BMI = body mass index; BMD = bone mineral density.
Bone mineral density
At week 104 (or at early treatment discontinuation), BMD of the lumbar spine (L1–L4) was improved significantly for both bazedoxifene treatment groups compared with the placebo group (p < .001; Fig. 2A); no significant difference was observed between the bazedoxifene treatment groups. The mean percent changes [95% confidence intervals (CIs)] were 2.43% (1.75% to 3.12%) with bazedoxifene 20 mg, 2.74% (2.04% to 3.45%) with bazedoxifene 40 mg, and −0.65% (−1.35% to 0.05%) with placebo. Treatment with bazedoxifene 20 and 40 mg was associated with a significant increase from baseline in lumbar spine BMD as early as week 24 (p < .001 versus placebo), which was sustained throughout the remainder of the study (Fig. 2A). Although BMD of the lumbar spine for the placebo group showed minimal change from baseline through week 52, there was a significant decrease from baseline at week 104 (p = .039). Similar results were observed for BMD analysis of the lumbar spine (L2–L4).
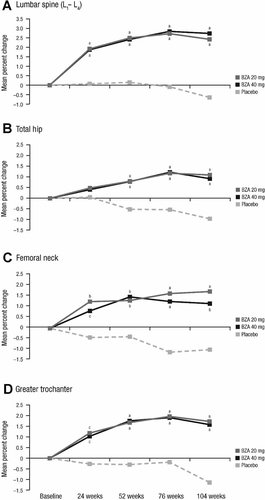
Mean percent change from baseline in BMD response at (A) the lumbar spine (L1–L4), (B) the total hip, (C) the femoral neck, and (D) the greater trochanter. BZA = bazedoxifene. ap ≤ .001 versus placebo. bp < .01 versus placebo. cp < .05 versus placebo.
Bazedoxifene 20 and 40 mg also showed significantly greater improvements in BMD of the total hip, femoral neck, and greater trochanter at week 104 (or at early treatment discontinuation) relative to placebo (p ≤ .001 for each comparison; Fig. 2B–D); no significant differences were observed between the bazedoxifene treatment groups. For bazedoxifene 20 and 40 mg and placebo, respectively, the mean percent changes from baseline were 1.10%, 0.93%, and −0.97% for the total hip; 1.73%, 1.16%, and −1.01% for the femoral neck; and 1.73%, 1.58%, and −1.14% for the greater trochanter. As observed for the lumbar spine, BMD of the hip increased from baseline for bazedoxifene 20 and 40 mg by week 24 and was maintained thereafter (Fig. 2B–D).
Markers of bone turnover
For all four markers of bone turnover (serum CTX, serum NTX, serum osteocalcin, and urinary NTX), significant reductions from baseline were seen in both bazedoxifene treatment groups compared with the placebo group as early as week 12 (p < .05; Fig. 3). At week 104, the median percent changes from baseline in serum CTX were −33.2% for bazedoxifene 20 mg and −32.6% for bazedoxifene 40 mg compared with −7.1% for placebo (p ≤ .001 for both comparisons). The median percent changes from baseline in serum NTX were −16.3% and −16.3% for bazedoxifene 20 and 40 mg, respectively, compared with −3.4% for the placebo group (p = .007 and p < .001, respectively). The median percent changes from baseline in serum osteocalcin were −25.8% for bazedoxifene 20 mg and −25.1% for bazedoxifene 40 mg compared with −3.0% for placebo (p < .001 for both comparisons). For urinary NTX, the median percent changes were −30.1% and −31.0% for bazedoxifene 20 and 40 mg, respectively, compared with +6.2% for placebo (p < .001 for both comparisons).
