Trabecular and Cortical Microstructure and Fragility of the Distal Radius in Women
ABSTRACT
Fragility fractures commonly involve metaphyses. The distal radius is assembled with a thin cortex formed by fusion (corticalization) of trabeculae arising from the periphery of the growth plate. Centrally positioned trabeculae reinforce the thin cortex and transfer loads from the joint to the proximal thicker cortical bone. We hypothesized that growth- and age-related deficits in trabecular bone disrupt this frugally assembled microarchitecture, producing bone fragility. The microarchitecture of the distal radius was measured using high-resolution peripheral quantitative computed tomography in 135 females with distal radial fractures, including 32 girls (aged 7 to 18 years), 35 premenopausal women (aged 18 to 44 years), and 68 postmenopausal women (aged 50 to 76 years). We also studied 240 fracture-free controls of comparable age and 47 healthy fracture-free premenopausal mother-daughter pairs (aged 30 to 55 and 7 to 20 years, respectively). In fracture-free girls and pre- and postmenopausal women, fewer or thinner trabeculae were associated with a smaller and more porous cortical area (r = 0.25 to 0.71 after age, height, and weight adjustment, all p < 0.05). Fewer and thinner trabeculae in daughters were associated with higher cortical porosity in their mothers (r = 0.30 to 0.47, all p < 0.05). Girls and premenopausal and postmenopausal women with forearm fractures had 0.3 to 0.7 standard deviations (SD) fewer or thinner trabeculae and higher cortical porosity than controls in one or more compartment; one SD trait difference conferred odds ratio (95% confidence interval) for fracture ranging from 1.56 (1.01–2.44) to 4.76 (2.86–7.69). Impaired trabecular corticalization during growth, and cortical and trabecular fragmentation during aging, may contribute to the fragility of the distal radius. © 2014 American Society for Bone and Mineral Research.
Introduction
Long-bone diaphyses (shafts), predominantly cortical structures, are usually sites of traumatic fractures. By contrast, fragility fractures, resulting from low or moderate trauma, commonly involve regions rich in both cortical and trabecular bone like the metaphyses (ends) of the distal radius, proximal humerus, distal tibia, and proximal femur.1, 2 For example, one third of children have fractures and ∼50% of the fractures involve the distal forearm.3 Forearm fractures are also the most common fractures in pre- and postmenopausal women.1, 4 The reasons for the susceptibility to fragility of metaphyses are uncertain but may involve concessions made during skeletal development so that these regions can serve paradoxical functions.
Bone must be stiff, able to resist bending to function as a lever, and yet be flexible, able to absorb energy by shortening or lengthening at the microscopic level.5, 6 Bone must also be strong, able to tolerate loading, yet light to facilitate mobility.5 Strength can be achieved by bulk, but bulk takes time to grow, is costly to maintain, and limits agility and coordination, important functions of the limbs. To circumvent the need for bulk, mass is minimized during growth by taking advantage of the strength conferred by bone's microarchitecture and the spatial configuration of its mineralized bone matrix, not only its mass.7
The spatial configuration of the microarchitecture of a region is determined by the relative proportions of mineralized bone matrix and void volumes. The greater the proportion of void volume, the lighter the structure assembled but the more vulnerable it is to becoming fragile. By convention, cortical (compact) bone is composed of >70% mineralized bone matrix and <30% void volume formed mainly by the canals traversing it (seen as porosity in cross section).8 Trabecular (spongy) bone is composed of 10% to 30% mineralized bone matrix fashioned as plates and rods occupying the medullary canal, which constitutes 70% to 90% void volume.9