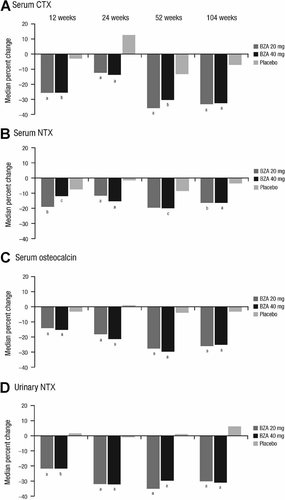
Median percent changes from baseline in serum levels of bone turnover markers, including (A) serum CTX, (B) serum NTX, (C) serum osteocalcin, and (D) urinary NTX. CTX = C-telopeptide; NTX = N-telopeptide; BZA = bazedoxifene. ap ≤ .001 versus placebo. bp ≤ .01 versus placebo. cp < .05 versus placebo.
Incidence of fractures
At week 104 (or at early treatment discontinuation), the incidences of new vertebral fractures were 3.8% (n = 5) with bazedoxifene 20 mg, 2.4% (n = 3) with bazedoxifene 40 mg, and 4.7% (n = 6) with placebo. Although the rates of vertebral fractures were lower in the bazedoxifene treatment groups compared with the placebo group, the differences were not statistically significant. The incidences of new nonvertebral fractures were 3.8% (n = 5) with bazedoxifene 20 mg, 2.4% (n = 3) with bazedoxifene 40 mg, and 3.1% (n = 4) with placebo, with no significant differences between each treatment group.
Lipid metabolism
Total cholesterol levels were significantly decreased from baseline at each time point for both bazedoxifene treatment groups (p < .001). At week 104 (or at early treatment discontinuation), the reductions from baseline in total cholesterol were significantly greater (p < .05) for both bazedoxifene groups compared with the placebo group (Table 2). The levels of LDL cholesterol also were decreased significantly from baseline at each time point for both bazedoxifene treatment groups (p < .05), and these reductions were significantly greater than those in the placebo group (p < .05 for both comparisons). At week 104 (or early treatment discontinuation), the increase from baseline in HDL cholesterol levels was greater with bazedoxifene 20 and 40 mg compared with placebo, but these differences were not statistically significant. Changes from baseline in triglyceride levels were small and showed no clear trends among groups. Levels of lipoprotein(a) were generally lower in both bazedoxifene groups compared with the placebo group throughout the study period. The percent change from baseline to week 104 (or early treatment discontinuation) was statistically significant in the bazedoxifene 20- and 40-mg groups (p < .001 and p = .001, respectively), but not in the placebo group.
| Parameter | Bazedoxifene 20 mg (n = 105) | Bazedoxifene 40 mg (n = 109) | Placebo (n = 99) |
|---|---|---|---|
| Total cholesterol | −4.9b,c | −6.3b,d | −0.5 |
| LDL cholesterol | −2.7c,e | −5.9b,d | 3.4 |
| HDL cholesterol | 3.1 | 2.3 | 1.3 |
| Triglycerides | 2.9 | 0.3 | 0.2 |
| Lipoprotein(a) | −3.6b | −1.4f | 1.5 |
- LDL = low-density lipoprotein; HDL = high-density lipoprotein.
- a Percent changes calculated using data for subjects with lipid assessments at baseline and week 104 (or at early treatment discontinuation).
- b p < .001 versus baseline.
- c p < .01 versus placebo.
- d p < .001 versus placebo.
- e p < .05 versus baseline.
- f p = .001 versus baseline.
Safety and tolerability
Both doses of bazedoxifene were generally safe and well tolerated during the 2-year study. The overall incidence of treatment-emergent AEs, serious AEs, and discontinuations because of AEs was similar among the bazedoxifene and placebo groups (Table 3). No deaths occurred during the study. The number of cardiovascular AEs was low and similar among groups (Table 3). Angina pectoris was reported by one subject in each group. There were no reports of coronary occlusion, myocardial infarction, myocardial ischemia, or venous thromboembolic events in any treatment group. There were two cases of cerebral infarction (one each with bazedoxifene 20 and 40 mg), both of which were found incidentally on brain imaging (reasons for central nervous system imaging: one subject had reported slight numbness in the right upper and lower limbs, and the other subject had experienced tinnitus); however, these events did not correlate with imaging findings and were not considered to be related to study medication by the investigator. The incidence of hot flushes was low among all treatment groups but higher with bazedoxifene 20 mg than with bazedoxifene 40 mg or placebo (overall p < .05).
| Subjects, n (%) | Bazedoxifene 20 mg (n = 143) | Bazedoxifene 40 mg (n = 140) | Placebo (n = 140) |
|---|---|---|---|
| Any treatment-emergent AE | 134 (93.7) | 126 (90.0) | 126 (90.0) |
| Any serious AE | 9 (6.3) | 9 (6.4) | 14 (10.0) |
| Discontinuations due to AEs | 21 (14.7) | 12 (8.6) | 13 (9.3) |
| Selected AEs | |||
| Angina pectoris | 1 (0.7) | 1 (0.7) | 1 (0.7) |
| Carotid artery thrombosis | 0 | 1 (0.7) | 0 |
| Cerebral infarction | 1 (0.7) | 1 (0.7) | 0 |
| Coronary occlusion | 0 | 0 | 0 |
| Hot flushes | 6 (4.2) | 0 | 2 (1.4) |
| Myocardial infarction | 0 | 0 | 0 |
| Myocardial ischemia | 0 | 0 | 0 |
| Venous thromboembolic events | 0 | 0 | 0 |
- AE = adverse event.
The most commonly reported treatment-emergent AEs (≥5% of subjects in any treatment group) are shown in Table 4. The incidence of gastrointestinal events was not different among subjects treated with bazedoxifene or placebo, with the exception of vomiting (overall p < .05). Reports of muscle spasm generally were higher with bazedoxifene 20 or 40 mg than with placebo.
| Subjects, n (%) | Bazedoxifene 20 mg (n = 143) | Bazedoxifene 40 mg (n = 140) | Placebo (n = 140) |
|---|---|---|---|
| Gastrointestinal disorder | 67 (46.9) | 68 (48.6) | 67 (47.9) |
| Abdominal pain (upper) | 4 (2.8) | 9 (6.4) | 13 (9.3) |
| Constipation | 17 (11.9) | 17 (12.1) | 10 (7.1) |
| Dental caries | 10 (7.0) | 12 (8.6) | 9 (6.4) |
| Diarrhea | 11 (7.7) | 7 (5.0) | 11 (7.9) |
| Gastritis | 8 (5.6) | 7 (5.0) | 4 (2.9) |
| Gingivitis | 3 (2.1) | 7 (5.0) | 5 (3.6) |
| Stomach discomfort | 7 (4.9) | 13 (9.3) | 8 (5.7) |
| Stomatitis | 3 (2.1) | 8 (5.7) | 3 (2.1) |
| Vomitinga | 8 (5.6) | 2 (1.4) | 1 (0.7) |
| Infectious and parasitic disease | 101 (70.6) | 85 (60.7) | 84 (60.0) |
| Cystitisa | 15 (10.5) | 3 (2.1) | 8 (5.7) |
| Nasopharyngitis | 86 (60.1) | 75 (53.6) | 74 (52.9) |
| Pharyngitis | 10 (7.0) | 7 (5.0) | 3 (2.1) |
| Injury, addiction, and treatment complication | 34 (23.8) | 37 (26.4) | 39 (27.9) |
| Arthropod sting | 5 (3.5) | 7 (5.0) | 5 (3.6) |
| Contusion | 11 (7.7) | 11 (7.9) | 12 (8.6) |
| Musculoskeletal and connective tissue disorder | 70 (49.0) | 56 (40.0) | 65 (46.4) |
| Arthralgia | 23 (16.1) | 16 (11.4) | 12 (8.6) |
| Back pain | 29 (20.3) | 21 (15.0) | 27 (19.3) |
| Pain in extremity | 5 (3.5) | 7 (5.0) | 9 (6.4) |
| Periarthritis | 8 (5.6) | 7 (5.0) | 4 (2.9) |
| Muscle spasm | 8 (5.6) | 5 (3.6) | 2 (1.4) |
| Musculoskeletal stiffness | 6 (4.2) | 11 (7.9) | 9 (6.4) |
| Nervous system disorder | 39 (27.3) | 37 (26.4) | 43 (30.7) |
| Dizziness | 11 (7.7) | 12 (8.6) | 14 (10.0) |
| Headache | 16 (11.2) | 13 (9.3) | 14 (10.0) |
| Hypoesthesia | 8 (5.6) | 4 (2.9) | 3 (2.1) |
| Mental disorder | 13 (9.1) | 11 (7.9) | 11 (7.9) |
| Insomnia | 11 (7.7) | 8 (5.7) | 8 (5.7) |
| Reproductive and breast disorder | 16 (11.2) | 16 (11.4) | 19 (13.6) |