Although historically entrenched, this binary anatomical division of bone “compartments” is an oversimplification that obscures the pathophysiology of the region's assembly during growth and deterioration after menopause. For example, the compartments are not independent. Metaphyseal cortical bone is trabecular in origin. During longitudinal endochondral ossification, trabeculae arise from the cartilaginous growth plate.10, 11 Those arising from its periphery thicken and coalesce (corticalize), forming a thin cortical shell while centrally placed trabeculae form trabecular bone (Fig. 1).11-13 In any cross section, the transition between corticalizing trabeculae laterally and trabeculae centrally is not distinct. This cortico-medullary junctional zone (or transitional zone) is a region of vigorous remodeling activity that distributes similar amounts of mineralized bone matrix volume with varying amounts of void volume as cortical, transitional, and trabecular bone, depending on the location of a cross section along the metaphysis (Fig. 1, inset).14-16
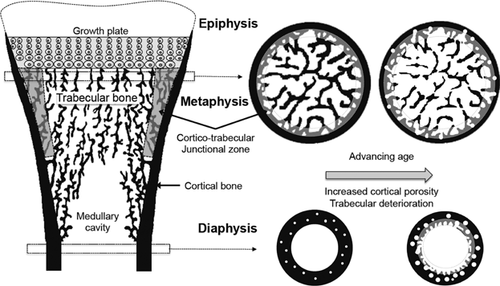
The use of minimal mass to frugally assemble this microstructure may come at a price of a low safety margin. Trabeculae abutting the thin cortical shell reinforce it like struts of a bridge and transfer loads proximally, from the joint to the predominantly cortical metaphyseal-diaphyseal junction.17 Without this reinforcement, excessive loads are imposed on the distal thin cortical bone.18 Fragility may result when growth-related, genetic, or environmental factors produce fewer trabeculae, thinner trabeculae, or prevent trabecular coalescence, causing cortical porosity and failed thickening upon the inner surface of the cortex. Fragility may also result when unbalanced and rapid bone remodeling after menopause erode trabeculae, cavitate, and fragment (trabecularize) the cortex, enlarging the transitional zone.19
Given the trabecular origin of metaphyseal cortical and trabecular bone, we hypothesized that 1) trabecular and cortical morphology correlate in healthy women and in daughter-mother pairs, reflecting the heritability of these traits; 2) women with forearm fractures have fewer and thinner trabeculae and higher cortical porosity than controls; and 3) deficits are likely to be growth related in children and premenopausal women but age-related in postmenopausal women.
Materials and Methods
Subjects
Forty healthy girls aged 7 to 18 years and 70 postmenopausal women aged ≥50 years with a distal forearm fracture after minimal trauma were recruited from the fracture clinic of Austin Health, Melbourne, Australia. In addition, 40 premenopausal women younger than 45 years with distal forearm fracture were recruited at Beth Israel Deaconess Medical Center (BIDMC) and Brigham and Women's Hospital (Boston, MA, USA).20 Each case was controlled by two fracture-free females of comparable age, height, and weight (plus Tanner pubertal stage for girls) living in the same city. Control girls had no history of fracture at any site, and pre- and postmenopausal women controls had no history of fracture during their adulthood. Fracture and medical histories were documented during an interview. Subjects receiving medication that could affect bone metabolism (glucocorticoids and immunosuppressive medications, hormone-replacement therapy, bisphosphonates, parathyroid hormone, selective estrogen receptor modulators, or aromatase inhibitors) were excluded. Standing height was measured using a stadiometer to the nearest 0.1 cm. Body weight was measured using an electronic scale to the nearest 0.1 kg. Pubertal stage was self-evaluated by providing the participants with images depicting the development of pubic hair and breast in female. Menopause was defined as amenorrhea >12 months. To study heritability of bone structure, a cohort of premenopausal daughters (aged 7 to 20 years) and their premenopausal mothers (aged 30 to 55 years) were recruited in Melbourne (47 pairs).10 The study was approved by the ethics committees of Austin Health and BIDMC. Informed consent was obtained from the subjects (or their parents in the case of the children).
Measurement of microarchitecture
Microarchitecture was assessed at the nonfractures arm for the cases or nondominant distal forearm for the controls using HR-pQCT (XtremeCT, Scanco Medical AG, Brüttisellen, Switzerland). Quality was monitored by daily scans of hydroxyapatite phantoms. Stacks of 110 images (9.2 mm) were acquired with an isotropic voxel size of 82 µm.21 The first slice was 9.5 mm from the distal end of the radius in adults, whereas it was set at 4% of the radius length in girls. The compact-appearing cortical areas, outer and inner transitional zones, and trabecular (Tb) compartments were segmented using the StrAx1.0 algorithm.16 Total, cortical, and medullary cross-sectional areas (CSAs), volumetric density (vBMD), cortical porosity (Ct.Po), and trabecular bone volume fraction (BV/TV), number (Tb.N), and thickness (Tb.Th) were quantified.16, 22 CSAs are reported as the ratio of the bone compartment's CSA over the total CSA unless otherwise stated. We used the cortical CSA/total CSA ratio to express the amount of cortical bone accounting for the total bone size. The reproducibility errors for segmentation and quantification of porosity expressed as root mean square coefficients of variation ranged from 0.54% to 3.98% and were <1.5% for vBMD.16
Statistical methods
The quality of the scans was assessed using the criterion published by Pialat and colleagues, and scans with a grade >3 were discarded.23 After exclusion of poor-quality images resulting from movement artefacts, analyses included 32 cases and 78 controls in girls, 35 cases and 65 controls in the premenopausal women, and 68 cases and 97 controls in postmenopausal women. R values reported correspond to Pearson's correlation coefficient used to compute age-, height-, and weight-adjusted correlations between morphologic traits among the different age groups or between daughters and their mothers. Comparisons between cases and controls in girls and pre- and postmenopausal women were made using two-sample t tests if the data were normally distributed; otherwise, the nonparametric Mann-Whitney test was used. The analyses were carried out unadjusted for other covariates and then adjusted for age, height, and weight using generalized additive models. The p values were controlled for type 1 error because of the multiplicity of the comparisons using the false discovery rate (FDR). Associations between microarchitecture (predictors) and fractures (outcome) were assessed using univariate and multivariate logistic regression adjusted for age, height, and weight. All analyses were conducted using Stata (version 11; StataCorp., College Station, TX, USA). Sample size estimates were derived from prior data comparing bone microarchitecture assessed by HR-pQCT in subjects with or without fracture at the distal radius in children and postmenopausal women. For the primary outcome variables that included cortical and trabecular microstructure assessed by HR-pQCT, a sample size of 32 cases among the girls and 30 cases among the adults allow the detection of a 0.5 SD difference with a power of 0.80 and a α = 0.05.
Results
Associations between trabecular and cortical morphology
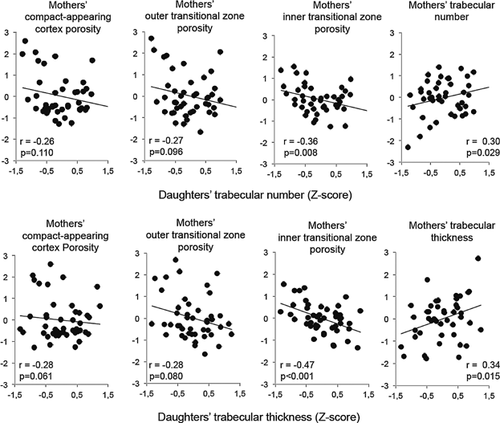
In healthy girls and pre- and postmenopausal women, thinner or fewer trabeculae were associated with a lower cortical area, a reciprocally larger medullary cavity area (r from 0.27 to 0.50, all p < 0.05) and a higher porosity in at least one of the cortical compartments (r from 0.25 to 0.73, all p < 0.05) (Fig. 2, Supplemental Figs. S1 and S2). Moreover, a larger medullary cavity was associated with a higher porosity in all of the cortical compartments (r from 0.49 to 0.80, all p < 0.001) (Supplemental Fig. S3). Fewer or thinner trabeculae in daughters were associated with fewer and thinner trabeculae and higher porosity in at least one cortical compartment (r from 0.30 to 0.47, all p < 0.05) in their mothers (Fig. 3).