| Vaginal discharge | 3 (2.1) | 3 (2.1) | 8 (5.7) |
| Respiratory, chest, and mediastinal disorder | 26 (18.2) | 24 (17.1) | 17 (12.1) |
| Cough | 4 (2.8) | 8 (5.7) | 2 (1.4) |
| Skin and subcutaneous tissue disorder | 41 (28.7) | 27 (19.3) | 36 (25.7) |
| Contact dermatitis | 5 (3.5) | 5 (3.6) | 8 (5.7) |
| Eczema | 17 (11.9) | 7 (5.0) | 13 (9.3) |
- AE = adverse event.
- a Overall p < .05, chi-square test.
Clinical laboratory testing showed a significant reduction from baseline in the serum levels of calcium, phosphorus, and alkaline phosphatase in both bazedoxifene treatment groups compared with the placebo group (p < .001). No significant or clinically meaningful differences in the levels of alanine aminotransferase, aspartate aminotransferase, and total bilirubin were found among treatment groups. PTH levels at week 104 were significantly increased from baseline for all treatment groups (p < .001) and were significantly higher with bazedoxifene 20 and 40 mg compared with placebo (p < .001 and p = .018, respectively). Mean (SD) changes from baseline in PTH levels at week 104 were 12.4 (11.2) pg/mL with bazedoxifene 20 mg, 10.8 (12.9) pg/mL with bazedoxifene 40 mg, and 6.9 (10.9) pg/mL with placebo. There were no abnormal clinically important values in any group or other clinically meaningful differences between placebo and either bazedoxifene dose.
There were no differences in the incidences of reproductive system and breast-related AEs among the bazedoxifene and placebo groups (Table 5). In addition, no safety concerns were identified based on gynecologic examination, and no significant differences in mammography or breast ultrasonography were noted among the groups. There was one report of breast neoplasm with bazedoxifene 20 mg; the lesion from this subject was found to be both estrogen receptor– and progesterone receptor–positive. No major changes in the number of ovarian cysts or in ovarian volume were observed in any treatment group. A small number of subjects had abnormalities on uterine cell examination (Papanicolaou test results of class III or IIIa: bazedoxifene 20 mg, n = 2; bazedoxifene 40 mg, n = 1; placebo, n = 1); follow-up examinations showed normal cervical Papanicolaou test results for all these subjects. There was no case of cervical or endometrial cancer reported in this study. At 2 years, TVU data showed no significant change from baseline in endometrial thickness in the bazedoxifene treatment groups (bazedoxifene 20 mg, 0.06 mm; bazedoxifene 40 mg, −0.03 mm; placebo, −0.22 mm). Two subjects (bazedoxifene 20 mg, n = 1; placebo, n = 1) experienced genital hemorrhage; both events were mild, transient, and nonserious and were considered possibly related to the study drug. Neither subject had endometrial hyperplasia.