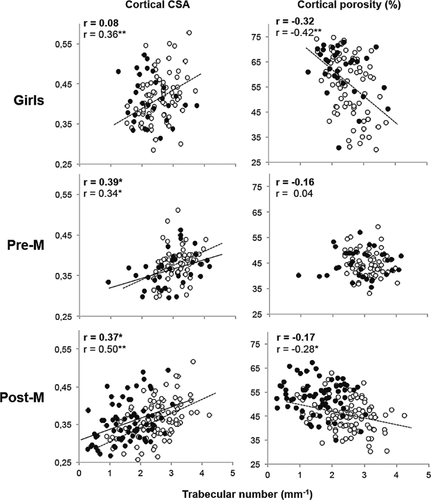
Deficits in microarchitecture in females with forearm fracture
Similar associations between trabecular and cortical morphology were found in females with distal radial fractures (Fig. 2, Supplemental Figs. S1 and S2). However, the abnormalities relative to controls depended on the age group studied. In girls with forearm fractures, trabecular BV/TV was 0.5 SD lower than nonfracture controls because of 0.4 SD fewer trabeculae. The compact-appearing cortical area was also 0.4 SD smaller, the outer transitional zone was no different, and the inner transitional zone was 0.4 SD larger in fracture cases than controls (each p < 0.05), whereas porosity was 0.3, 0.4, and 0.5 SD higher in the respective cortical compartments (all p < 0.01) (Table 1).
| Girls | Premenopausal women | Postmenopausal women | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases (n = 32) | Controls (n = 78) | Cases (n = 35) | Controls (n = 65) | Cases (n = 68) | Controls (n = 97) | ||||||||||
| Mean | SD | Mean | SD | p Value | Mean | SD | Mean | SD | p Value | Mean | SD | Mean | SD | p Value | |
| Age (years) | 11.3 | 3.1 | 11.9 | 2.9 | 0.353 | 29.4 | 7.8 | 27.8 | 5.9 | 0.289 | 66.5 | 9.1 | 64.9 | 7.4 | 0.329 |
| Weight (kg) | 42.3 | 13.8 | 43.0 | 14.5 | 0.833 | 69.3 | 15.9 | 67.3 | 13.6 | 0.513 | 71.3 | 12.5 | 66.1 | 11.9 | 0.012 |
| Height (cm) | 148.0 | 15.4 | 148.0 | 15.4 | 0.879 | 165.4 | 5.9 | 163.2 | 7.8 | 0.144 | 159.4 | 7.6 | 160.2 | 8.0 | 0.458 |
| Cross-sectional area (mm2) | |||||||||||||||
| Total | 153.5 | 48.1 | 150.4 | 37.9 | 0.720 | 214.5 | 37.4 | 205.8 | 36.0 | 0.260 | 208.9 | 39.3 | 201.7 | 34.07 | 0.217 |
| Medullary | 92.1 | 36.6 | 88.8 | 27.0 | 0.644 | 137.3 | 31.2 | 128.9 | 29.2 | 0.186 | 135.9 | 33.1 | 130.2 | 29.6 | 0.248 |
| Total cortex | 61.3 | 13.6 | 61.6 | 14.3 | 0.940 | 77.2 | 9.3 | 76.9 | 9.6 | 0.870 | 72.9 | 10.7 | 71.5 | 9.7 | 0.380 |
| Compact-appearing cortex | 37.2 | 9.7 | 39.1 | 10.8 | 0.401 | 52.7 | 6.5 | 51.9 | 7.1 | 0.571 | 51.3 | 8.5 | 50.8 | 7.0 | 0.722 |
| Outer transitional zone | 5.2 | 1.4 | 5.2 | 1.5 | 0.862 | 6.9 | 1.0 | 6.9 | 1.0 | 0.871 | 7.2 | 1.2 | 6.8 | 1.0 | 0.013* |
| Inner transitional zone | 19.0 | 4.9 | 17.3 | 4.3 | 0.073* | 17.6 | 4.3 | 18.1 | 4.0 | 0.595 | 14.4 | 4.6 | 13.9 | 3.7 | 0.391 |
| Volumetric BMD (g/cm3) | |||||||||||||||
| Total | 327.1 | 65.2 | 353.8 | 86.5 | 0.234 | 373.3 | 53.1 | 401.6 | 48.1 | 0.008* | 315.1 | 64.5 | 354.8 | 66.0 | <0.001* |
| Cortical | 623.4 | 102.0 | 677.3 | 122.1 | 0.012* | 753.6 | 42.5 | 759.2 | 39.6 | 0.513 | 653.6 | 59.8 | 734.1 | 61.0 | <0.001* |
| Trabecular | 130.8 | 36.6 | 135.7 | 33.9 | 0.516 | 145.1 | 37.3 | 176.8 | 34.0 | <0.001* | 111.8 | 44.9 | 130.4 | 41.9 | 0.007* |
| Cortical porosity (%) | |||||||||||||||
| Compact-appearing cortex | 46.5 | 10.9 | 40.9 | 12.9 | 0.023* | 29.0 | 3.8 | 28.5 | 3.8 | 0.537 | 41.5 | 7.2 | 33.3 | 7.3 | <0.001* |
| Outer transitional zone | 72.1 | 7.3 | 67.7 | 8.8 | 0.020* | 59.9 | 3.9 | 58.7 | 3.6 | 0.100 | 67.1 | 4.7 | 61.2 | 5.1 | <0.001* |
| Inner transitional zone | 89.8 | 2.9 | 87.1 | 3.7 | <0.001* | 82.4 | 2.7 | 80.6 | 2.5 | 0.002* | 86.2 | 2.8 | 82.8 | 2.5 | <0.001* |
| Trabecular bone microstructure | |||||||||||||||
| Tb.BV/TV (%) | 2.74 | 1.35 | 3.67 | 1.70 | 0.016 | 1.49 | 0.88 | 2.57 | 1.46 | <0.001* | 2.00 | 1.16 | 3.08 | 1.79 | <0.001* |
| Tb.N (mm-1) | 2.24 | 0.50 | 2.54 | 0.48 | 0.048 | 2.89 | 0.73 | 3.11 | 0.42 | 0.054* | 1.76 | 0.50 | 2.47 | 0.68 | <0.001* |
| Tb.Th (mm) | 0.06 | 0.02 | 0.06 | 0.02 | 0.228 | 0.05 | 0.02 | 0.07 | 0.02 | <0.001* | 0.04 | 0.02 | 0.05 | 0.02 | <0.001* |
- CSA = cross-sectional area; BMD = bone mineral density; Tb.BV/TV = trabecular bone volume fraction; Tb.N = trabecular number; Tb.Th = trabecular thickness.
- * p < 0.05 after adjusting for age, height, and weight and correcting for multiple testing, using false discovery rate (FDR).
In premenopausal women with forearm fractures, trabecular BV/TV was 0.5 SD lower than controls because of 0.3 SD fewer and 0.5 SD thinner trabeculae (all p < 0.05). No deficits in cortical compartment areas were detected, whereas porosity was 0.4 SD higher in fracture cases in the inner transitional zone only (p < 0.05) (Table 1).
In postmenopausal women with forearm fractures, trabecular BV/TV was 0.4 SD lower than controls because of 0.7 SD fewer and 0.7 SD thinner trabeculae. Only the outer transitional zone area was 0.3 SD larger (p < 0.001). Porosity was 0.6 SD higher in each of the three cortical compartments (all p < 0.001) (Table 1).