| Subjects, n (%) | Bazedoxifene 20 mg (n = 143) | Bazedoxifene 40 mg (n = 140) | Placebo (n = 140) |
|---|---|---|---|
| Endometrial hyperplasia | 1 (0.7) | 0 | 0 |
| Ovarian cyst | 1 (0.7) | 0 | 0 |
| Cervical polyp | 0 | 0 | 1 (0.7) |
| Uterine polyp | 1 (0.7) | 0 | 0 |
| Genital hemorrhage | 1 (0.7) | 0 | 1 (0.7) |
| Breast cancer | 1 (0.7) | 0 | 1 (0.7) |
| Breast cyst | 0 | 1 (0.7) | 0 |
| Fibrocystic breast disease | 3 (2.1) | 4 (2.9) | 1 (0.7) |
| Breast pain | 0 | 2 (1.4) | 1 (0.7) |
| Benign breast neoplasm | 1 (0.7) | 0 | 0 |
- AE = adverse event.
Discussion
This study evaluated the efficacy and safety of bazedoxifene in postmenopausal Japanese women with osteoporosis over 2 years of therapy. Both doses of bazedoxifene (20 and 40 mg) were associated with significant improvements in BMD compared with placebo at multiple skeletal sites (ie, lumbar spine, total hip, femoral neck, and greater trochanter). These improvements in BMD reached statistical significance as early as 6 months for the lumbar spine and total hip and were sustained throughout the remainder of the study. No significant differences in BMD response were observed between the bazedoxifene groups at any skeletal site. Both bazedoxifene doses were associated with significantly greater reductions in the levels of bone turnover markers (including urinary NTX and serum CTX, NTX, and osteocalcin) and improvements in lipid parameters (total cholesterol and LDL cholesterol) compared with placebo.
The favorable effects of bazedoxifene on BMD, bone turnover, and lipid metabolism seen in postmenopausal Japanese women are consistent with those seen in two large prospective phase 3 studies5, 6 conducted worldwide. This study showed BMD increases from baseline of more than 2% at the lumbar spine with bazedoxifene 20 and 40 mg, which was somewhat higher than that reported in the pivotal 3-year study6 of postmenopausal women with osteoporosis. The greater BMD responses of Japanese women observed in this study compared with the global phase 3 study may be attributed to the lower mean BMI (21.4 versus 26.5 kg/m2, respectively)6 and other differences in study population. A raloxifene study11 showed a similar increase in lumbar spine BMD (3.5%) of postmenopausal Japanese women with osteoporosis after 1 year of treatment; the trends for bone turnover markers and serum lipids observed with bazedoxifene in this study also were comparable with those reported with raloxifene in this population.11
The global osteoporosis treatment study6 showed a significant reduction in new vertebral fracture risk with bazedoxifene 20 and 40 mg compared with placebo, which was similar to that seen with raloxifene 60 mg. In addition, independent reanalyses of data from that study showed that bazedoxifene significantly decreased the risk of all clinical morphometric vertebral and nonvertebral fractures in women at or above a Fracture Risk Assessment Tool–based fracture probability threshold.12, 13 The incidence of nonvertebral fractures was significantly reduced with bazedoxifene 20 mg compared with placebo and raloxifene 60 mg in a subgroup of women at higher risk.6 Although the current study (N < 400, efficacy analyses) showed no differences in the incidence of new vertebral or nonvertebral fractures in the bazedoxifene groups compared with the placebo group, it should be noted that the study was not powered for evaluation of changes in fracture risk.