Associations between microarchitecture and forearm fractures
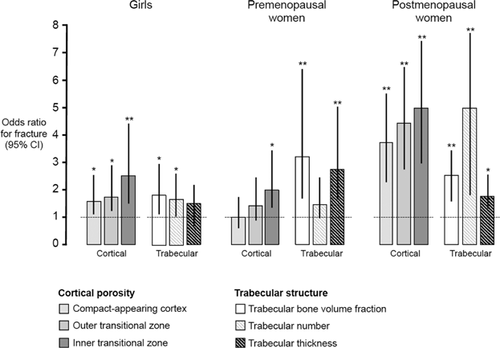
In univariate regression analyses, lower trabecular BV/TV as well as fewer or thinner trabeculae were associated with fracture in all three groups (OR for 1 SD deficit [95% CI] ranged from 1.56 [1.01–2.44] to 4.76 [2.86–7.69], all p < 0.05). Higher porosity of each of the three cortical compartments was associated with forearm fracture in girls and postmenopausal women (OR for 1 SD increase [95% CI] ranged from 1.64 [1.03–2.62] to 4.71 [2.85–7.78], all p < 0.05); only porosity of the inner transitional zone was associated with forearm fracture in premenopausal women (OR [95% CI] = 2.05 (1.26–3.32), p = 0.004).
In multivariate regression analyses, independent associations with fracture were observed for inner transitional zone porosity in girls, trabecular BV/TV in premenopausal women, and compact-appearing cortical porosity and trabecular number in postmenopausal women, with OR ranging from 2.49 to 3.44 for a 1 SD difference in the trait of interest (Fig. 4, Supplemental Fig. S4).
Discussion
Our aim was to identify the microstructural deficits in bone that lead to skeletal fragility. We report correlations between trabecular and cortical morphology in healthy girls and pre- and postmenopausal women. In each age group, fewer or thinner trabeculae were associated with a smaller cortical area, a reciprocally larger medullary canal area, and a higher cortical porosity. In addition, a larger medullary area was associated with higher porosity. Moreover, fewer and thinner trabeculae in healthy daughters were associated with fewer and thinner trabeculae and higher cortical porosity in their mothers. Most of these features were amplified in girls and women with forearm fractures; trabeculae were fewer or thinner and porosity was higher in one or more cortical compartments than in controls.
Studies of bone histomorphometry in rabbits demonstrate that trabecular bone forms both trabecular and cortical bone of the metaphyseal region.13 Studies of bone microarchitecture in baboons demonstrate that ∼60% of the variation in cortical bone microstructure is accounted for by genetic factors.24, 25 Likewise, studies in human subjects show that identical twins have higher same-trait correlations (eg, cortical porosity in one twin versus cortical porosity in the co-twin) than nonidentical twins, and higher different-trait correlations (eg, cortical porosity in one twin versus medullary area in the co-twin) than nonidentical twins.26 These studies, and the associations between trabecular morphology in daughters and cortical morphology in mothers, suggest that differences in trabecular and cortical morphology between individuals are largely the result of shared genes, shared environment, or both.24, 25, 27
As reported in another cohort, medullary area and cortical porosity also correlated.28 Assembly of the medullary cavity void space and intracortical Haversian and Volkmann canal voids confer strength and minimize the amount of material needed to do so by altering the spatial configuration of the mineralized bone matrix. Endocortical resorption offsets cortical thickening and enlarges the medullary canal, which shifts the increasingly porous cortex radially. Wider bones can be constructed with less mass relative to their size because this spatial arrangement of mass optimizes the resistance of the bone to bending and torsional loading.29
Deficits in trabecular bone volume fraction, number, or thickness consistently observed in girls and pre- and postmenopausal women with fractures relative to controls suggest that abnormalities in the formation of trabeculae from the growth plate may play a pivotal role in the assembly of this fracture-prone region, but the contribution differs according to age.30, 31 Girls with forearm fractures had fewer trabeculae and high porosity in each cortical compartment, but only porosity of the inner transitional zone was independently associated with fracture. Eighty percent to 90% of the adult length of the radius is the result of rapid longitudinal growth at its distal metaphysis.11, 32 We suggest that this longitudinal growth outpaces trabecular apposition and thickening, which delays coalesecence-corticalization of adjacent trabeculae, leaving a transiently thinner more porous compact-appearing cortex and a reciprocally larger and more porous transitional zone. Fractures are more likely should a fall occur during this transitory phase of bone fragility. When longitudinal growth slows with closure of the epiphyses at puberty, trabecular apposition “catches up,” trabeculae thicken and coalesce, and porosity is “infilled,” thickening the cortex from within with a reciprocal reduction in the transitional zone area and medullary area.33-35
Premenopausal women with forearm fractures had deficits in trabecular morphology with normal cortical areas and only small deficits in cortical morphology; porosity was higher than in controls but only in the inner transitional zone.
Only deficits in trabecular bone volume fraction correlated independently with fracture. We cannot determine whether these deficits are the result of reduced accrual, bone loss, or both, but we suggest that reduced accrual during growth is more likely because remodeling imbalance has not been reported before midlife.36, 37 Nevertheless, if trabecular bone loss does occur before menopause as reported by some investigators,38, 39 it is plausible that remodeling in the high normal range may remove thinner trabeculae because resorption depth, 50 to 120 microns, is close to the thickness of trabeculae.9, 40
Postmenopausal women with forearm fractures had fewer and thinner trabeculae and high porosity in each cortical compartment. Unbalanced and rapid remodeling is more likely to be responsible for the structural deficits than reduced accrual.36, 37, 41 However, the deficit in trabecular number and thickness may also arise from reduced accrual of peak trabecular number and thickness and a high peak cortical porosity. This is plausible because the intercept of the regression line of porosity on age in females with fractures was ∼0.5 SD higher than in controls, without a difference in slope.42
Our study has limitations. The study was cross sectional. We cannot establish whether deficits in morphology are the result of reduced accrual, bone loss, or both. However, bone loss is unlikely to occur in children and premenopausal women. Further work is needed before we can attribute daughter-mother correlations between trabecular and cortical morphology to shared genetic or environmental factors. Bone remodeling markers were not available, so we cannot assess whether the lower trabecular bone volume fraction in premenopausal women is associated with higher remodeling rates, an observation reported based on female twins.43, 44 The premenopausal women with fractures were scanned in Boston. Although calibration of scanners may differ,42 the analysis using a nonthreshold-based image analysis avoids interscanner differences.16 We did not stratify subjects according risk factors for skeletal fragility such as dietary, lifestyle factors, or family history of fracture. Most subjects were white. Only about 10% were Asian, so the findings cannot be generalized to other populations with different proportions of different ethnic groups. Finally, the regions measured in the children and the adults may differ anatomically. Adults were scanned using the default mode (9.5 mm from the distal end of the radius), whereas the children were measured at 4% of their radius length. However, the different age groups were not compared, and in the mother-daughter cohort, this is likely to underestimate the trait correlation.10
In conclusion, assembly of a lighter skeleton is advantageous in youth and young adulthood. However, configuring the microarchitecture of the skeleton during growth with less mineralized bone matrix volume and a reciprocally more void volume becomes a liability with longevity. Reduced numbers of trabeculae arising from the growth plate, reduced thickening of trabeculae, or both impair corticalization of the metaphyseal region, producing a smaller, more porous cortical compartment and a larger medullary canal containing fewer and thinner trabeculae. In the setting of a higher porosity and a larger medullary canal, more intracortical and endocortical surface is available for unbalanced and accelerated remodeling to be initiated upon after menopause. Erosion of this precariously assembled structure leaves it susceptible to becoming fragile and to fracture should a fall occur.
Disclosures
RMZD, AGZ, and ES are inventors of the StrAx1.0 software. All other authors state that they have no conflicts of interest.
Acknowledgments
Authors' roles: Drafting manuscript: YB and ES. Revising manuscript content: YB, AGZ, RMZD, TDR, SI, QW, QMB, XFW, MLB, and ES. Approving final version of manuscript: YB, AGZ, RMZD, TDR, SI, QW, QMB, XFW, MLB, and ES. QMB and YB take responsibility for the data analysis. ES takes responsibility for the integrity of the data analysis.