Both doses of bazedoxifene were well tolerated over 2 years of therapy in this population of postmenopausal Japanese women with osteoporosis, with a safety profile similar to that of placebo. Overall, the incidences of AEs, serious AEs, and discontinuations due to AEs with bazedoxifene were not different from those with placebo. Increased incidences of hot flushes and venous thromboembolic events have been reported in previous studies of SERMs14-17; however, this study found no significant differences in the incidence of hot flushes between the bazedoxifene and placebo treatment groups. This finding is consistent with the low incidence of hot flushes seen with raloxifene in postmenopausal Japanese women11 and reports that Asian women are generally less likely to report hot flushes and other menopausal symptoms than white women.18-21 Additionally, there were no reports of venous thromboembolic events. The numbers of cardiovascular and cerebrovascular AEs were low and similarly distributed among treatment groups. The levels of liver enzymes also were similar among treatment groups, indicating that bazedoxifene is not associated with liver toxicity. At 2 years, bazedoxifene treatment was associated with increased PTH levels, which likely occurred in response to the small reductions in serum calcium levels attributable to decreased bone resorption. It should be noted that PTH levels in the bazedoxifene groups were not different from those in the placebo group after 3 years in the global study of postmenopausal women with osteoporosis (data on file), indicating that long-term treatment with bazedoxifene is unlikely to have clinically relevant effects on the PTH-calcium axis.
The favorable effects of bazedoxifene on the endometrium, breasts, and ovaries observed in this study were consistent with reproductive tract safety evaluations22, 23 in the global phase 3 studies of osteoporosis prevention and treatment. The incidence of breast cancer was low in this study (one subject each with bazedoxifene 20 mg and placebo), and there were no reports of cervical or endometrial cancer.
In conclusion, in this population of postmenopausal Japanese women with osteoporosis, bazedoxifene improved BMD, reduced bone turnover, and was associated with a favorable safety and tolerability profile. Collectively, these findings suggest that bazedoxifene may provide a safe and effective new therapy for the treatment of osteoporosis in postmenopausal Japanese women.
Disclosures
AI reports serving as a consultant for Wyeth, Chugai, and Ohtsuka. KY reports serving as a consultant for Wyeth and Eli Lilly. TM reports serving as a consultant for Wyeth. IG reports serving as a consultant for Wyeth and as a speaker for Chugai and Eli Lilly. TS reports serving as a consultant for Wyeth, Asahi Kasei, and Daiichi-Sankyo. HM reports serving as a consultant for Wyeth, Chugai, Shiseido, Ono, and Aska. HO reports serving as a consultant for Wyeth, Pola-Pharma, Otsuka, Teijin Pharma, Eisai, Novartis, Banyu, Tsumura, Bayer, Chugai, and Takeda. AAC, HO, and GDC were employees of Wyeth, which was acquired by Pfizer Inc, in October 2009. HS, IG, TS, HM, and HO served as members of the safety monitoring committee for this study. KY, TM, and MT served as members of the fracture evaluation committee for this study. All authors had full and unrestricted access to the study data and were involved in the analysis and interpretation of data and development/critical revising of the manuscript and approved the final version submitted.
Acknowledgements
This study was sponsored by Wyeth, which was acquired by Pfizer Inc, in October 2009. Medical writing support was provided by Kimberly Brooks, PhD, of MedErgy and was funded by Wyeth.
Unique trial number: NCT00238745; trial registration date: October 12, 2005. Principal Investigators and Investigational Sites: Hiroshi Wada (Wada Women's Clinic); Akira Honda, Hisashi Kudo, and Testu Shozawa (Niigata Medicalcare Cooperative Kido Hospital); Susumu Tsukikawa (Tsukikawa Lady's Clinic); Naoki Fujita (Nanju Kinen Clinic); Hiroshige Itakura (Shinagawa East One Medical Clinic); Hikaru Ishii (Shin-Nihonbashi Ishii Clinic); Masanari Omata (Oimachi Orthopaedic Clinic); Hiroshi Shimomura (Masashino Clinic); Jui-Tung Chen (Jui-Tung Chen Clinic); Masataka Karube (NS Clinic); Minoru Tsuboi and Chino Nakamura (Yokohama Minoru Clinic); Osamu Chaki and Kentaro Kurasawa (Yokohama City University Hospital); Kenji Hirota (Nissay Hospital); Sumitada Okamoto (KS Okamoto Clinic); Sumiaki Okamoto (Sanyo Osteoporosis Research Foundation Okamoto Clinic).




